Hepatic Metabolic Dysregulation as a Potential Amplifier of Leukemogenesis Following mRNA Vaccination: A Novel Mechanistic Hypothesis
Abstract
1. Introduction
1.1. Mechanistic Framework and Biological Rationale
1.2. Hepato-Hematopoietic Metabolic Crosstalk
1.3. Metabolic Vulnerabilities in Leukemogenesis
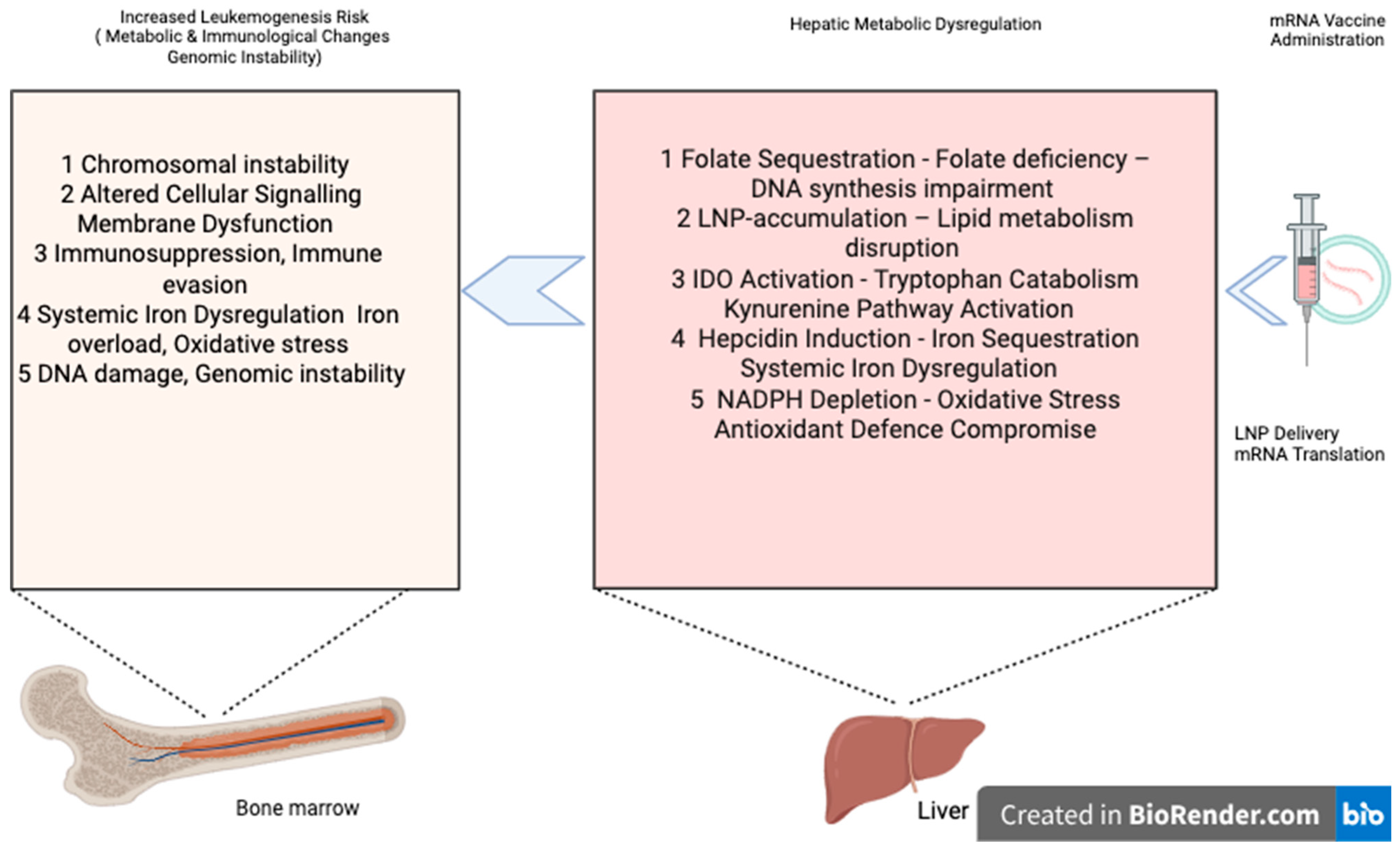
2. Proposed Mechanisms of Hepatic Metabolic Amplification in Leukemogenesis
2.1. Competitive Folate Sequestration and One-Carbon Metabolism Dysregulation
2.1.1. Established Knowledge: Mechanistic Basis
2.1.2. COVID-19 mRNA Vaccines, Spike Protein, and Folate Metabolism
3. Hypothesis
3.1. Hepatic Lipid Processing Overload and Membrane Lipid Dysregulation
3.1.1. Established Knowledge: Mechanistic Basis
3.1.2. Leukemogenic Implications
Hypothesis
3.2. Cytokine-Mediated Indoleamine 2,3-Dioxygenase Upregulation and Tryptophan Catabolism
3.2.1. Established Knowledge: Mechanistic Basis
3.2.2. Leukemogenic Implications
Hypothesis
3.3. Inflammatory Hepcidin Induction and Iron Homeostasis Disruption
3.3.1. Established Knowledge: Mechanistic Basis
3.3.2. Leukemogenic Implications
Hypothesis
3.4. Hepatic NADPH Demand and Redox Homeostasis Perturbation
3.4.1. Established Knowledge: Mechanistic Basis
3.4.2. Leukemogenic Implications
Hypothesis
3.5. Vaccine-Induced Metabolic Perturbations
3.5.1. SARS-CoV-2 Spike Protein and NADPH Depletion
3.5.2. Testable Predictions of Vaccine-Induced Metabolic Perturbations
4. Mechanistic Integration and Synergistic Effects of Our Hypothesis
4.1. Temporal Dynamics and Cascade Amplification
4.2. Clinical Implications and Translational Opportunities
4.3. Corroborating the Mechanistic Framework: Insights from Clinical Metabolomics
4.4. Discussion and Critical Analysis
4.5. Future Directions
4.6. Limitations
4.6.1. Clinical Significance for Vaccine Safety and Population Health
4.6.2. Experimental Framework and Research Priorities
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greaves, M. Leukaemia ‘firsts’ in cancer research and treatment. Nat. Rev. Cancer 2016, 16, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Erdoğdu, B.; Kaplan, O.; Fidan, B.B.; Çelebier, M.; Malkan, Ü.Y.; Haznedaroglu, I.C. Metabolomic Profiling of Leukemic Hematopoiesis: Effects of BNT162b2 mRNA COVID-19 Vaccine Administration. Curr. Mol. Med. 2025; in press. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef]
- Mishra, S.K.; Millman, S.E.; Zhang, L. Metabolism in acute myeloid leukemia: Mechanistic insights and therapeutic targets. Blood 2023, 141, 1119–1135. [Google Scholar] [CrossRef]
- Jones, C.L.; Inguva, A.; Jordan, C.T. Targeting Energy Metabolism in Cancer Stem Cells: Progress and Challenges in Leukemia and Solid Tumors. Cell Stem Cell 2021, 28, 378–393. [Google Scholar] [CrossRef]
- Hosseini-Kharat, M.; Bremmell, K.E.; Prestidge, C.A. Why do lipid nanoparticles target the liver? Understanding of biodistribution and liver-specific tropism. Mol. Ther. Methods Clin. Dev. 2025, 33, 101436. [Google Scholar] [CrossRef]
- Shi, Y.; Shi, M.; Wang, Y.; You, J. Progress and prospects of mRNA-based drugs in pre-clinical and clinical applications. Signal Transduct. Target. Ther. 2024, 9, 322. [Google Scholar] [CrossRef]
- Alhumaid, S.; Al Mutair, A.; Rabaan, A.A.; Alshakhs, F.M.; Choudhary, O.P.; Yong, S.J.; Nainu, F.; Khan, A.; Muhammad, J.; Alhelal, F.; et al. New-onset and relapsed liver diseases following COVID-19 vaccination: A systematic review. BMC Gastroenterol. 2022, 22, 433. [Google Scholar] [CrossRef] [PubMed]
- Eens, S.; Van Hecke, M.; Favere, K.; Tousseyn, T.; Guns, P.J.; Roskams, T.; Heidbuchel, H. B-cell lymphoblastic lymphoma following intravenous BNT162b2 mRNA booster in a BALB/c mouse: A case report. Front. Oncol. 2023, 13, 1158124. [Google Scholar] [CrossRef]
- Zamfir, M.-A.; Moraru, L.; Dobrea, C.; Scheau, A.-E.; Iacob, S.; Moldovan, C.; Scheau, C.; Caruntu, C.; Caruntu, A. Hematologic Malignancies Diagnosed in the Context of the mRNA COVID-19 Vaccination Campaign: A Report of Two Cases. Medicina 2022, 58, 874. [Google Scholar] [CrossRef] [PubMed]
- Ang, S.-Y.; Huang, Y.-F.; Chang, C.-T. pH-positive B-cell acute lymphoblastic leukemia occurring after receipt of bivalent SARS-CoV-2 mRNA vaccine booster: A case report. Medicina 2023, 59, 627. [Google Scholar] [CrossRef]
- Paulusma, C.C.; Lamers, W.H.; Broer, S.; van de Graaf, S.F. Amino acid metabolism, transport and signalling in the liver revisited. Biochem. Pharmacol. 2022, 201, 115074. [Google Scholar] [CrossRef]
- Soares-da-Silva, F.; Peixoto, M.; Cumano, A.; Pinto-Do-Ó., P. Crosstalk between the hepatic and hematopoietic systems during embryonic development. Front. Cell Dev. Biol. 2020, 8, 612. [Google Scholar] [CrossRef] [PubMed]
- Meynard, D.; Babitt, J.L.; Lin, H.Y. The liver: Conductor of systemic iron balance. Blood 2014, 123, 168–176. [Google Scholar] [CrossRef]
