1. Introduction
Alzheimer’s disease (AD) is a neurodegenerative disorder amongst humans, which is characterized by loss of memory and cognition, mental depression and early mortality [
1]. Though AD is a concern amongst the ageing human population, several recent reports suggest that the disease affects an increasing number of younger people [
2,
3]. The production of Aβ-oligomers has been identified as a primary cause of neuro-degeneration in AD patients [
4]. The toxic effects of Aβ-oligomers are unlikely to decrease in the absence of effective preventive and curative measures. Unfortunately, there is no effective drug in the market for the treatment of AD. Among major AD therapies, reversing Aβ (Aβ
1-42) toxicity has been the main target for drug development [
5]. Aβ-aggregation has also been linked to increased oxidative stress causing neuronal injury and death. It is believed that preventing deposition of Aβ oligomers, reduction of oxidative stresses, or activation of disease modifying pathways could reduce the onset of AD [
6,
7]. In this regard, it is reasonable to explore the effect of natural compounds from a number of sources to evaluate their effects on the reduction of pathologic markers for AD.
A select number of seaweeds (
i.e., red, green and brown) are important components of human diets in several parts of the world, predominantly Southeast Asia. A number of
in vitro and
in vivo studies have revealed beneficial health-promoting effects of extracts and compounds isolated from certain seaweeds [
8,
9]. The wide variety of health-promoting effects of seaweeds is primarily due to their structurally diverse bioactive molecules. For instance, compounds isolated from seaweeds have been shown to possess a wide variety of biological activities, including anti-oxidant, anti-microbial, anti-cancer, anti-inflammatory, anti-coagulant and anti-obesity activities [
10]. There is a considerable interest in identifying neuro-protective compounds from seaweeds [
11,
12]. A large number of seaweed species are yet to be explored for their neuro-protective effects, particularly against β-amyloid toxicity in whole animal studies.
Red seaweeds (Rhodophyta) are known producers of bioactive proteins, sulphated polysaccharides (such as agarans, xylans and carrageenans), vitamins, minerals, pigments and several other components [
10,
13,
14]. However, this group of seaweeds remains a relatively under-utilized resource for mining pharmaceutical, nutraceutical and functional food benefits. A commercial strain of cultivated red seaweed (
Chondrus crispus or Irish Moss) is used as food and has also been demonstrated to possess significant beneficial bioactivity [
15].
C. crispus is known to contain several micro and macro-elements, various fatty acids, sterols and polysaccharides such as carrageenans [
16]. Recent studies showed the presence of bioactive peptides and prebiotics in selected seaweeds, including
C. crispus, suggesting their potential health benefits [
17,
18].
Caenorhabditis elegans is a highly suitable animal model for the study of the effects of bioactive components which might have relevance to human health. This is, in part, due to its high level of homology with the human genome [
19]. Many of the stress-induced pathways, and their components studied in
C. elegans are similar to those of humans (e.g., the insulin-like growth factor IGF-1 signal transduction pathway, and the insulin/IGF-1 signal (IIS) transduction pathway regulated SKN-1, an orthologue of human Nrf1Nrf1/2/3 [
20]). Moreover,
C. elegans has a short lifespan and can be cultured with ease in the laboratory. These characteristics make the nematode a convenient model to study biochemical and molecular responses to a variety of environmental stresses. Mechanisms of aging and age-associated neuro-degenerative diseases have been elucidated using
C. elegans [
21]. Transgenic
C. elegans strains which express human β-amyloid (Aβ), facilitate further enhanced understanding of the mechanisms of Aβ-toxicity in biological systems and can be used in the screening of therapeutic agents
in vivo [
22,
23]. Natural products such as extracts from ginkgo leaves (
Ginkgo biloba), cinnamon bark (
Cinnamomum cassia), ground coffee seeds (
Coffea spp.), green tea leaves (
Camellia sinensis), and others were found to reduce Aβ-induced paralysis in
C. elegans. Furthermore, using this model, the molecular mechanism of activity of these products was also revealed [
1,
23,
24].
In this study, we investigated the effects of chemical components from a cultivated strain of the red seaweed C. crispus against Aβ-induced toxicity using a transgenic C. elegans model for AD. Bioassay-guided fractionation of an organic extract of C. crispus resulted in identification of lead molecules which protected C. elegans against Aβ-toxicity. The molecular mechanism(s) of biological activity of these compounds are also discussed.
3. Discussion
Neurodegenerative Alzheimer’s disease (AD) largely associated with ageing has been difficult to treat, as information on etiology of the disease is not clear. Nutritionally rich food has been proposed to mitigate neurological diseases such as AD [
26]. Seaweeds are being explored for their neuro-protective potential, particularly due to the presence of abundant and diverse bioactive components, which are reported to impart numerous animal and human health benefits. In this paper, we evaluated the effects of extracts from a commercially cultivated strain of the red seaweed,
C. crispus on Aβ-induced toxicity using a transgenic model
C. elegans expressing human Aβ
1-42. We demonstrated that organic components of the seaweed, in particular glycolipids, delayed the onset of β-amyloid-induced paralysis, which, in turn, reduced low molecular weight Aβ species in the worm, and enhanced the transcript abundance of a number of stress-induced genes, which subsequently reduced ROS levels in the treated
