Structural and Immunological Activity Characterization of a Polysaccharide Isolated from Meretrix meretrix Linnaeus
Abstract
:1. Introduction
2. Results and Discussion
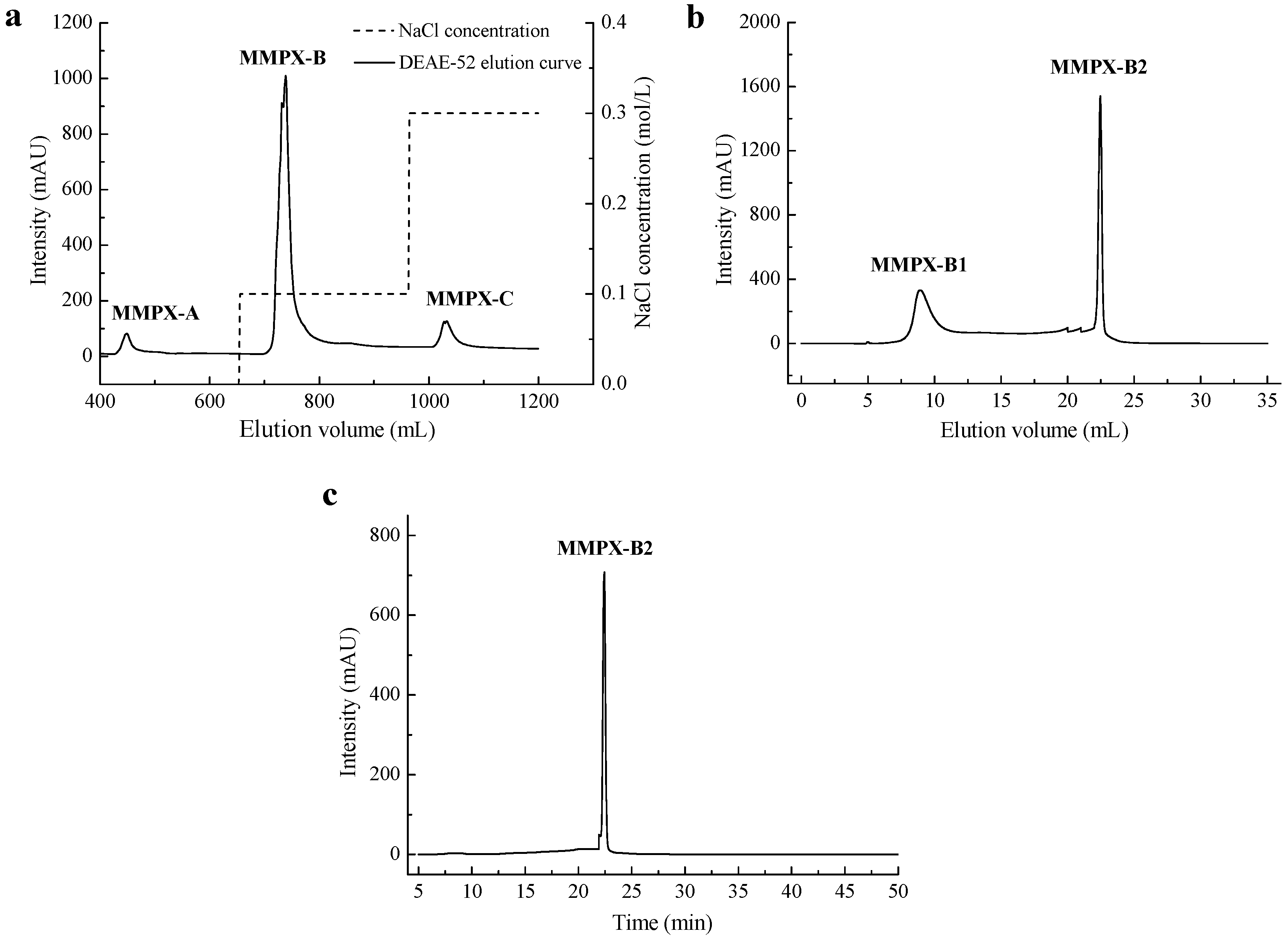
2.1. Extraction and Purification of MMPX-B2
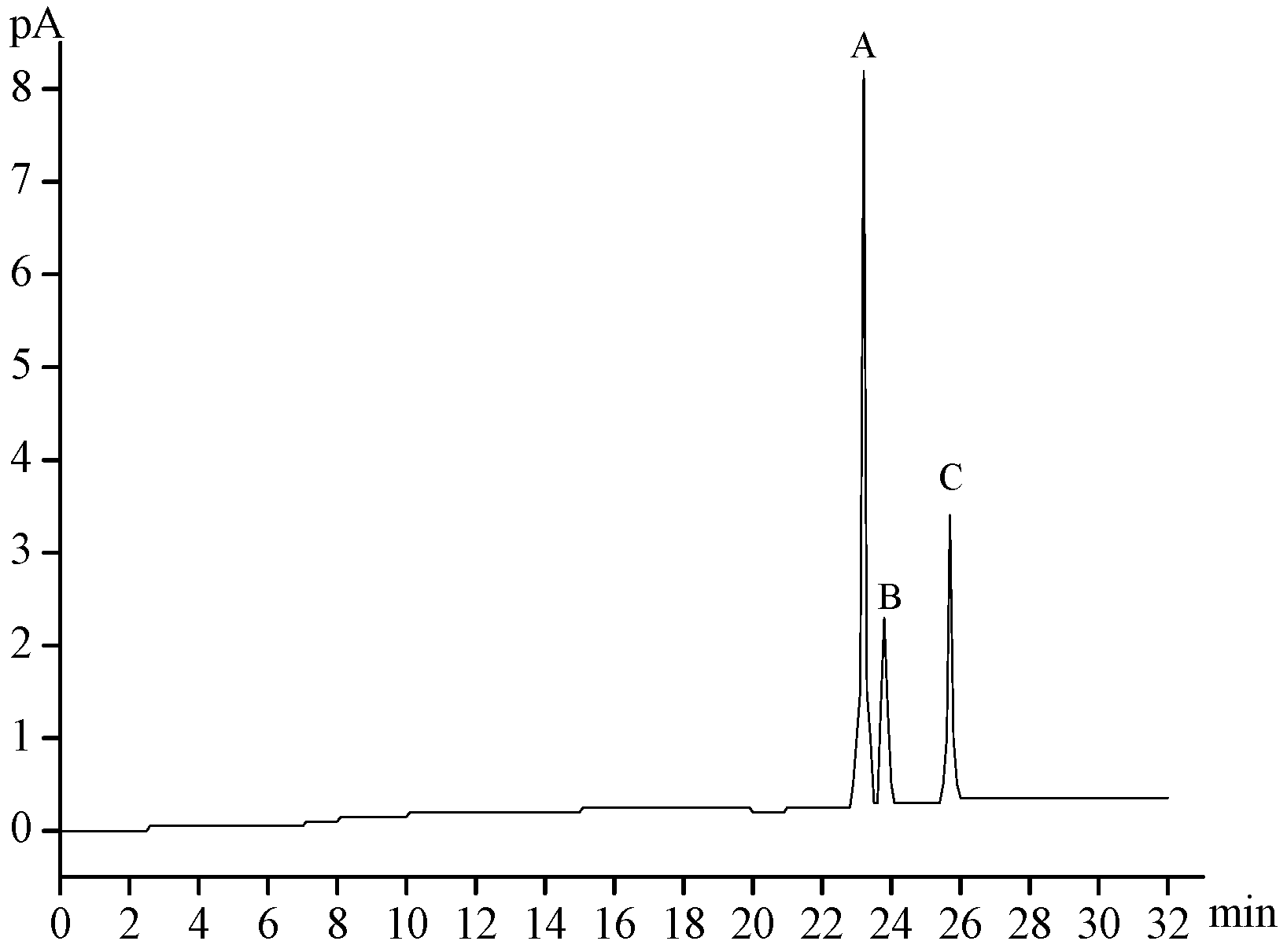
2.2. Chemical Composition of MMPX-B2
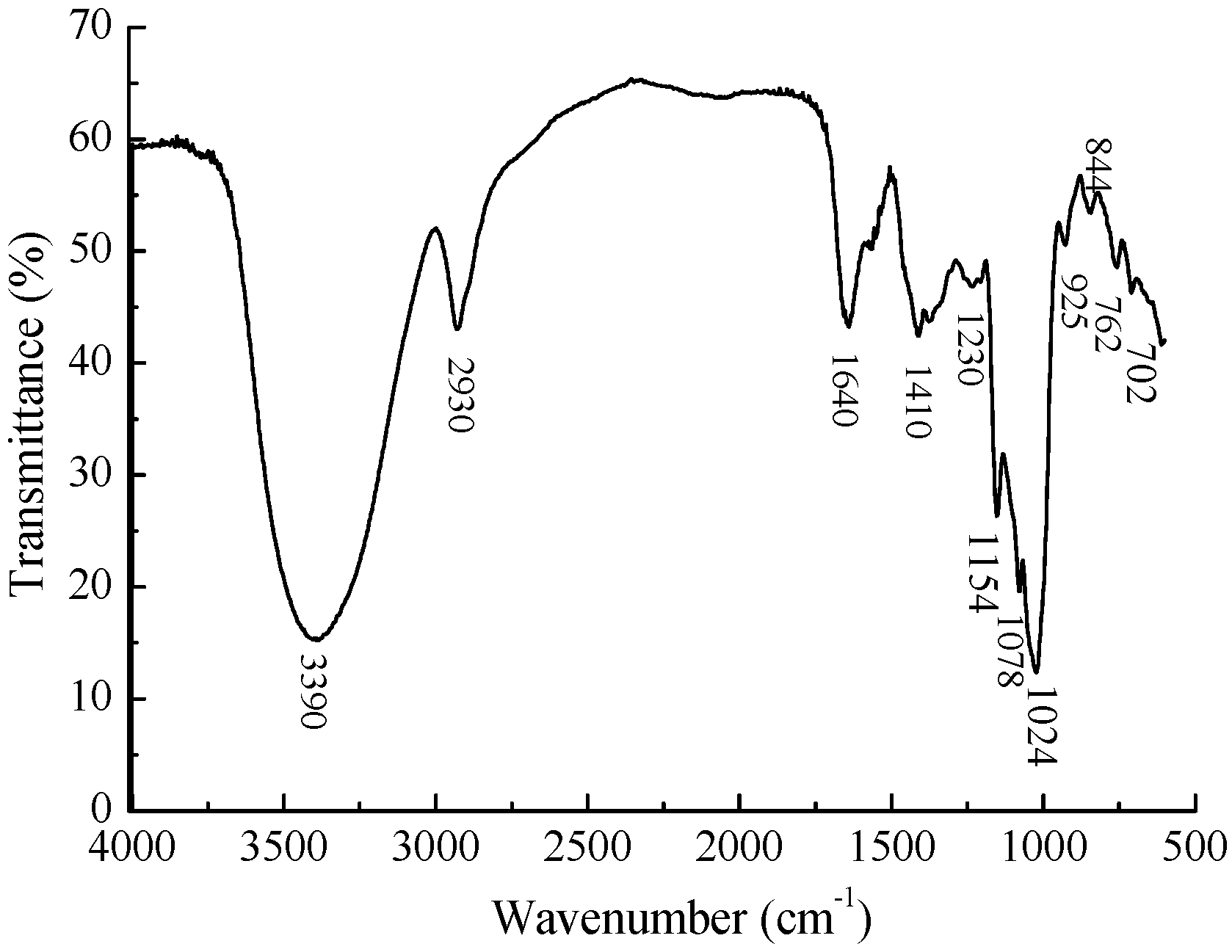
2.3. Linkage Analysis and Structure Speculation
| Methylated Sugar Residue | Molar Ratio | Ratio (%) | Type of Linkage |
|---|---|---|---|
| 2,3,4,6-Me4-Gal | 1.03 | 17.64 | Galp-(1→ |
| 2,4,6-Me3-Gal | 0.30 | 5.14 | →3)-Galp-(1→ |
| 2,3,6-Me3-Glc | 3.51 | 60.10 | →4)-Glcp-(1→ |
| 2,3-Me2-Glc | 1.00 | 17.12 | →4,6)-Glcp-(1→ |
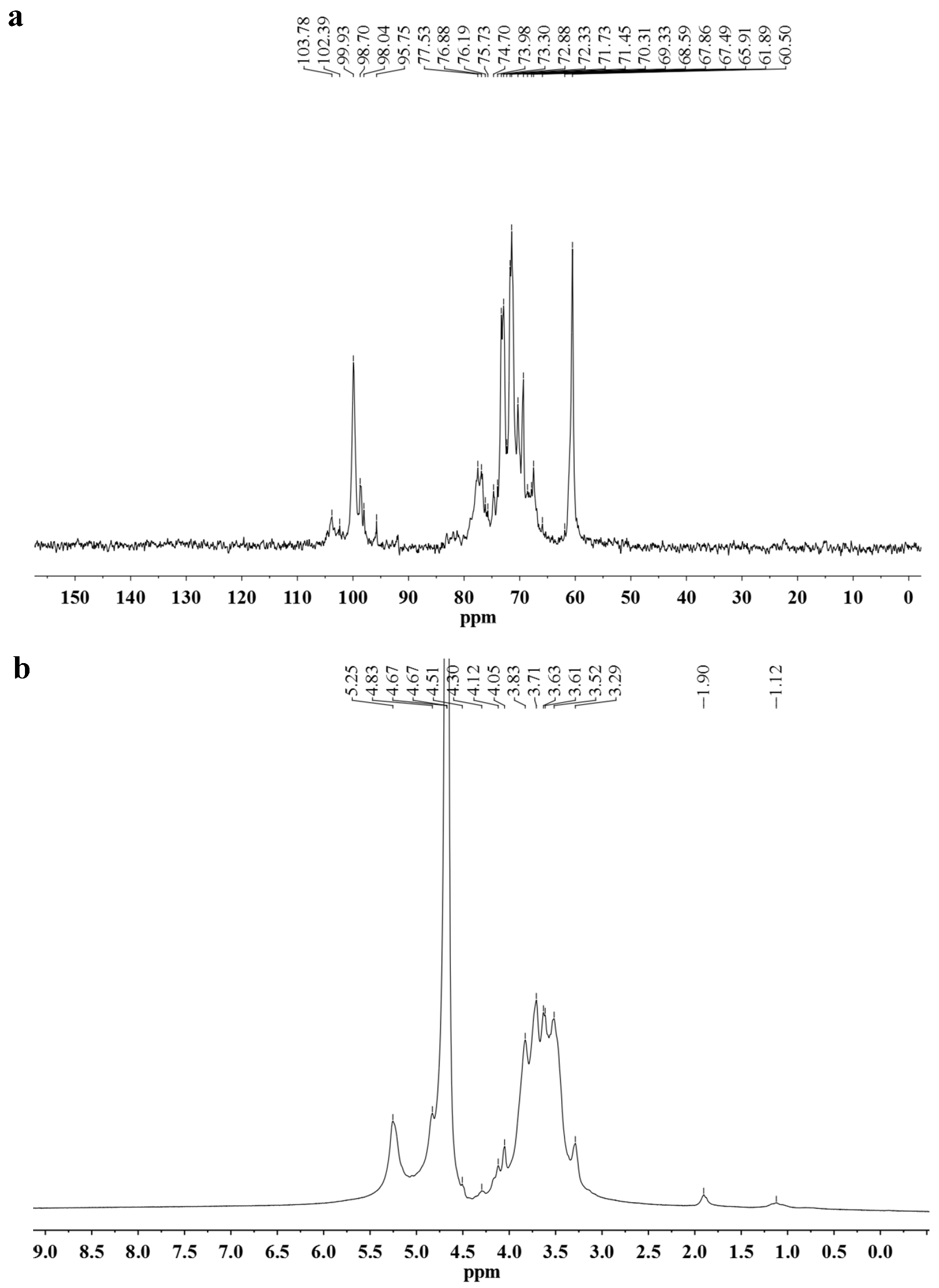
| Residues | Chemical Shift (δ, ppm) | |||||
|---|---|---|---|---|---|---|
| C1/H1 | C2/H2 | C3/H3 | C4/H4 | C5/H5 | C6/H6 | |
| →4)-α-Glcp-(1→ | 99.92/5.25 | 71.45/3.63 | 73.30/3.97 | 76.88/3.63 | 71.25/3.83 | 60.50/3.71 |
| →4,6)-α-Glcp-(1→ | 98.70/5.30 | 72.33/3.52 | 74.70/3.71 | 78.45/3.61 | 71.28/3.63 | 61.20/3.45 |
| β-Galp-(1→ | 103.78/4.49 | 72.30/3.54 | 74.10/3.63 | 69.94/4.12 | 76.70/3.71 | 61.90/3.73 |
| →3)-β-Galp-(1→ | 102.39/4.75 | 71.73/3.83 | 83.10/3.90 | 69.90/4.17 | 75.73/3.73 | 61.90/3.71 |

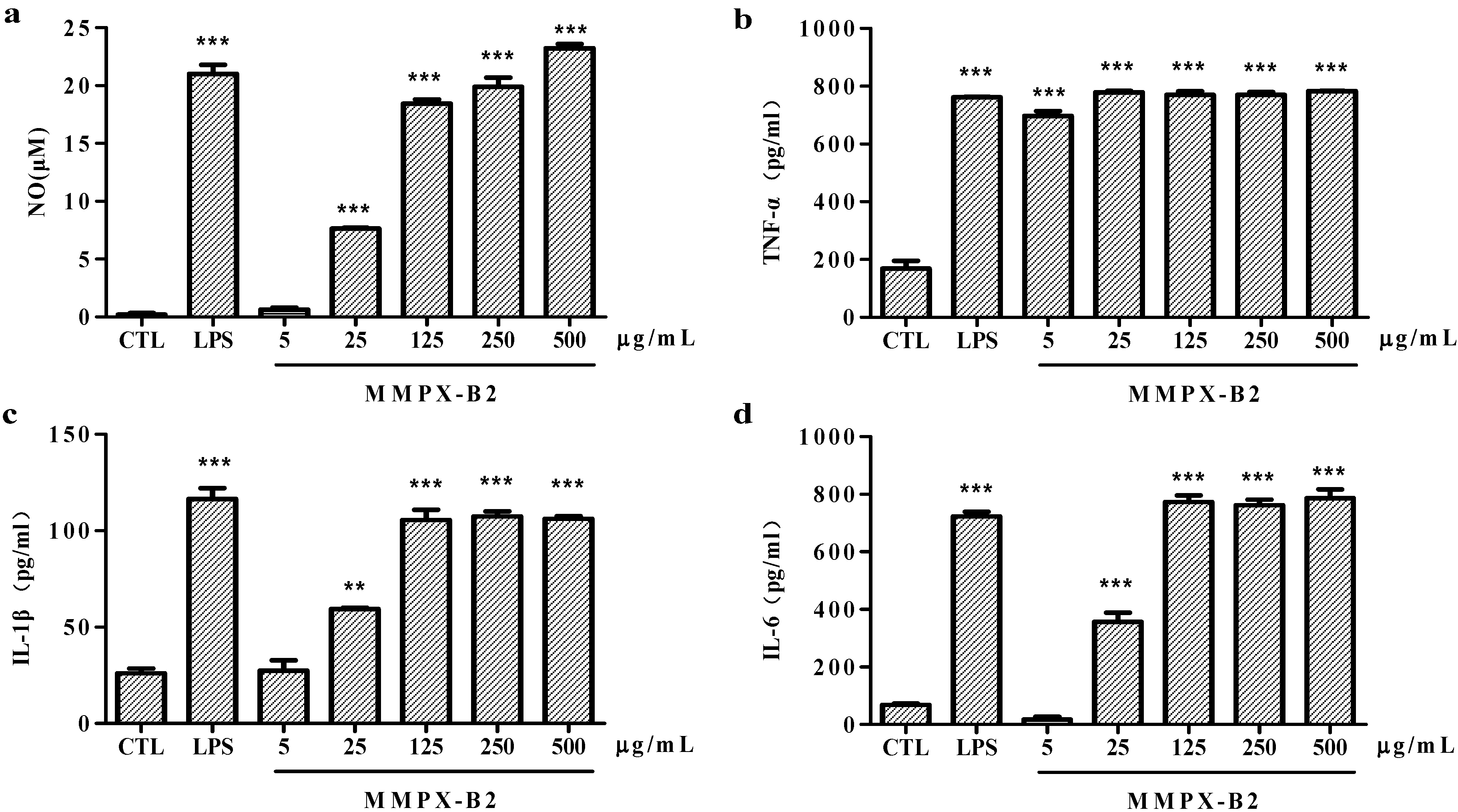
2.4. Cell-Mediated Immunological Activity
3. Experimental Section
3.1. Materials
3.2. Extraction, Isolation and Purification of MMPX-B2
3.3. Homogeneity and Molecular Weight Determination