- Nakamura-Ishizu, A.; Ito, K.; Suda, T. Hematopoietic stem cell metabolism during development and aging. Dev. Cell 2020, 54, 239–255. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Noda, M.; Ono, A.; Kamiya, M.; Matsumoto, M.; Tsurumaru, M.; Mizukami, S.; Mukai, H.; Kawakami, S. Effect of Cholesterol Content of Lipid Composition in mRNA-LNPs on the Protein Expression in the Injected Site and Liver After Local Administration in Mice. J. Pharm. Sci. 2023, 112, 1401–1410. [Google Scholar] [CrossRef]
- Vasileva, O.; Zaborova, O.; Shmykov, B.; Ivanov, R.; Reshetnikov, V. Composition of lipid nanoparticles for targeted delivery: Application to mRNA therapeutics. Front. Pharmacol. 2024, 15, 1466337. [Google Scholar] [CrossRef]
- Wang, J.; Ding, Y.; Chong, K.; Cui, M.; Cao, Z.; Tang, C.; Tian, Z.; Hu, Y.; Zhao, Y.; Jiang, S. Recent Advances in Lipid Nanoparticles and Their Safety Concerns for mRNA Delivery. Vaccines 2024, 12, 1148. [Google Scholar] [CrossRef]
- Parrett, B.J.; Yamaoka, S.; Barry, M.A. Reducing off-target expression of mRNA therapeutics and vaccines in the liver with microRNA binding sites. Mol. Ther. Methods Clin. Dev. 2025, 33, 101402. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Tuyishime, S.; Muramatsu, H.; Kariko, K.; Mui, B.L.; Tam, Y.K.; Madden, T.D.; Hope, M.J.; Weissman, D. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J. Control. Release 2015, 217, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Kalita, T.; Dezfouli, S.A.; Pandey, L.M.; Uludag, H. siRNA Functionalized Lipid Nanoparticles (LNPs) in Management of Diseases. Pharmaceutics 2022, 14, 2520. [Google Scholar] [CrossRef]
- Kalola, U.K.; Pellegrini, M.V. Patisiran; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Jiang, K.; Tian, K.; Yu, Y.; Wu, E.; Yang, M.; Pan, F.; Qian, J.; Zhan, C. Kupffer cells determine intrahepatic traffic of PEGylated liposomal doxorubicin. Nat. Commun. 2024, 15, 6136. [Google Scholar] [CrossRef]
- Xie, F.; Ding, X.; Zhang, Q.Y. An update on the role of intestinal cytochrome P450 enzymes in drug disposition. Acta Pharm. Sin. B 2016, 6, 374–383. [Google Scholar] [CrossRef]
- Pasquiers, B.; Benamara, S.; Felices, M.; Nguyen, L.; Declèves, X. Review of the Existing Translational Pharmacokinetics Modeling Approaches Specific to Monoclonal Antibodies (mAbs) to Support the First-In-Human (FIH) Dose Selection. Int. J. Mol. Sci. 2022, 23, 12754. [Google Scholar] [CrossRef]
- Abufares, H.I.; Zenati, R.A.; Soares, N.C.; El-Huneidi, W.; Dahabiyeh, L.A.; Al-Hroub, H.M.; Alqudah, M.A.Y.; Abuhelwa, A.Y.; Alzoubi, K.H.; Abu-Gharbieh, E.; et al. A non-targeted metabolomics comparative study on plasma of pfizer and sinopharm COVID-19 vaccinated individuals, assessed by (TIMS-QTOF) mass spectrometry. Heliyon 2024, 10, e35443. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Pfeifle, A.; Lansdell, C.; Frahm, G.; Cecillon, J.; Tamming, L.; Gravel, C.; Gao, J.; Thulasi Raman, S.N.; Wang, L.; et al. The Expression Kinetics and Immunogenicity of Lipid Nanoparticles Delivering Plasmid DNA and mRNA in Mice. Vaccines 2023, 11, 1580. [Google Scholar] [CrossRef] [PubMed]
- Pateev, I.; Seregina, K.; Ivanov, R.; Reshetnikov, V. Biodistribution of RNA Vaccines and of Their Products: Evidence from Human and Animal Studies. Biomedicines 2024, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Yada, E.; Terai, Y.; Takahashi, T.; Nakanishi, H.; Tanaka, H.; Akita, H.; Itaka, K. Comprehensive Evaluation of Lipid Nanoparticles and Polyplex Nanomicelles for Muscle-Targeted mRNA Delivery. Pharmaceutics 2023, 15, 2291. [Google Scholar] [CrossRef]
- Di, J.; Du, Z.; Wu, K.; Jin, S.; Wang, X.; Li, T.; Xu, Y. Biodistribution and Non-linear Gene Expression of mRNA LNPs Affected by Delivery Route and Particle Size. Pharm. Res. 2022, 39, 105–114. [Google Scholar] [CrossRef]
- Schinas, G.; Polyzou, E.; Dimakopoulou, V.; Tsoupra, S.; Gogos, C.; Akinosoglou, K. Immune-mediated liver injury following COVID-19 vaccination. World J. Virol. 2023, 12, 100–108. [Google Scholar] [CrossRef]
- Palmer, M.; Seekins, D.; Avigan, M.; Marcinak, J.; Rockey, D.C.; Regev, A.; Shastri, V.K.; Lewis, J.H.; Dash, A. The Impact of COVID-19 and COVID-19 Vaccination on Detection, Assessment, and Management of Suspected Acute Drug-Induced Liver Injury Occurring during Clinical Trials: Consensus Recommendations from the IQ DILI Initiative. Drug Saf. 2025, 48, 879–890. [Google Scholar] [CrossRef]
- Guardiola, J.; Lammert, C.; Teal, E.; Chalasani, N. Unexplained liver test elevations after SARS-CoV-2 vaccination. J. Hepatol. 2022, 77, 251–253. [Google Scholar] [CrossRef]
- Lv, K.; Yu, Z.; Wang, J.; Li, N.; Wang, A.; Xue, T.; Wang, Q.; Shi, Y.; Han, L.; Qin, W. Discovery of Ketal-Ester Ionizable Lipid Nanoparticle with Reduced Hepatotoxicity, Enhanced Spleen Tropism for mRNA Vaccine Delivery. Adv. Sci. 2024, 11, 2404684. [Google Scholar] [CrossRef]
- Ng, A.J.J.; Teo, D.C.H.; Dorajoo, S.R.; Yap, A.J.Y.; Chow, W.C.; Ng, N.K.M.; Soh, S.B.L. Acute autoimmune hepatitis following COVID-19 mRNA vaccination: A population-based study using electronic health records in Singapore. Vaccine 2024, 42, 126462. [Google Scholar] [CrossRef] [PubMed]
- Ghielmetti, M.; Schaufelberger, H.D.; Mieli-Vergani, G.; Cerny, A.; Dayer, E.; Vergani, D.; Terziroli Beretta-Piccoli, B. Acute autoimmune-like hepatitis with atypical anti-mitochondrial antibody after mRNA COVID-19 vaccination: A novel clinical entity? J. Autoimmun. 2021, 123, 102706. [Google Scholar] [CrossRef]