C. elegans.
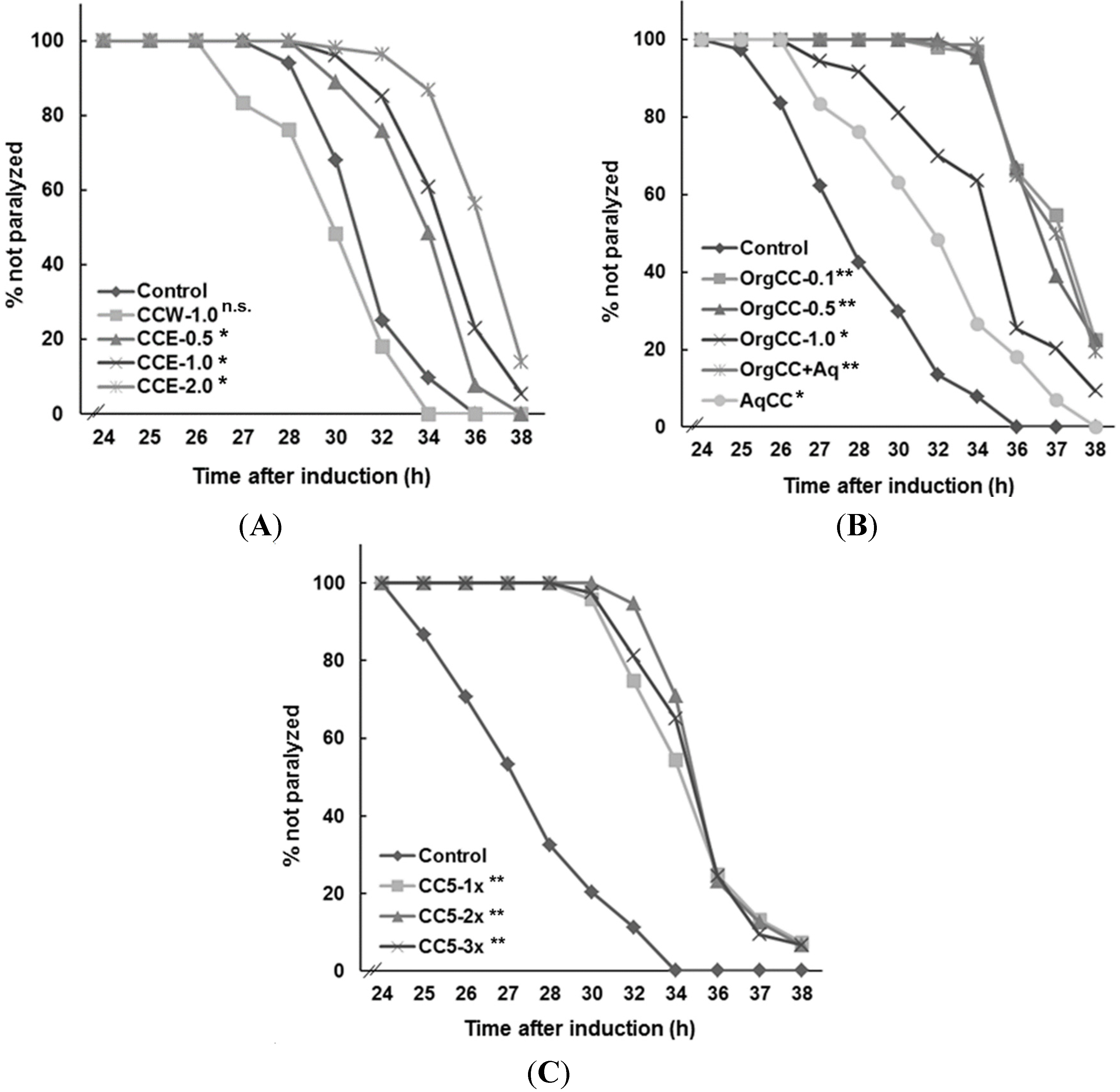
This study investigated whether the chemical components present in
C. crispus extracts offered protective effects against Aβ-toxicity in
C. elegans expressing human Aβ
1-42 [
4]. The methanol extract of
C. crispus delayed the paralysis induced by Aβ-toxicity, while the water extract was not effective. Bioassay guided fractionation of the methanol extract was effective in delaying the onset of Aβ-amyloid-induced paralysis in
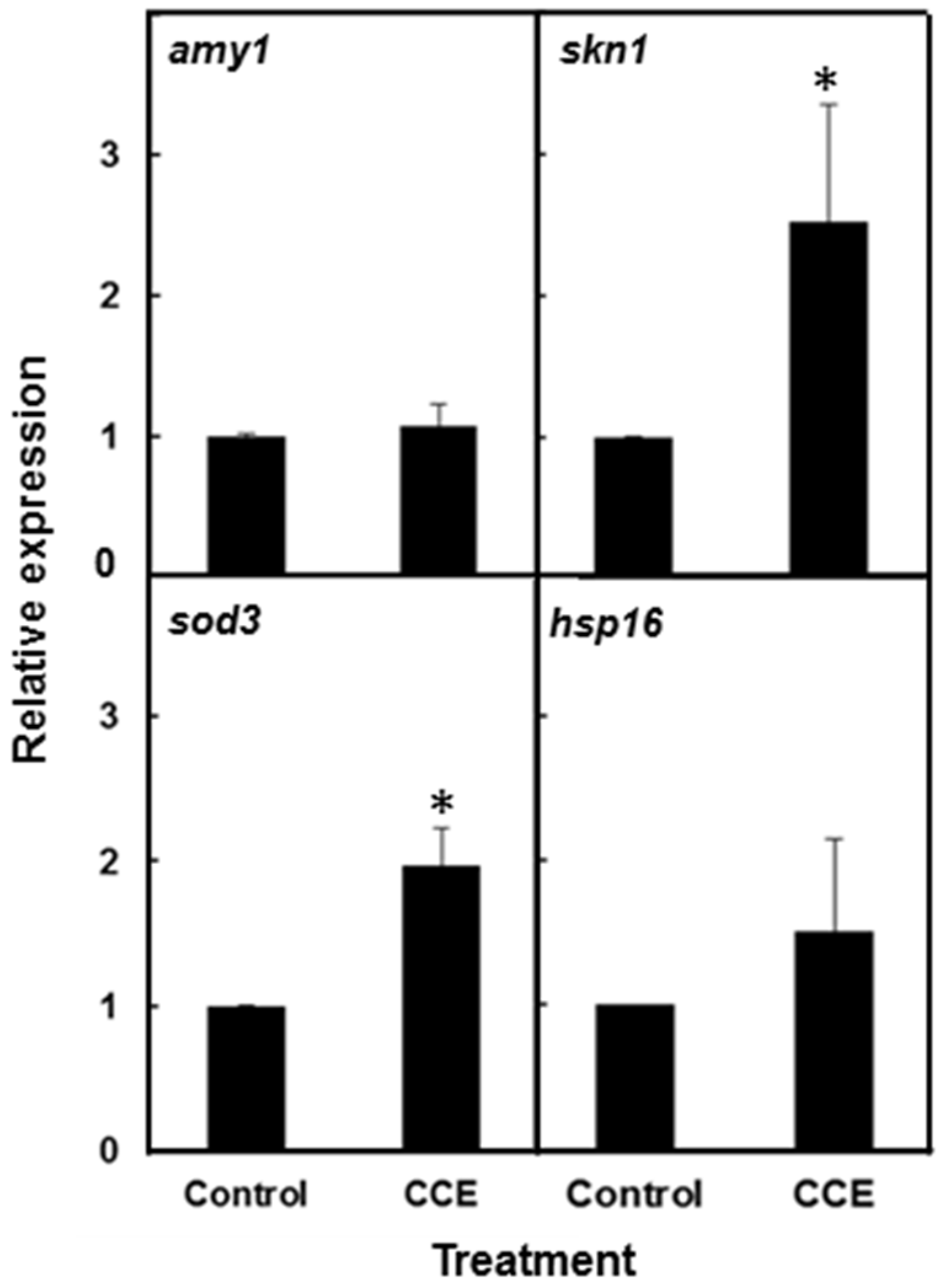
C. elegans strain CL4176. However, this fraction did not suppress
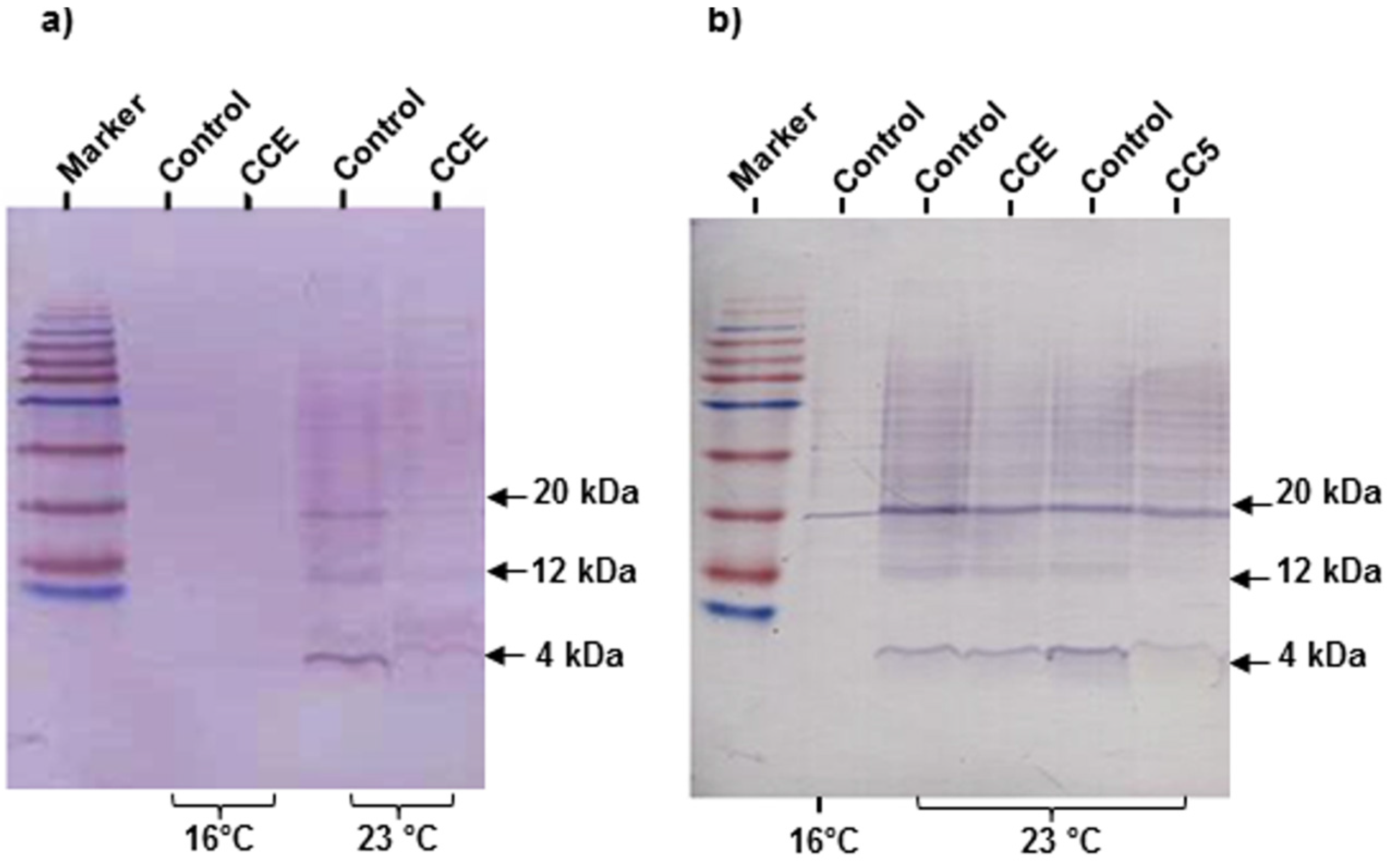
amy1 transcript abundance in the nematode, thereby suggesting that the biological effect of the chemical component of the fraction was acting at a post-transcriptional level. An examination of Aβ in
C. elegans strain CL4176 revealed that the concentrations of Aβ species were reduced in the treatment as compared to the control. A similar decrease in the Aβ species has been reported in response to treatment with natural products while the
amy-1 transcripts were not affected or reduced [
1]. However, CCE did not reverse the paralysis phenotype caused by deposition of Aβ plaques. The results suggested that the CCE-mediated protection against Aβ toxicity was, to some extent, through a reduction in the Aβ plaque deposition and lower amyloid species in the worms.