3.4. Analysis of Monosaccharide Composition
3.5. FT-IR Analysis
3.6. Periodate Oxidation and Smith Degradation
3.7. Methylation Analysis
3.8. NMR Spectroscopy
3.9. Hydrolysis of MMPX-B2 with α-Amylase
3.10. Assay of Cell-Mediated Immunity
3.10.1. Cell Culture
3.10.2. Quantitative Determination of NO and Cytokines
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Supatra, K.; Soottawat, B.; Hideki, K.; Yung-Hsiang, T. Chemical compositions and nutritional value of Asian hard clam (Meretrix lusoria) from the coast of Andaman Sea. Food Chem. 2013, 141, 4138–4145. [Google Scholar]
- Xie, W.Y.; Chen, C.; Liu, X.S.; Wang, B.; Sun, Y.; Ma, Y.; Zhang, X. Meretrix meretrix: Active components and their bioactivities. Life Sci. J. 2012, 9, 756–762. [Google Scholar]
- Jiang, C.X.; Xiong, Q.P.; Gan, D.; Jiao, Y.; Liu, J.; Ma, L.; Zeng, X. Antioxidant activity and potential hepatoprotective effect of polysaccharides from Cyclina sinensis. Carbohydr. Polym. 2013, 91, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.X.; Wang, M.; Liu, J.; Gan, D.; Zeng, X. Extraction, preliminary characterization, antioxidant and anticancer activities in vitro of polysaccharides from Cyclina sinensis. Carbohydr. Polym. 2011, 84, 851–857. [Google Scholar] [CrossRef]
- Xu, X.L.; Li, T.M.; Zhang, C.R. Experimental study on the stability of thrombin activity. Chin. J. Biochem. Pharm. 1999, 20, 298–299. [Google Scholar]
- Xie, H.; Zhong, Z.W.; Zhu, W.C.; Cao, K.G. Process of research on the medicinal value of Meretrix meretrix Linnaeus. J. Chengde Pet. Coll. 2005, 7, 9–12. [Google Scholar]