- Kim, J.H.; Chae, H.B.; Woo, S.; Song, M.S.; Kim, H.-J.; Woo, C.G. Clinicopathological characteristics of autoimmune-like hepatitis induced by COVID-19 mRNA vaccine (Pfizer-BioNTech, BNT162b2): A case report and literature review. Int. J. Surg. Pathol. 2023, 31, 1156–1162. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Aragao, A.P.; Ding, X. COVID-19-associated Autoimmune Hepatitis: A Case Report and Literature Review. J. Clin. Transl. Pathol. 2025. [Google Scholar] [CrossRef]
- Efe, C.; Uzun, S.; Matter, M.S.; Terziroli Beretta-Piccoli, B. Autoimmune-Like Hepatitis Related to SARS-CoV-2 Vaccination: Towards a Clearer Definition. Liver Int. 2025, 45, e16209. [Google Scholar] [CrossRef]
- Chen, C.; Xie, D.; Xiao, J. Real-world evidence of autoimmune hepatitis following COVID-19 vaccination: A population-based pharmacovigilance analysis. Front. Pharmacol. 2023, 14, 1100617. [Google Scholar] [CrossRef] [PubMed]
- Chow, K.W.; Pham, N.V.; Ibrahim, B.M.; Hong, K.; Saab, S. Autoimmune Hepatitis-Like Syndrome Following COVID-19 Vaccination: A Systematic Review of the Literature. Dig. Dis. Sci. 2022, 67, 4574–4580. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.; Yoshimoto, M.; Takebe, T. Fetal liver hematopoiesis: From development to delivery. Stem Cell Res. Ther. 2021, 12, 139. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, Z.; Ong, B.; Sahu, C.; Zeng, H.; Ruan, H.B. Bone marrow adipose tissue-derived stem cell factor mediates metabolic regulation of hematopoiesis. Haematologica 2019, 104, 1731–1743. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, X.; Huang, J.; Zeng, Y.; Liu, W.; Geng, C.; Li, K.W.; Yang, D.; Wu, S.; Wei, H. Relationships between hematopoiesis and hepatogenesis in the midtrimester fetal liver characterized by dynamic transcriptomic and proteomic profiles. PLoS ONE 2009, 4, e7641. [Google Scholar] [CrossRef]
- Gao, B.; Li, Z.T.; Xue, D.B.; Zhang, W.H. A novel mechanism of abnormal hematological indices in liver cirrhosis: Bone marrow endothelial cell dysfunction caused by humoral inhibitor affects the hematopoietic function of bone marrow. Med. Hypotheses 2014, 82, 282–285. [Google Scholar] [CrossRef]
- Ganz, T.; Nemeth, E. Hepcidin and iron homeostasis. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2012, 1823, 1434–1443. [Google Scholar] [CrossRef]
- Clara, C.; Antonella, N.; Laura, S. Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica 2020, 105, 260–272. [Google Scholar] [CrossRef]
- Xu, Y.; Alfaro-Magallanes, V.M.; Babitt, J.L. Physiological and pathophysiological mechanisms of hepcidin regulation: Clinical implications for iron disorders. Br. J. Haematol. 2021, 193, 882–893. [Google Scholar] [CrossRef] [PubMed]
- Tufail, M.; Jiang, C.-H.; Li, N. Altered metabolism in cancer: Insights into energy pathways and therapeutic targets. Mol. Cancer 2024, 23, 203. [Google Scholar] [CrossRef]
- Sayın, S.; Yıldırım, M.; Erdoğdu, B.; Kaplan, O.; Koç, E.; Bulduk, T.; Cömert, M.; Güney, M.; Çelebier, M.; Aylı, M. Metabolomic Profiling and Bioanalysis of Chronic Myeloid Leukemia: Identifying Biomarkers for Treatment Response and Disease Monitoring. Metabolites 2025, 15, 376. [Google Scholar] [CrossRef]
- Rattigan, K.M.; Zarou, M.M.; Helgason, G.V. Metabolism in stem cell–driven leukemia: Parallels between hematopoiesis and immunity. Blood 2023, 141, 2553–2565. [Google Scholar]
- Tuval, A.; Shlush, L.I. Evolutionary trajectory of leukemic clones and its clinical implications. Haematologica 2019, 104, 872–880. [Google Scholar] [CrossRef]
- Joo, L.; Bradley, C.C.; Lin, S.H.; Scheet, P.A.; Nead, K.T. Causes of Clonal Hematopoiesis: A Review. Curr. Oncol. Rep. 2023, 25, 211–220. [Google Scholar] [CrossRef]
- Corces, M.R.; Chang, H.Y.; Majeti, R. Preleukemic hematopoietic stem cells in human acute myeloid leukemia. Front. Oncol. 2017, 7, 263. [Google Scholar] [CrossRef] [PubMed]
- Shuvalov, O.; Petukhov, A.; Daks, A.; Fedorova, O.; Vasileva, E.; Barlev, N.A. One-carbon metabolism and nucleotide biosynthesis as attractive targets for anticancer therapy. Oncotarget 2017, 8, 23955. [Google Scholar] [CrossRef] [PubMed]
- Kemna, K.; van der Burg, M.; Lankester, A.; Giera, M. Hematopoietic stem cell metabolism within the bone marrow niche—Insights and opportunities. Bioessays 2025, 47, e2400154. [Google Scholar] [CrossRef]
- Zarou, M.M.; Vazquez, A.; Vignir Helgason, G. Folate metabolism: A re-emerging therapeutic target in haematological cancers. Leukemia 2021, 35, 1539–1551. [Google Scholar] [CrossRef]
- Zaitsev, A.V.; Martinov, M.V.; Vitvitsky, V.M.; Ataullakhanov, F.I. Rat liver folate metabolism can provide an independent functioning of associated metabolic pathways. Sci. Rep. 2019, 9, 7657. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, S.E.; Campbell, C.L.; Hillman, R.S. Kinetics of the normal folate enterohepatic cycle. J. Clin. Investig. 1979, 64, 83–88. [Google Scholar] [CrossRef]