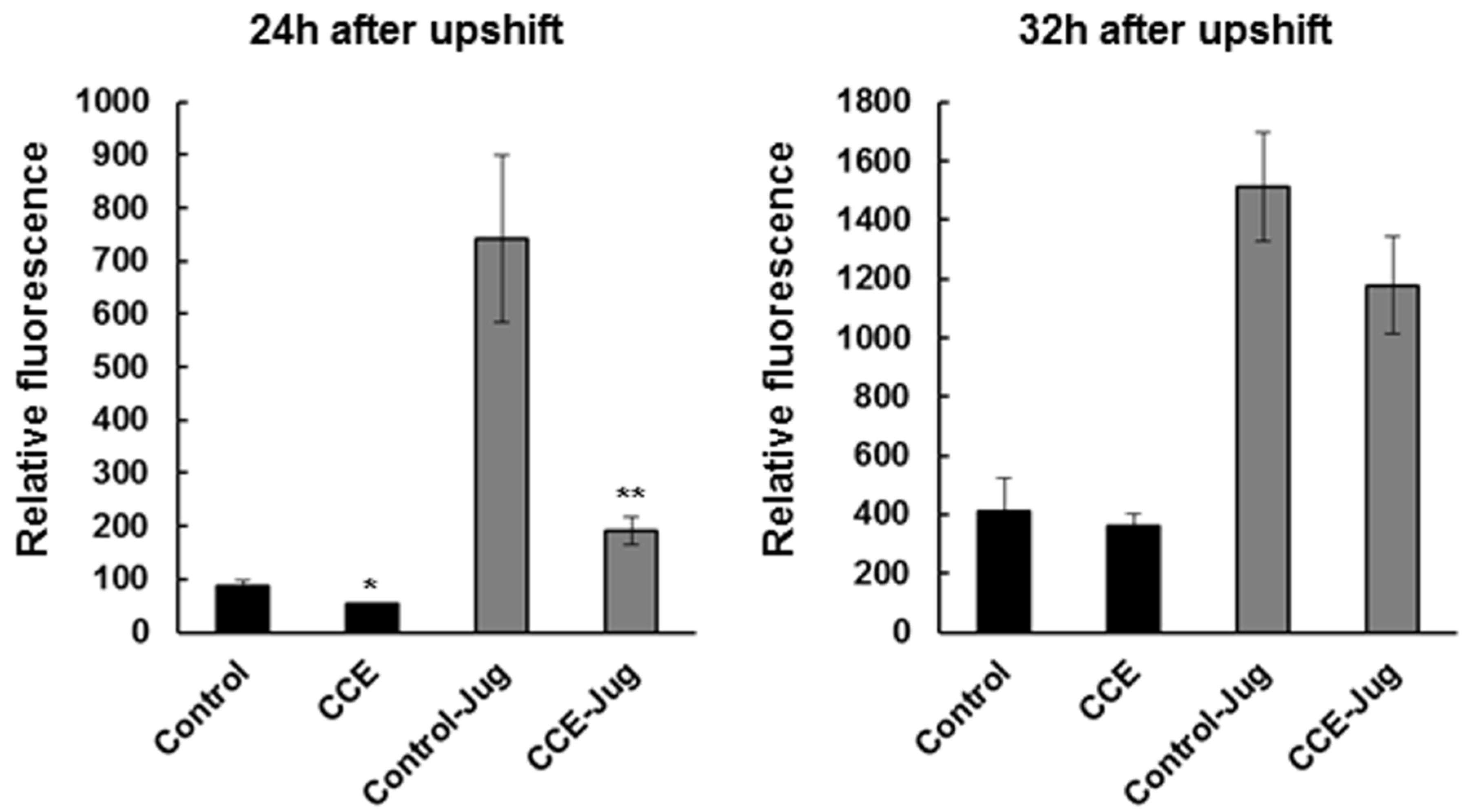
AD pathogenesis, specifically Aβ plaques, leads to oxidative stress which is associated with ROS accumulation that can further aggravate the pathological condition in AD patients [
27]. We observed here that CCE treatment reduced ROS species in the worms expressing Aβ (CL4176), which might have been either due to the reduction in the formation of toxic Aβ species or induction of antioxidant systems in the worm, thereby reducing the negative effects of Aβ. Antioxidant therapies have been suggested as a potential route to mitigate pathology associated AD [
28]. Earlier work suggested that
C. crispus extracts had mild anti-oxidative potential [
15]. In N2 wild type, treatment with a methanol extract of
C. crispus showed a strong protection against Juglone-induced oxidative stress [
15]. Natural products have been shown to reduce ROS, and are also shown to be neuro-protective in
C. elegans. Our results support the hypothesis that the ROS reducing activity of CCE might be one of the mechanisms by which it protected
C. elegans against Aβ toxicity. The induction of superoxide dismutase 3 (
sod-3) transcript in the
C. elegans strain CL4176, with CCE clearly demonstrated that antioxidant systems were activated in the treated worms and that might have acted to protect the worms against Aβ induced stress. Induction of small heat-shock proteins HSP-16 by Aβ was also reported to offer protection against
in vivo Aβ peptide toxicity in
C. elegans [
29]. However, we observed that CCE treatment caused only a small increase in
hsp-16.2 transcript. Surprisingly, the