- Zhang, L.X.; Fan, X.; Niu, R.L. Immunomodulatory activity determination of ethanol extracts from some marine invertebrates. Chin. J. Immunol. 2003, 19, 739–743. [Google Scholar]
- Flint, H.J.; Bayer, E.A.; Rincon, M.T.; Lamed, R.; White, B.A. Polysaccharide utilization by gut bacteria: Potential for new insights from genomic analysis. Nat. Rev. Microbiol. 2008, 6, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Barlow, G.M.; Yu, A.; Mathur, R. Role of the gut microbiome in obesity and diabetes mellitus. Nutr. Clin. Pract. 2015, 30, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Lin, C.S.; Lu, C.C.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Tseng, S.F.; Wu, T.R.; Chen, Y.Y.M.; Young, J.D.; et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 2015, 6, 7489–7506. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.X.; Xiong, Q.P.; Li, S.L.; Zhao, X.R.; Zeng, X.X. Structural characterization, sulfation and antitumor activity of a polysaccharide fraction from Cyclina sinensis. Carbohydr. Polym. 2015, 115, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Liao, N.B.; Chen, S.G.; Ye, X.Q.; Zhong, J.J.; Wu, N.; Dong, S.L.; Yang, B.; Liu, D.H. Antioxidant and antitumor activity of a polysaccharide from freshwater clam Corbicula flumineat. Food Funct. 2013, 4, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Vidhyanandhini, R.; Vairamani, S. The anticoauulant activity and structural characterization of fractionated and purified glycosaminoglycans from venerid clam Meretrix casta (Chemnitz). J. Liq. Chromatogr. Relat. Technol. 2014, 37, 917–929. [Google Scholar] [CrossRef]