- Cantarella, C.D.; Ragusa, D.; Giammanco, M.; Tosi, S. Folate deficiency as predisposing factor for childhood leukaemia: A review of the literature. Genes. Nutr. 2017, 12, 14. [Google Scholar] [CrossRef]
- Duthie, S.J. Folate and cancer: How DNA damage, repair and methylation impact on colon carcinogenesis. J. Inherit. Metab. Dis. 2011, 34, 101–109. [Google Scholar] [CrossRef]
- LeBlanc, D.P.; Behan, N.A.; O’Brien, J.M.; Marchetti, F.; MacFarlane, A.J. Folate deficiency increases chromosomal damage and mutations in hematopoietic cells in the transgenic mutamouse model. Environ. Mol. Mutagen. 2018, 59, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Koury, M.J.; Park, D.J.; Martincic, D.; Horne, D.W.; Kravtsov, V.; Whitlock, J.A.; del Pilar Aguinaga, M.; Kopsombut, P. Folate Deficiency Delays the Onset But Increases the Incidence of Leukemia in Friend Virus-Infected Mice. Blood 1997, 90, 4054–4061. [Google Scholar] [CrossRef]
- Zheng, Y.; Cantley, L.C. Toward a better understanding of folate metabolism in health and disease. J. Exp. Med. 2019, 216, 253–266. [Google Scholar] [CrossRef]
- Beetstra, S.; Thomas, P.; Salisbury, C.; Turner, J.; Fenech, M. Folic acid deficiency increases chromosomal instability, chromosome 21 aneuploidy and sensitivity to radiation-induced micronuclei. Mutat. Res. 2005, 578, 317–326. [Google Scholar] [CrossRef]
- Lamm, N.; Maoz, K.; Bester, A.C.; Im, M.M.; Shewach, D.S.; Karni, R.; Kerem, B. Folate levels modulate oncogene-induced replication stress and tumorigenicity. EMBO Mol. Med. 2015, 7, 1138–1152. [Google Scholar]
- Duthie, S.J. Folic acid deficiency and cancer: Mechanisms of DNA instability. Br. Med. Bull. 1999, 55, 578–592. [Google Scholar] [CrossRef] [PubMed]
- Crider, K.S.; Yang, T.P.; Berry, R.J.; Bailey, L.B. Folate and DNA methylation: A review of molecular mechanisms and the evidence for folate’s role. Adv. Nutr. 2012, 3, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Bills, N.D.; Koury, M.J.; Clifford, A.J.; Dessypris, E.N. Ineffective hematopoiesis in folate-deficient mice. Blood 1992, 79, 2273–2280. [Google Scholar][Green Version]
- Henry, C.J.; Nemkov, T.; Casás-Selves, M.; Bilousova, G.; Zaberezhnyy, V.; Higa, K.C.; Serkova, N.J.; Hansen, K.C.; D’Alessandro, A.; DeGregori, J. Folate dietary insufficiency and folic acid supplementation similarly impair metabolism and compromise hematopoiesis. Haematologica 2017, 102, 1985–1994. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, R.; Kim, S.H.; Shah, H.; Zhang, S.; Liang, J.H.; Fang, Y.; Gentili, M.; Leary, C.N.O.; Elledge, S.J.; et al. SARS-CoV-2 hijacks folate and one-carbon metabolism for viral replication. Nat. Commun. 2021, 12, 1676. [Google Scholar] [CrossRef]
- Trougakos, I.P.; Terpos, E.; Alexopoulos, H.; Politou, M.; Paraskevis, D.; Scorilas, A.; Kastritis, E.; Andreakos, E.; Dimopoulos, M.A. Adverse effects of COVID-19 mRNA vaccines: The spike hypothesis. Trends Mol. Med. 2022, 28, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pang, Y.; Xu, B.; Chen, X.; Liang, S.; Hu, J.; Luo, X. Folic acid restricts SARS-CoV-2 invasion by methylating ACE2. Front. Microbiol. 2022, 13, 980903. [Google Scholar] [CrossRef]
- Teijaro, J.R.; Farber, D.L. COVID-19 vaccines: Modes of immune activation and future challenges. Nat. Rev. Immunol. 2021, 21, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Reinscheid, M.; Luxenburger, H.; Karl, V.; Graeser, A.; Giese, S.; Ciminski, K.; Reeg, D.B.; Oberhardt, V.; Roehlen, N.; Lang-Meli, J.; et al. COVID-19 mRNA booster vaccine induces transient CD8+ T effector cell responses while conserving the memory pool for subsequent reactivation. Nat. Commun. 2022, 13, 4631. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. Publisher Correction: COVID-19 vaccine BNT162b1 elicits human antibody and T(H)1 T cell responses. Nature 2021, 590, E17. [Google Scholar] [CrossRef]
- Abe, I.; Shirato, K.; Hashizume, Y.; Mitsuhashi, R.; Kobayashi, A.; Shiono, C.; Sato, S.; Tachiyashiki, K.; Imaizumi, K. Folate-deficiency induced cell-specific changes in the distribution of lymphocytes and granulocytes in rats. Environ. Health Prev. Med. 2013, 18, 78–84. [Google Scholar] [CrossRef]
- Courtemanche, C.; Elson-Schwab, I.; Mashiyama, S.T.; Kerry, N.; Ames, B.N. Folate Deficiency Inhibits the Proliferation of Primary Human CD8+ T Lymphocytes In Vitro1. J. Immunol. 2004, 173, 3186–3192. [Google Scholar] [CrossRef]
- Bayer, A.L.; Fraker, C.A. The folate cycle as a cause of natural killer cell dysfunction and viral etiology in type 1 diabetes. Front. Endocrinol. 2017, 8, 302005. [Google Scholar] [CrossRef]
- Dagla, I.; Iliou, A.; Benaki, D.; Gikas, E.; Mikros, E.; Bagratuni, T.; Kastritis, E.; Dimopoulos, M.A.; Terpos, E.; Tsarbopoulos, A. Plasma metabolomic alterations induced by COVID-19 vaccination reveal putative biomarkers reflecting the immune response. Cells 2022, 11, 1241. [Google Scholar] [CrossRef] [PubMed]
- Masoodi, M.; Peschka, M.; Schmiedel, S.; Haddad, M.; Frye, M.; Maas, C.; Lohse, A.; Huber, S.; Kirchhof, P.; Nofer, J.-R.; et al. Disturbed lipid and amino acid metabolisms in COVID-19 patients. J. Mol. Med. 2022, 100, 555–568. [Google Scholar] [CrossRef]