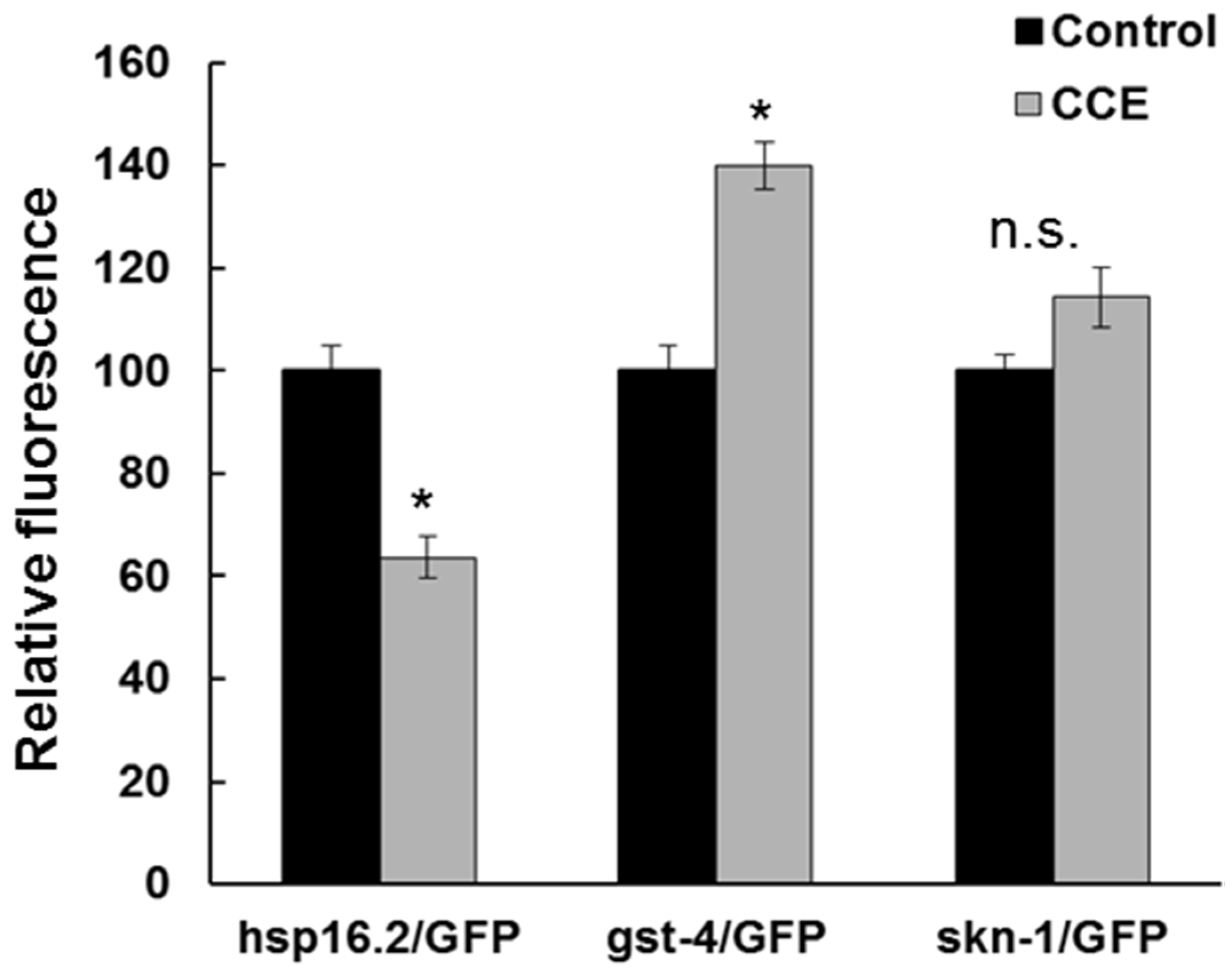
hsp-16.2/GFP fluorescence intensity in CCE treatment was lower than in the control worms. Thus, it appears
hsp-16.2 had a marginal role in CCE-mediated tolerance to Aβ toxicity in the nematode and shifted the focus of this study towards other mechanisms perhaps functioning to enhance plaque clearance and modulate oligomerization of Aβ.
It appears that SKN-1 in
C. elegans was affected with CCE treatment. First of all, CCE treatment increased the expression of the
gst-4/GFP which is regulated by the SKN-1 transcription factor. Gene expression results also showed increased expression of
skn-1. Further, induction of
skn-1/GFP was enhanced in transgenic worms with CCE treatment. This evidence, taken together, suggests that the protection from Aβ toxicity in
C. elegans could have resulted from the downstream induction of effectors in the SKN-1 pathway, which might function as antioxidants [
1]. SKN-1 is a functionally analogous to Nrf2 [
1], which is known to be involved in phase II detoxification response genes and also in cellular responses to oxidative stress. SKN-1 might be acting in a way similar to Nrf2, with CCE treatment. The induced response of Nrf2 was reported to protect an amyloid precursor protein/presenilin-1 (APP/PS1) transgenic mouse model [
30] and neuronal cells, exposed to exogenous Aβ. In
C. elegans, similar results were reported by Dostal
et al., [
1], attributing the role of
skn-1 in coffee extract-mediated protection, against Aβ toxicity. These findings suggest that the role of CCE-mediated protection against Aβ-toxicity is at least in part, through the SKN-1 pathway in