- Saravanan, R.; Shanmugam, A. Isolation and characterization of heparin sulfate from marine scallop Amusium pleuronectes (Linne) an alternative source of heparin. Carbohydr. Polym. 2011, 86, 1082–1084. [Google Scholar] [CrossRef]
- Pan, T.T.; Peng, S.H.; Xu, Z.L.; Xiong, B.; Wen, C.R.; Yao, M.; Pang, J. Synergetic degradation of konjacglucomannan by γ-ray irradiation and hydrogen peroxide. Carbohydr. Polym. 2013, 93, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.B.; Liang, H.P.; Wu, Y. Isolation, purification and structural characterization of polysaccharide from Acanthopanax brachypus. Carbohydr. Polym. 2015, 127, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.R.; Xie, L.L.; Chen, Y.; Zhang, H. Purification, characterization and anticoagulant activity of the polysaccharides from green tea. Carbohydr. Polym. 2013, 92, 1086–1090. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.P.; Dong, Q.; Chen, H.J.; Cong, Q.F.; Ding, K. Structural characterization of a polysaccharide from Chrysanthemum morifolium flowers and its antioxidant activity. Carbohydr. Polym. 2015, 130, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.T.; Li, N.S.; Wan, J.B.; Zhang, D.Z.; Yan, C.Y. Structural characterization and antioxidant activity of a novel heteropolysaccharide from the submerged fermentation mycelia of Ganoderma capense. Carbohydr. Polym. 