- Krum, B.; Huerta, N.; Chiou, V.; Welner, R.; Patel, S.B.; Nemkov, T.; Beaudin, A.E. Metabolic programming of hematopoietic stem cell function by prenatal folate. Blood 2023, 142, 5. [Google Scholar] [CrossRef]
- Karakousis, N.D.; Gourgoulianis, K.I.; Kotsiou, O.S. The Role of Folic Acid in SARS-CoV-2 Infection: An Intriguing Linkage under Investigation. J. Pers. Med. 2023, 13, 561. [Google Scholar] [CrossRef]
- Škrbić, R.; Travar, M.; Stojiljković, M.P.; Djuric, D.M.; Suručić, R. Folic Acid and Leucovorin Have Potential to Prevent SARS-CoV-2-Virus Internalization by Interacting with S-Glycoprotein/Neuropilin-1 Receptor Complex. Molecules 2023, 28, 2294. [Google Scholar]
- Bliek-Bueno, K.; Mucherino, S.; Poblador-Plou, B.; González-Rubio, F.; Aza-Pascual-Salcedo, M.; Orlando, V.; Clerencia-Sierra, M.; Ioakeim-Skoufa, I.; Coscioni, E.; Carmona-Pírez, J. Baseline drug treatments as indicators of increased risk of COVID-19 mortality in Spain and Italy. Int. J. Environ. Res. Public Health 2021, 18, 11786. [Google Scholar]
- Sheybani, Z.; Dokoohaki, M.H.; Negahdaripour, M.; Dehdashti, M.; Zolghadr, H.; Moghadami, M.; Masoompour, S.M.; Zolghadr, A.R. The interactions of folate with the enzyme furin: A computational study. RSC Adv. 2021, 11, 23815–23824. [Google Scholar] [CrossRef]
- Jiang, X.; Li, D.; Maghsoudloo, M.; Zhang, X.; Ma, W.; Fu, J. Targeting furin, a cellular proprotein convertase, for COVID-19 prevention and therapeutics. Drug Discov. Today 2024, 29, 104026. [Google Scholar] [CrossRef]
- McCaddon, A.; Regland, B. COVID-19: A methyl-group assault? Med. Hypotheses 2021, 149, 110543. [Google Scholar] [CrossRef] [PubMed]
- Rinchai, D.; Deola, S.; Zoppoli, G.; Kabeer, B.S.A.; Taleb, S.; Pavlovski, I.; Maacha, S.; Gentilcore, G.; Toufiq, M.; Mathew, L.; et al. High-temporal resolution profiling reveals distinct immune trajectories following the first and second doses of COVID-19 mRNA vaccines. Sci. Adv. 2022, 8, eabp9961. [Google Scholar] [CrossRef] [PubMed]
- Jukic, I.; Heffernan, A.; Schelling, A.F.; Kokic Males, V.; Savicevic, N.J.; Kovacic, V. Association between COVID-19 Infection or Vaccination Outcomes and Methylenetetrahydrofolate Reductase Gene Polymorphism: A Systematic Review of the Literature. J. Pers. Med. 2023, 13, 1687. [Google Scholar] [CrossRef]
- Reichmuth, A.M.; Oberli, M.A.; Jaklenec, A.; Langer, R.; Blankschtein, D. mRNA vaccine delivery using lipid nanoparticles. Ther. Deliv. 2016, 7, 319–334. [Google Scholar] [CrossRef]
- Hui, Y.; Yi, X.; Wibowo, D.; Yang, G.; Middelberg, A.P.; Gao, H.; Zhao, C.-X. Nanoparticle elasticity regulates phagocytosis and cancer cell uptake. Sci. Adv. 2020, 6, eaaz4316. [Google Scholar] [CrossRef]
- Kiaie, S.H.; Majidi Zolbanin, N.; Ahmadi, A.; Bagherifar, R.; Valizadeh, H.; Kashanchi, F.; Jafari, R. Recent advances in mRNA-LNP therapeutics: Immunological and pharmacological aspects. J. Nanobiotechnol. 2022, 20, 276. [Google Scholar] [CrossRef]
- Nikolova-Karakashian, M.N.; Reid, M.B. Sphingolipid metabolism, oxidant signaling, and contractile function of skeletal muscle. Antioxid. Redox Signal. 2011, 15, 2501–2517. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, S.; Meyers-Needham, M.; Senkal, C.E.; Saddoughi, S.A.; Sentelle, D.; Selvam, S.P.; Salas, A.; Ogretmen, B. Sphingolipids and cancer: Ceramide and sphingosine-1-phosphate in the regulation of cell death and drug resistance. Future Oncol. 2010, 6, 1603–1624. [Google Scholar] [CrossRef] [PubMed]
- Oskouian, B.; Saba, J.D. Cancer treatment strategies targeting sphingolipid metabolism. Adv. Exp. Med. Biol. 2010, 688, 185–205. [Google Scholar] [CrossRef]
- Binici, B.; Rattray, Z.; Zinger, A.; Perrie, Y. Exploring the impact of commonly used ionizable and pegylated lipids on mRNA-LNPs: A combined in vitro and preclinical perspective. J. Control Release 2025, 377, 162–173. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Ferraresso, F.; Strilchuk, A.W.; Juang, L.J.; Poole, L.G.; Luyendyk, J.P.; Kastrup, C.J. Comparison of DLin-MC3-DMA and ALC-0315 for siRNA Delivery to Hepatocytes and Hepatic Stellate Cells. Mol. Pharm. 2022, 19, 2175–2182. [Google Scholar] [CrossRef]
- Álvarez-Benedicto, E.; Farbiak, L.; Márquez Ramírez, M.; Wang, X.; Johnson, L.T.; Mian, O.; Guerrero, E.D.; Siegwart, D.J. Optimization of phospholipid chemistry for improved lipid nanoparticle (LNP) delivery of messenger RNA (mRNA). Biomater. Sci. 2022, 10, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.F.; Galam, D.; Gao, L.; Tan, B.C.; Wong, B.H.; Chua, G.L.; Loke, R.Y.; Lim, Y.C.; Wenk, M.R.; Lim, M.S.; et al. Blood-derived lysophospholipid sustains hepatic phospholipids and fat storage necessary for hepatoprotection in overnutrition. J. Clin. Investig. 2023, 133, e171267. [Google Scholar] [CrossRef]
- Papadopoulos, C.; Tentes, I.; Papazoglou, D.; Anagnostopoulos, K. Lysophospholipid metabolism and signalling in non-alcoholic fatty liver disease. Folia Medica 2022, 64, 7–12. [Google Scholar] [CrossRef]
- Tan, S.T.; Ramesh, T.; Toh, X.R.; Nguyen, L.N. Emerging roles of lysophospholipids in health and disease. Prog. Lipid Res. 2020, 80, 101068. [Google Scholar] [CrossRef]