C. elegans.
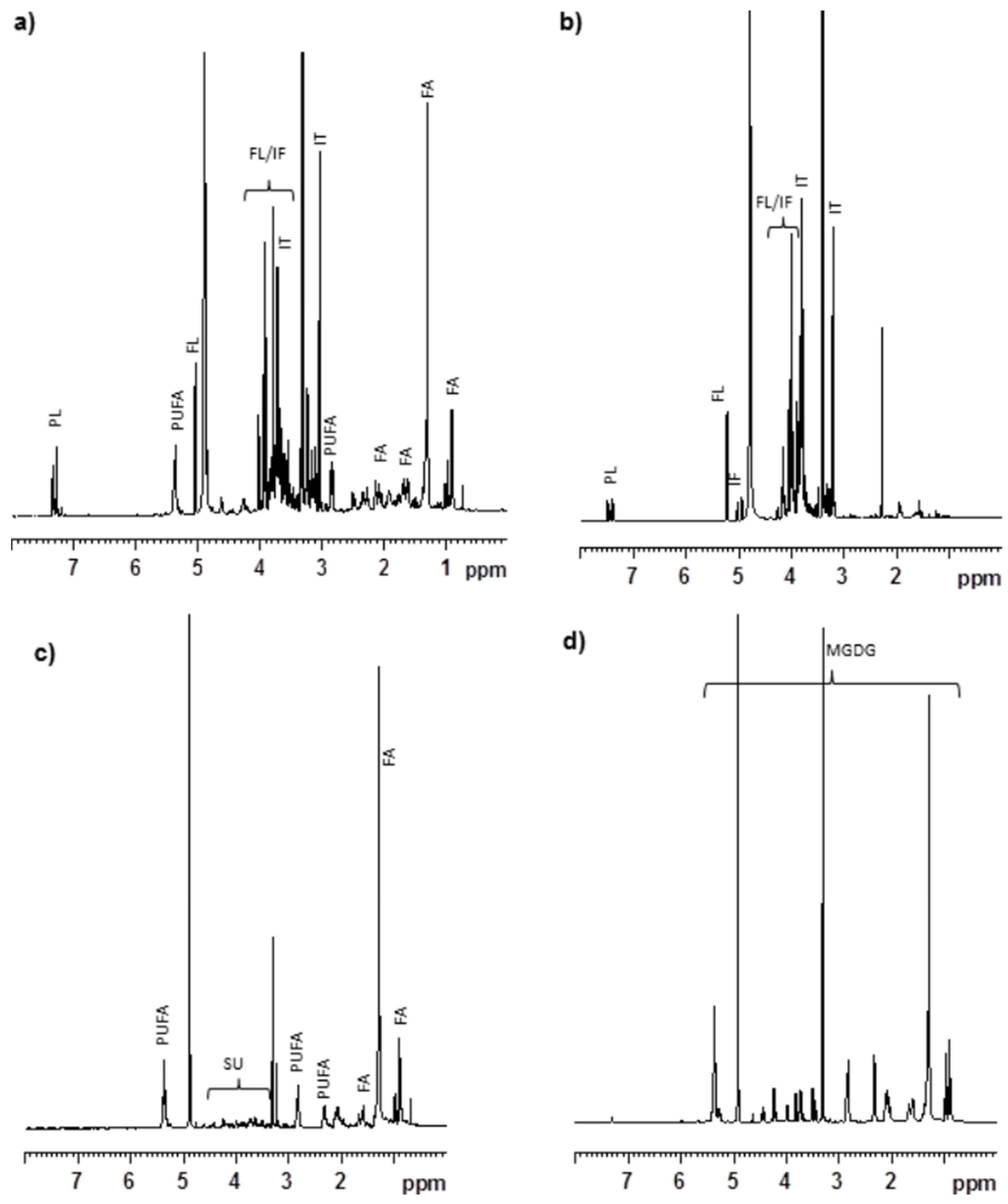
In an earlier study, we reported that the MeOH extract of
C. crispus contained mainly floridoside, isethionic acid, taurine, phenylalanine and other lipophilic metabolites including polyunsaturated fatty acids [
15]. Further
1H NMR study suggested that the organic soluble portion (
i.e., OrgCC) had signals corresponding to polyunsaturated fatty acids, and other lipids which would have shown protective activity against Aβ toxicity. The AqCC on the other hand contained primarily floridoside, isethionic acid and phenylalanine, which displayed no protective activities. Sub-fraction 5 (CC5) eluted with CHCl
3/MeOH (9:1), that exhibited the most protective activity against Aβ-toxicity in
C. elegans, and contained predominantly MGDG (
Figure 5C), were identified as (2
S)-1,2-
bis-
O-eicosapentaenoyl-3-
O-β-
d-galactopyranosylglycerol, (2
S)-1-
O-eicosapentaenoyl-2-
O-arachidonoyl-3-
O-β-
d-galactopyranosylglycerol, (2
S)-1-
O-(6
Z,9
Z,12
Z,15
Z-octadecatetranoyl)-2-
O-palmitoyl-3-
O-β-
d-galactopyranosylglycerol, (2
S)-1-
O-eicosapentaenoyl-2-
O-palmitoyl-3-
O-β-
d-galactopyranosylglycerol, (2
S)-1,2-
bis-
O-arachidonoyl-3-
O-β-
d-galactopyranosylglycerol and (2
S)-1-
O-arachidonoyl-2-
O-palmitoyl-3-
O-β-
d-galactopyranosylglycerol [
25] and accounts for over 71.2% of the fraction. Lipid-containing seaweed extract fractions are known to have an abundance of polyunsaturated fatty acids (PUFAs), glycolipids and unique blends of pigments. Glycolipids constitute an important class of membrane lipids in red seaweeds and possess a broad spectrum of biological activities. Even though water-soluble components such as sulphated polysaccharides, floridoside and iso-floridoside have properties such as cell protection [
31,
32], we did not observe any positive effect against Aβ-toxicity in
C. elegans by CCW whereas AqCC showed only a mild effect in delaying the paralysis phenotype. When tested, purified water soluble components were not effective against Aβ toxicity (data not shown). These results supported the conclusion that the lipid rich portion of the MeOH extract of the commercially cultivated
C. crispus was responsible for the protective effects against Aβ-amyloid toxicity in
C. elegans. The MGDG-rich fraction was the most active class of lipids present in the OrgCC suggesting that the bioactivity of the
C. crispus extracts was due to this special class of lipids. MGDG and DGDG are major constituents of chloroplast lipids of plants and algae, it will be interesting to investigate if MGDGs from plants also alleviate Aβ related toxicity in
C. elegans, similar to the effect as observed with MGDG enriched fractions of
C. crispus.
In conclusion, CCE has protective effects against β-amyloid toxicity in C. elegans through reduced deposition of amyloid species, increased antioxidant activity, and the activated SKN1 pathway in the transgenic worm model. The bioactivity of CCE was primarily attributed to the MGDG-enriched fraction of the extract. The results also suggest the further need to investigate whether these health benefits are observed with dietary C. crispus in animal models.
4. Materials and Methods
4.1. Chemicals
H2DCF-DA (2′,7′-dichlorodihydrofluorescein diacetate), Juglone (5-hydroxy-1,4-naphthoquinone), MGDG, quercetin, secondary anti-mouse IgG alkaline phosphatase conjugate and SigmaFast™ BCIP®/NBT tablets were purchased from Sigma-Aldrich (St. Louis, MO, USA). Bradford Reagent was purchased from Biorad (Mississauga, ON, Canada), secondary alkaline phosphatase conjugate and amyloid antibody 6E10 was purchased from Covance (Montreal, QC, Canada). G. biloba extract (EGb761) was a kind gift from Dr. Willmar Schwabe Pharmaceuticals, Germany.