2015, 134, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.Z.; Luo, Z.; Liu, D.; Ning, Z.X.; Yang, J.G.; Ren, J.Y. Structure characterization of a novel polysaccharide from Dictyophora indusiata and its macrophage immunomodulatory activities. J. Agric. Food Chem. 2015, 63, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Zhao, Y.Y.; Su, T.T.; Zhang, J.; Wang, F. Characterization and antioxidant activity in vitro and in vivo of polysaccharide purified from Rana chensinensis skin. Carbohydr. Polym. 2015, 126, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; You, L.J.; Fu, X.; Huang, Q.; Yu, S.J.; Liu, R.H. Structural characterization and immunomodulatory activity of a new hetero polysaccharide from Prunella vulgaris. Food Funct. 2015, 6, 1557–1567. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.N.; Niu, Y.G.; Lv, J.L.; Xie, Z.H.; Jin, L.; Yao, W.B.; Gao, X.D.; Yu, L.L. Characterization of a hetero polysaccharide isolated from diploid Gynostemma pentaphyllum Makino. Carbohydr. Polym. 2013, 92, 2111–2117. [Google Scholar]
- Dobruchowska, J.M.; Meng, X.F.; Leemhuis, H.; Gerwig, G.J.; Dijkhuizen, L.; Kamerling, J.P. Glucooligomers initially formed by the reuteransucrase enzyme of Lactobacillus reuteri 121 incubated with sucrose and malto-oligosaccharides. Glycobiology 2013, 23, 1084–1096. [Google Scholar] [CrossRef] [PubMed]