- Giraldo-Lorza, J.M.; Leidy, C.; Manrique-Moreno, M. The Influence of Cholesterol on Membrane Targeted Bioactive Peptides: Modulating Peptide Activity Through Changes in Bilayer Biophysical Properties. Membranes 2024, 14, 220. [Google Scholar] [CrossRef] [PubMed]
- Ikonen, E. Cellular cholesterol trafficking and compartmentalization. Nat. Rev. Mol. Cell Biol. 2008, 9, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Escalona-Rayo, O.; Zeng, Y.; Knol, R.A.; Kock, T.J.F.; Aschmann, D.; Slütter, B.; Kros, A. In vitro and in vivo evaluation of clinically-approved ionizable cationic lipids shows divergent results between mRNA transfection and vaccine efficacy. Biomed. Pharmacother. 2023, 165, 115065. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Osborne, T.F.; Seo, Y.-K.; Jeon, T.-I. SREBP-1c Ablation Protects Against ER Stress-induced Hepatic Steatosis by Preventing Impaired Fatty Acid Oxidation. J. Life Sci. 2021, 31, 796–805. [Google Scholar]
- Pinto, B.A.S.; França, L.M.; Laurindo, F.R.M.; Paes, A.M.A. Unfolded Protein Response: Cause or Consequence of Lipid and Lipoprotein Metabolism Disturbances? Adv. Exp. Med. Biol. 2019, 1127, 67–82. [Google Scholar] [CrossRef]
- Baral, A. Endoplasmic Reticulum Stress Signaling in the Regulation of Hepatic Pathological Responses. Stresses 2024, 4, 481–504. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, H.; Han, F.; Guo, P. Reactive Oxygen Species as Key Molecules in the Pathogenesis of Alcoholic Fatty Liver Disease and Nonalcoholic Fatty Liver Disease: Future Perspectives. Curr. Issues Mol. Biol. 2025, 47, 464. [Google Scholar] [CrossRef]
- Liang, W.; Tonini, G.; Mulder, P.; Kelder, T.; van Erk, M.; van den Hoek, A.M.; Mariman, R.; Wielinga, P.Y.; Baccini, M.; Kooistra, T. Coordinated and interactive expression of genes of lipid metabolism and inflammation in adipose tissue and liver during metabolic overload. PLoS ONE 2013, 8, e75290. [Google Scholar]
- Błachnio-Zabielska, A.U.; Sadowska, P.; Zdrodowski, M.; Laudański, P.; Szamatowicz, J.; Kuźmicki, M. The Interplay between Oxidative Stress and Sphingolipid Metabolism in Endometrial Cancer. Int. J. Mol. Sci. 2024, 25, 10243. [Google Scholar] [CrossRef]
- Green, C.D.; Maceyka, M.; Cowart, L.A.; Spiegel, S. Sphingolipids in metabolic disease: The good, the bad, and the unknown. Cell Metab. 2021, 33, 1293–1306. [Google Scholar] [CrossRef]
- Van Brocklyn, J.R.; Williams, J.B. The control of the balance between ceramide and sphingosine-1-phosphate by sphingosine kinase: Oxidative stress and the seesaw of cell survival and death. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2012, 163, 26–36. [Google Scholar]
- Köberlin, M.S.; Snijder, B.; Heinz, L.X.; Baumann, C.L.; Fauster, A.; Vladimer, G.I.; Gavin, A.-C.; Superti-Furga, G. A Conserved Circular Network of Coregulated Lipids Modulates Innate Immune Responses. Cell 2015, 162, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Rufail, M.L.; Bassi, R.; Giussani, P. Sphingosine-1-Phosphate Metabolic Pathway in Cancer: Implications for Therapeutic Targets. Int. J. Mol. Sci. 2025, 26, 1056. [Google Scholar] [CrossRef] [PubMed]
- Chalfant, C.E.; Spiegel, S. Sphingosine 1-phosphate and ceramide 1-phosphate: Expanding roles in cell signaling. J. Cell Sci. 2005, 118, 4605–4612. [Google Scholar] [CrossRef]
- Anselmo, S.; Bonaccorso, E.; Gangemi, C.; Sancataldo, G.; Conti Nibali, V.; D’Angelo, G. Lipid Rafts in Signalling, Diseases, and Infections: What Can Be Learned from Fluorescence Techniques? Membranes 2025, 15, 6. [Google Scholar] [CrossRef] [PubMed]
- Preta, G. New insights into targeting membrane lipids for cancer therapy. Front. Cell Dev. Biol. 2020, 8, 571237. [Google Scholar] [CrossRef]
- Marques-da-Silva, D.; Lagoa, R. Rafting on the Evidence for Lipid Raft-like Domains as Hubs Triggering Environmental Toxicants’ Cellular Effects. Molecules 2023, 28, 6598. [Google Scholar] [CrossRef]
- Morán, G.A.G.; Parra-Medina, R.; Cardona, A.G.; Quintero-Ronderos, P.; Rodríguez, É.G. Cytokines, chemokines and growth factors. In Autoimmunity: From Bench to Bedside [Internet]; El Rosario University Press: Bogotá, Colombia, 2013. [Google Scholar]
- Alomari, M.; Almohazey, D.; Almofty, S.A.; Khan, F.A.; Al Hamad, M.; Ababneh, D. Role of Lipid Rafts in Hematopoietic Stem Cells Homing, Mobilization, Hibernation, and Differentiation. Cells 2019, 8, 630. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, M.; Su, J.; Li, Y.; Long, J.; Chu, J.; Wan, X.; Cao, Y.; Li, Q. Lipid Metabolism and Cancer. Life 2022, 12, 784. [Google Scholar] [CrossRef]
- Zhang, L.; Chang, N.; Liu, J.; Liu, Z.; Wu, Y.; Sui, L.; Chen, W. Reprogramming lipid metabolism as potential strategy for hematological malignancy therapy. Front. Oncol. 2022, 12, 987499. [Google Scholar] [CrossRef]
- Nguyen, V.; Zhang, Y.; Gao, C.; Cao, X.; Tian, Y.; Carver, W. The Spike Protein of SARS-CoV-2 Impairs Lipid Metabolism and Increases Susceptibility to Lipotoxicity: Implication for a Role of Nrf2. Cells 2022, 11, 1916. [Google Scholar]