4.2. Nematode Strains
The wild-type
C. elegans strain N2 (Bristol) and transgenic worms, TJ375 (
hsp-16.2/GFP), CL2166 (
gst-4/GFP) CL2166 (dvIs19[pAF15(gst-4::GFP::NLS)]), LD1 (
skn-1/GFP), and CL4176 (smg-1ts [
myo-3/Aβ1–42 long 3′-untranslated region (UTR)]) were purchased from the
Caenorhabditis Genetics Center (University of Minnesota, Minneapolis, MN, USA). The transgenic nematode strains CL4176 expressed muscle-specific Aβ
1–42 [
4] leading to a paralysis phenotype of the worm under non-permissive conditions. All
C. elegans strains were maintained at 20 °C, except strain CL4176, which was maintained at 16 °C, on solid nematode growth medium (NGM), seeded with live
E. coli (OP50) as a food source.
4.3. Seaweed Extraction and the Preparation of NGM
Both water (CCW) and methanol (CCE) extracts were prepared from a commercially cultivated strain of the red seaweed
C. crispus [
33]. The strain is proprietary and owned by the National Reseach Council (NRC) of Canada, and exclusively cultivated in Nova Scotia by Acadian Seaplants Limited. This strain of
C. crispus is cultivated in the world’s largest on-land cultivation facility for the Asian food market (marketed as Hana-Tsunomata™). A voucher specimen of the sample is kept at the Marine Bioproducts Laboratory, Department of Environmental Sciences, Dalhousie University, Canada. The
C. crispus was collected from production tanks, rinsed with distilled water and immediately lyophilized and vacuum sealed. For preparation of the extracts, the lyophilized seaweed (1000 g) was extracted with Methanol (MeOH) (5L × 3), stirring at room temperature for 1 h, followed by sonication for 15 min. The combined MeOH extract was evaporated under reduced pressure to yield
C. crispus MeOH extract (CCE). The MeOH extract (50 g) was suspended in water (600 mL) and extracted with ethyl acetate (EtOAc) (600 mL × 2). Both aqueous (AqCC) and organic (OrgCC) extracts were dried under reduced pressure and lyophilized yielding 45.0 g and 5.0 g, respectively. OrgCC (4.0 g) was further fractionated by solid phase extraction, using a silica gel cartridge (Strata SI-1, 70 g, Phenomenex, Torrance, CA, USA) into 7 sub-fractions, eluting with hexane/EtoAc/CHCl
3/MeOH gradients [sub-fraction 1, hexane/EtOAc (3:1), 284 mg; sub-fraction 2, hexane/EtOAc (1:1), 678 mg; sub-fraction 3, hexane/EtOAc (1:1), 106 mg; sub-fraction 4, EtOAc, 18 mg; sub-fraction 5, CHCl
3/MeOH (9:1), 222 mg; sub-fraction 6, CHCl
3/MeOH (1:1); 925 mg; sub-fraction 7, MeOH, 1035 mg]. The seaweed powder (10 g), in a separate process, was extracted with boiling water (30 min each, total water 200 mL), water was then removed at reduced pressure and dried at 70 °C to yield the water extract (CCW).
The seaweed extracts were mixed in nematode growth medium (NGM) to a final range of concentrations form 0.1 to 2.0 mg/mL of NGM using 0.05%–0.1% methanol, just before dispensing in Petri plates. The organic sub-fractions, or pure compounds, were added to NGM as 1 mg equivalent. To inhibit microbial contamination, gentamycin sulfate was added to the NGM at a concentration of 30 µg/mL. The E. coli OP50, spread on to the NGM, served as food for C. elegans. Plain NGM or NGM + MeOH served as the controls. An extract of G. biloba (EGb761; 0.1–1 mg/mL NGM), and in some experiments, quercetin (200 µg/mL NGM) were also used for comparison.
4.4. Bioassays for β-Amyloid Induced Paralysis
To determine if
C. crispus extracts suppressed or delayed the onset of β-amyloid-induced, progressive paralysis of the
C. elegans strain CL4176, expressing muscle-specific Aβ
1–42 [
4], synchronized eggs were transferred onto Petri dishes containing solidified NGM, either mixed with extract or the controls (water or MeOH). Paralysis was induced in the worms following the method described in Sangha
et al. [
34]. Worms were scored as paralyzed if they failed to move their body or moved only the head when touched with a platinum loop. Each experiment was performed with at least 90 worms. The data represent the mean of three independent experiments with three replications (
N = 270).
4.5. Western Blot Analysis of Aβ Species in C. elegans
Worms were harvested in ddH
2O containing 1× protease inhibitor cocktail (Sigma, Oakville, ON, Canada), flash frozen in to liquid nitrogen and stored at −80 °C, until use. For protein extraction, worms were boiled at 105 °C for 10 min in a lysis buffer (
i.e., 62 mM Tris-HCl pH 6.8, 2% SDS (w/v), 10% glycerol (v/v), 4% β-mercaptoethanol (v/v) and 1× protease inhibitor cocktail), and then cooled, on ice, and centrifuged for 5 min at 14,000 G at 4 °C. The protein in the supernatant was quantified using Bradford Reagent (Bio-Rad, Mississauga, ON, Canada). Electrophoresis and Western blot analysis was performed following a previously published method [
34]. Equal loading was determined by replicated, non-transferred Coomassie Blue stained, SDS-PAGE gel. The data was expressed as the mean of 5 biological replicates, using 4 and 20 kD bands in the analysis.