- Bushneva, O.A.; Ovodova, R.G.; Shashkov, A.S.; Ovodov, Y.S. Structural studies on hairy region of pectic polysaccharide from campion Silene vulgaris (Obernabehen). Carbohydr. Polym. 2002, 49, 471–478. [Google Scholar] [CrossRef]
- Amornrut, C.; Toida, T.; Imanari, T.; Woo, E.R.; Park, H.; Linhardt, R.; Wu, S.J.; Kim, Y.S. A new sulfated β-galactan from clams with anti-HIV activity. Carbohydr. Res. 1999, 321, 121–127. [Google Scholar] [CrossRef]
- Dai, Z.Y.; Zhang, H.; Zhang, Y.P.; Wang, H.H. Chemical properties and immunostimulatory activity of a water-soluble polysaccharide from the clam of Hyriopsis cumingii Lea. Carbohydr. Polym. 2009, 77, 365–369. [Google Scholar] [CrossRef]
- Papawee, S.; Kazuhiro, N.; Toshihiko, T.; Leo, J.L.D.; Van, G. Structural characterization and immunomodulatory effects of polysaccharides from Phellinus linteus and Phellinus igniarius on the IL-6/IL-10 cytokine balance of the mouse macrophage cell lines (RAW 264.7). Food Funct. 2015, 6, 2834–2844. [Google Scholar]
- Wasser, S. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. J. Microbiol. Biotechnol. 2002, 60, 258–274. [Google Scholar]
- Xu, X.; Wu, X.T.; Wang, Q.Q.; Cai, N.; Zhang, H.X.; Jiang, Z.D.; Wan, M.; Oda, T. Immunomodulatory effects of alginate oligosaccharides on murine macrophage RAW264.7 cells and their structure-activity relationships. Food Chem. 2014, 62, 3168–3176. [Google Scholar] [CrossRef] [PubMed]