- Hao, Y.; Zhang, Z.; Feng, G.; Chen, M.; Wan, Q.; Lin, J.; Wu, L.; Nie, W.; Chen, S. Distinct lipid metabolic dysregulation in asymptomatic COVID-19. iScience 2021, 24, 102974. [Google Scholar] [CrossRef]
- Munawar, W.A.S.W.A.; Elias, M.H.; Addnan, F.H.; Hassandarvish, P.; AbuBakar, S.; Roslan, N. Gene expression profiling of host lipid metabolism in SARS-CoV-2 infected patients: A systematic review and integrated bioinformatics analysis. BMC Infect. Dis. 2024, 24, 124. [Google Scholar] [CrossRef]
- Yang, Y.; Wei, Z.; Xiong, C.; Qian, H. Direct mechanisms of SARS-CoV-2-induced cardiomyocyte damage: An update. Virol. J. 2022, 19, 108. [Google Scholar] [CrossRef]
- Yonker, L.M.; Swank, Z.; Bartsch, Y.C.; Burns, M.D.; Kane, A.; Boribong, B.P.; Davis, J.P.; Loiselle, M.; Novak, T.; Senussi, Y.; et al. Circulating Spike Protein Detected in Post-COVID-19 mRNA Vaccine Myocarditis. Circulation 2023, 147, 867–876. [Google Scholar] [CrossRef]
- Bozkurt, B. Shedding Light on Mechanisms of Myocarditis With COVID-19 mRNA Vaccines. Circulation 2023, 147, 877–880. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Rao, Q.; Mei, Y.; Xing, H.; Gu, R.; Mou, J.; Chen, M.; Ding, F.; Xie, W.; et al. Targeting Fatty Acid Metabolism Abrogates the Differentiation Blockade in Preleukemic Cells. Cancer Res. 2024, 84, 4233–4245. [Google Scholar] [CrossRef] [PubMed]
- Lo Presti, C.; Yamaryo-Botté, Y.; Mondet, J.; Berthier, S.; Nutiu, D.; Botté, C.; Mossuz, P. Variation in Lipid Species Profiles among Leukemic Cells Significantly Impacts Their Sensitivity to the Drug Targeting of Lipid Metabolism and the Prognosis of AML Patients. Int. J. Mol. Sci. 2023, 24, 5988. [Google Scholar] [CrossRef] [PubMed]
- Raza, Y.; Atallah, J.; Luberto, C. Advancements on the Multifaceted Roles of Sphingolipids in Hematological Malignancies. Int. J. Mol. Sci. 2022, 23, 12745. [Google Scholar] [CrossRef]
- Salminen, A. Role of indoleamine 2,3-dioxygenase 1 (IDO1) and kynurenine pathway in the regulation of the aging process. Ageing Res. Rev. 2022, 75, 101573. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, X.; Yin, X.; Lv, J.; Tang, K.; Ma, J.; Ji, T.; Zhang, H.; Dong, W.; Jin, X.; et al. Blockade of IDO-kynurenine-AhR metabolic circuitry abrogates IFN-γ-induced immunologic dormancy of tumor-repopulating cells. Nat. Commun. 2017, 8, 15207. [Google Scholar] [CrossRef] [PubMed]
- Hellgren, F.; Rosdahl, A.; Cerveira, R.A.; Lenart, K.; Ols, S.; Gwon, Y.-D.; Kurt, S.; Delis, A.M.; Joas, G.; Evander, M. Modulation of innate immune response to mRNA vaccination after SARS-CoV-2 infection or sequential vaccination in humans. JCI Insight 2024, 9, e175401. [Google Scholar] [PubMed]
- Bergamaschi, C.; Terpos, E.; Rosati, M.; Angel, M.; Bear, J.; Stellas, D.; Karaliota, S.; Apostolakou, F.; Bagratuni, T.; Patseas, D.; et al. Systemic IL-15, IFN-γ, and IP-10/CXCL10 signature associated with effective immune response to SARS-CoV-2 in BNT162b2 mRNA vaccine recipients. Cell Rep. 2021, 36, 109504. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.W.; Williams, R.O. Modulation of T cells by tryptophan metabolites in the kynurenine pathway. Trends Pharmacol. Sci. 2023, 44, 442–456. [Google Scholar] [CrossRef]
- Basson, C.; Serem, J.C.; Hlophe, Y.N.; Bipath, P. The tryptophan–kynurenine pathway in immunomodulation and cancer metastasis. Cancer Med. 2023, 12, 18691–18701. [Google Scholar] [CrossRef]
- Hara, T.; Takuro, M.; Yuhei, S.; Nobuhiko, N.; Hiroshi, N.; Soranobu, N.; Junichi, K.; Yasuhito, N.; Masahito, S.; Hiroyasu, I.; et al. Prognostic value of the combination of serum l-kynurenine level and indoleamine 2,3-dioxygenase mRNA expression in acute myeloid leukemia. Leuk. Lymphoma 2016, 57, 2208–2211. [Google Scholar] [CrossRef]
- Leon-Letelier, R.A.; Dou, R.; Vykoukal, J.; Sater, A.H.A.; Ostrin, E.; Hanash, S.; Fahrmann, J.F. The kynurenine pathway presents multi-faceted metabolic vulnerabilities in cancer. Front. Oncol. 2023, 13, 1256769. [Google Scholar] [CrossRef]
- Shadboorestan, A.; Koual, M.; Dairou, J.; Coumoul, X. The role of the kynurenine/AhR pathway in diseases related to metabolism and cancer. Int. J. Tryptophan Res. 2023, 16, 11786469231185102. [Google Scholar]
- Arandi, N.; Ramzi, M.; Safaei, F.; Monabati, A. Overexpression of indoleamine 2,3-dioxygenase correlates with regulatory T cell phenotype in acute myeloid leukemia patients with normal karyotype. Blood Res. 2018, 53, 294–298. [Google Scholar] [CrossRef]
- Weiss-Tessbach, M.; Haider, T.; Gowran, A.; Schubert, L.; Mühlbacher, J.; Brankovic, J.; Wahrmann, M.; Jilma, B.; Boehm, T. COVID-19 mRNA-1273 vaccination induced mast cell activation with strongly elevated Th(2) cytokines in a systemic mastocytosis patient. Inflamm. Res. 2025, 74, 71. [Google Scholar] [CrossRef]
- Dehhaghi, M.; Heydari, M.; Panahi, H.K.S.; Lewin, S.R.; Heng, B.; Brew, B.J.; Guillemin, G.J. The roles of the kynurenine pathway in COVID-19 neuropathogenesis. Infection 2024, 52, 2043–2059. [Google Scholar] [CrossRef]
- Badawy, A.A.B. The kynurenine pathway of tryptophan metabolism: A neglected therapeutic target of COVID-19 pathophysiology and immunotherapy. Biosci. Rep. 2023, 43, BSR20230595. [Google Scholar] [CrossRef]