4.6. In Vivo Measurement of ROS in C. elegans
Intracellular ROS were measured in
C. elegans strain CL4176, using the 2′,7′-dichlorofluorescein diacetate (H2DCF-DA) method [
35]. Briefly, freshly laid eggs (60–65 eggs per plate) were transferred to the control or CCE (1 mg/mL) amended NGM plates and incubated for 36 h at 16 °C. To initiate amyloid-induced, progressive paralysis, the worms were shifted to an incubator set at 23 °C. The worms were harvested 24 and 32 h after the temperature shift using 500 μL of phosphate-buffered saline (PBS), washed twice with PBS to remove
E. coli (OP-50) cells and then transferred into 96-well plates (Costar) in 200 μL of PBS containing Tween 20 (0.01%) and H2DCF-DA (50 μM). In a parallel experiment to determine tolerance level during oxidative stress, juglone (300 μM) was added to the PBS-
C. elegans-H2DCF-DA mixture before placing the plate for fluorescence detection, which was quantified in a Synergy HT micro-plate reader (Bio-Tek Instruments, Winooski, VT, USA) for 6 h at 37 °C, using excitation at 485 nm, and emission at 530 nm. The data presented here represented the mean ± SE of three independent experiments, expressed as percentage fluorescence, relative to the MeOH control.
4.7. Fluorescence Microscopy of Reporter Gene Expression in CCE-Treated C. elegans
In order to determine the stress response mechanisms in
C. elegans that were induced by the CCE treatment, the expressions of
HSP-16.2,
GST-4 and
SKN-1 were studied in
C. elegans strains TJ375 (
hsp-16.2/GFP), CL2166 (
gst-4/GFP) and LD1 (skn-1/GFP), respectively. Synchronized TJ375 eggs were transferred on to NGM plates containing the extracts and incubated for 2 days at 20 °C. To induce the heat-shock response, L4 worms were shifted to 35 °C for 2 h, followed by recovery at 20 °C, before taking digital pictures. To determine the role of
gst-4 which encodes a glutathione-
S-transferase and is a downstream effector of the conserved
skn-1, phase II detoxification pathway [
36], freshly laid eggs of CL2166 were transferred to NGM plates with CCE (1 mg/mL) and grown at 16 °C until the L4 stage. A sub-section of the treated worms were shifted to freshly prepared NGM containing 300 µM juglone for 1 h and their fluorescence was recorded. To determine the role of
SKN-1, the
LD1 (skn-1/GFP) worms were reared on treatments as above. Pre-treated L4 worms were transferred to fresh NGM plates containing 100 µM juglone and incubated overnight before observed for fluorescence under a microscope.
For quantification of fluorescence, the worms were anesthetized with sodium azide (10 mM) on an agarose pad, on a glass slide and their fluorescence was recorded (Olympus, Japan) with excitations at 488 nm and emissions at 500–530 nm. The images were acquired with a CCD camera (Leica Microsystems, Richmond Hill, ON, Canada), and the intensity was analyzed using Image-J software. The experiments were repeated twice with 15–20 worms per group; data were presented as mean ± SE of two experiments.
4.8. Expression Analysis of Stress-Induced Genes in C. elegans
Quantitative Real Time-PCR was conducted on CL4176 worms treated with CCE vs. control in order to relate their phenotypic and biochemical responses with the molecular response, under conditions leading to expression of Aβ protein species in the worms. For this, the transcriptional response of β-amyloid transgene (amy-1), stress induced transcription factor (skn-1), superoxide dismutase 3 (sod-3) and small heat shock protein (hsp-16.2) was monitored. Eggs were transferred to treatment plates and incubated for 36 h at permissive (16 °C) temperatures before shifting to 23 °C to induce amy1 expression. The worms were sampled at 20 h after temperature up-shift. The worms (80–100) were transferred directly into TRIzol Reagent (100 µL; Invitrogen Life Technologies, Burlington, ON, Canada) and flash frozen in liquid nitrogen. Total RNA was extracted with TRIzol reagent following standard protocol and cDNA was synthesized with a High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Burlington, ON, Canada). The RT-PCR primers were as follows: amy-1 (forward, 5′-CCGACATGACTCAGGATATGAAGT-3′; reverse, 5′-CACCATGAGTCCAATGATTGCA-3′); small heat shock protein hsp-16.2 (forward, 5′-ACGCCAATTTGCTCCAGTCT-3′; reverse, 5′-GATGGCAAACTTTTGATCATTGTTA-3); sod-3 (forward, 5′-AGCATCATGCCACCTACGTGA-3′; reverse, 5′-CACCACCATTGAATTTCAGCG-3′); and skn-1 (forward, 5′-AGTGTCGGCGTTCCAGATTTC-3′; reverse, 5′-GTCGACGAATCTTGCGAATCA-3′). The gene ama-1 (forward, 5′-CTGACCCAAAGAACACGGTGA-3′; reverse, 5′-TCCAATTCGATCCGAAGAAGC-3′) was used as the internal control. The transcript abundance was assessed using StepOne™ Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) using an SYBR green reagent (Roche Diagnostics, Mississauga, ON, Canada). Data were analyzed from two independent runs (mean ± SE) and expressed as relative expression.
4.9. Chemical Profiling of CCE and Active Fractions
The NMR spectra were measured using a Bruker 500 or 700 MHz spectrometer with deuterated solvent. The CCE and CCW extracts (20 mg/mL) were dissolved in deuterated water containing 1 mM Sodium-(trimythylsilyl)-2,2′,3,3′-tetradeuratedpropionate (TMSP) and filtered through 4 mm, nylon 0.2 µm syringe filter (Canadian Life Science, Toronto, ON, Canada). Concentration of the major components present in the extracts and aqueous fraction were calculated based on the integration of individual protons as compared to the internal standard. The fatty acid profile of the active fraction was analyzed using LC/MS. Agilent 1200 Series HPLC coupled with 6100B Series Single Quadrupole LC/MS systems was used for LCMS analysis. The MGDG rich fraction (CC5) was subjected for LC/MS analysis using Synergi MAX-RP column (4 μm, 4.60 mm × 250 mm, Phenomenex, Torrance, CA, USA) with 95% MeOH/0.025M H2SO4 in H2O isocratic condition for 45 min at 1.0 mL/min.
4.10. Statistical Analyses
Statistical analyses were performed using JMP software (SAS institute Inc., Cary, NC, USA). One-way ANOVA was used for group comparisons using the Student’s t test. For β-amyloid-induced paralysis assays, the data were analyzed with Kaplan–Meier survival curves and the p values were calculated by log-rank comparison between the control and the treatments (* ≤ 0.05; ** < 0.0001).