- Mao, K.R.; Chen, S.Z.; Chen, M.K.; Ma, Y.L.; Wang, Y.; Huang, B.; He, Z.Y.; Zeng, Y.; Hu, Y.; Sun, S.H.; et al. Nitric oxide suppresses NLRP3 inflammasome activation and protects against LPS-induced septic shock. Cell Res. 2013, 23, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Yakut, E.; Jakobs, C.; Peric, A.; Michel, G.; Baal, N.; Bein, G.; Brüne, B.; Hornung, V.; Hackstein, H. Extracorporeal photopheresis promotes IL-1β production. J. Immunol. 2015, 194, 2569–2577. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.D.; Zhang, J.; Lan, R.; Yang, H.L.; Sun, Y.Y. A simple preparative method for isolation and purification of polysaccharides from mulberry (Morusalba, L.) leaves. Int. J. Food Sci. Technol. 2013, 48, 1275–1281. [Google Scholar] [CrossRef]
- Zhao, T.; Zhou, Y.; Mao, G.H.; Zou, Y.; Zhao, J.L.; Bai, S.Q.; Yang, L.Q.; Wu, X.Y. Extraction, purification and characterisation of chondroitin sulfate in Chinese sturgeon cartilage. J. Sci. Food Agric. 2013, 93, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.B.; Liu, X.M.; Tian, D.; Sun, X. Purification, chemical characterization and radical scavenging activities of alkali-extracted polysaccharide fractions isolated from the fruit bodies of Tricholoma matsutake. World J. Microbiol. Biotechnol. 2013, 29, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Li, N.S.; Yan, C.Y.; Hua, D.S.; Zhang, D.Z. Isolation, purification, and structural characterization of a novel polysaccharide from Ganoderma capense. Int. J. Biol. Macromol. 2013, 57, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.C.; Jiang, C.X.; Ma, L.P.; Zhang, Z.J.; Cao, L. Preparation, preliminary characterization and immunostimulatory activity of polysaccharide fractions from the peduncles of Hovenia dulcis. Food Chem. 2013, 138, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.; Lipkin, D. Spectrophotometric determination of vicinal glycols. Anal. Chem. 1954, 26, 1092–1093. [Google Scholar] [CrossRef]
- Ciucanu, I.; Kerek, F. A simple and rapid method for permethylation of carbohydrates. Carbohydr. Res. 1984, 131, 209–217. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Li, H.; Qian, J.; He, Y.; Zheng, J.; Lu, Z.; Xu, Z.; Shi, J. Structural and Immunological Activity Characterization of a Polysaccharide Isolated from Meretrix meretrix Linnaeus. Mar. Drugs 2016, 14, 6. https://doi.org/10.3390/md14010006
Li L, Li H, Qian J, He Y, Zheng J, Lu Z, Xu Z, Shi J. Structural and Immunological Activity Characterization of a Polysaccharide Isolated from Meretrix meretrix Linnaeus. Marine Drugs. 2016; 14(1):6. https://doi.org/10.3390/md14010006
Chicago/Turabian StyleLi, Li, Heng Li, Jianying Qian, Yongfeng He, Jialin Zheng, Zhenming Lu, Zhenghong Xu, and Jinsong Shi. 2016. "Structural and Immunological Activity Characterization of a Polysaccharide Isolated from Meretrix meretrix Linnaeus" Marine Drugs 14, no. 1: 6. https://doi.org/10.3390/md14010006
APA StyleLi, L., Li, H., Qian, J., He, Y., Zheng, J., Lu, Z., Xu, Z., & Shi, J. (2016). Structural and Immunological Activity Characterization of a Polysaccharide Isolated from Meretrix meretrix Linnaeus. Marine Drugs, 14(1), 6. https://doi.org/10.3390/md14010006