- Guo, L.; Appelman, B.; Mooij-Kalverda, K.; Houtkooper, R.H.; van Weeghel, M.; Vaz, F.M.; Dijkhuis, A.; Dekker, T.; Smids, B.S.; Duitman, J.W.; et al. Prolonged indoleamine 2,3-dioxygenase-2 activity and associated cellular stress in post-acute sequelae of SARS-CoV-2 infection. eBioMedicine 2023, 94, 104729. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, S.; Gou, J.; Wen, Y.; Fan, L.; Zhou, J.; Zhou, G.; Xu, G.; Zhang, Z. Spike-mediated ACE2 down-regulation was involved in the pathogenesis of SARS-CoV-2 infection. J. Infect. 2022, 85, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Montezano, A.C.; Camargo, L.L.; Mary, S.; Neves, K.B.; Rios, F.J.; Stein, R.; Lopes, R.A.; Beattie, W.; Thomson, J.; Herder, V.; et al. SARS-CoV-2 spike protein induces endothelial inflammation via ACE2 independently of viral replication. Sci. Rep. 2023, 13, 14086. [Google Scholar] [CrossRef]
- Stoian, I.; Manolescu, B.; Atanasiu, V.; Lupescu, O.; Buşu, C. IL-6—STAT-3—hepcidin: Linking inflammation to the iron metabolism. Rom. J. Intern. Med. 2007, 45, 305–309. [Google Scholar]
- Wrighting, D.M.; Andrews, N.C. Interleukin-6 induces hepcidin expression through STAT3. Blood 2006, 108, 3204–3209. [Google Scholar] [CrossRef]
- Rosati, M.; Terpos, E.; Homan, P.; Bergamaschi, C.; Karaliota, S.; Ntanasis-Stathopoulos, I.; Devasundaram, S.; Bear, J.; Burns, R.; Bagratuni, T. Rapid transient and longer-lasting innate cytokine changes associated with adaptive immunity after repeated SARS-CoV-2 BNT162b2 mRNA vaccinations. Front. Immunol. 2023, 14, 1292568. [Google Scholar] [CrossRef]
- Xie, F.; Zhao, M.F.; Zhu, H.B.; Lu, W.Y.; Xu, X.N.; Xiao, X.; Mu, J.; Liu, P.J.; Li, Y.M. Effects of oxidative stress on hematopoiesis of hematopoietic stem and progenitor cells with iron overload. Zhonghua Yi Xue Za Zhi 2011, 91, 3284–3288. [Google Scholar] [PubMed]
- Meneghini, R. Iron Homeostasis, Oxidative Stress, and DNA Damage. Free. Radic. Biol. Med. 1997, 23, 783–792. [Google Scholar] [CrossRef]
- Wang, F.; Lv, H.; Zhao, B.; Zhou, L.; Wang, S.; Luo, J.; Liu, J.; Shang, P. Iron and leukemia: New insights for future treatments. J. Exp. Clin. Cancer Res. 2019, 38, 406. [Google Scholar] [CrossRef] [PubMed]
- Yahata, T.; Takanashi, T.; Muguruma, Y.; Ibrahim, A.A.; Matsuzawa, H.; Uno, T.; Sheng, Y.; Onizuka, M.; Ito, M.; Kato, S.; et al. Accumulation of oxidative DNA damage restricts the self-renewal capacity of human hematopoietic stem cells. Blood 2011, 118, 2941–2950. [Google Scholar] [CrossRef]
- Nemeth, E.; Ganz, T. Hepcidin-Ferroportin Interaction Controls Systemic Iron Homeostasis. Int. J. Mol. Sci. 2021, 22, 6493. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Pereira-Reis, J.; Guerra, A.; Rivella, S.; Duarte, D. The Role of Iron in Benign and Malignant Hematopoiesis. Antioxid. Redox Signal. 2021, 35, 415–432. [Google Scholar] [CrossRef]
- Brissot, E.; Bernard, D.G.; Loréal, O.; Brissot, P.; Troadec, M.-B. Too much iron: A masked foe for leukemias. Blood Rev. 2020, 39, 100617. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Cho, S.-H.; Roh, G.; Park, H.-J.; Lee, Y.-J.; Jeon, H.-E.; Lee, Y.-S.; Bae, S.-H.; Youn, S.B.; et al. Assessing the impact of mRNA vaccination in chronic inflammatory murine model. NPJ Vaccines 2024, 9, 34. [Google Scholar] [CrossRef]
- Liakou, A.I.; Tsantes, A.G.; Routsi, E.; Agiasofitou, E.; Kalamata, M.; Bompou, E.-K.; Tsante, K.A.; Vladeni, S.; Chatzidimitriou, E.; Kotsafti, O.; et al. Could Vaccination against COVID-19 Trigger Immune-Mediated Inflammatory Diseases? J. Clin. Med. 2024, 13, 4617. [Google Scholar] [CrossRef]
- Wei, L.; Dong, C.; Zhu, W.; Wang, B.-Z. mRNA Vaccine Nanoplatforms and Innate Immunity. Viruses 2024, 16, 120. [Google Scholar] [CrossRef]
- Nemeth, E.; Rivera, S.; Gabayan, V.; Keller, C.; Taudorf, S.; Pedersen, B.K.; Ganz, T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J. Clin. Investig. 2004, 113, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.; Jung, C.L.; Gabayan, V.; Deng, J.C.; Ganz, T.; Nemeth, E.; Bulut, Y. Hepcidin induction by pathogens and pathogen-derived molecules is strongly dependent on interleukin-6. Infect. Immun. 2014, 82, 745–752. [Google Scholar] [CrossRef]
- Cobo, I.; Tanaka, T.; Glass, C.K.; Yeang, C. Clonal hematopoiesis driven by DNMT3A and TET2 mutations: Role in monocyte and macrophage biology and atherosclerotic cardiovascular disease. Curr. Opin. Hematol. 2022, 29, 1–7. [Google Scholar] [CrossRef]
- Vallelonga, V.; Gandolfi, F.; Ficara, F.; Della Porta, M.G.; Ghisletti, S. Emerging insights into molecular mechanisms of inflammation in myelodysplastic syndromes. Biomedicines 2023, 11, 2613. [Google Scholar] [CrossRef]
- Li, J.; Wang, C.; Liu, J.; Yu, Y.; Liu, Y.; Peng, Q.; Liu, H.; Guan, X. A feedback loop: Interactions between Inflammatory Signals and Clonal Hematopoiesis in Cardiovascular Disease. Mol. Biol. Rep. 2021, 48, 3785–3798. [Google Scholar] [CrossRef]
- Woo, J.; Lu, D.; Lewandowski, A.; Xu, H.; Serrano, P.; Healey, M.; Yates, D.P.; Beste, M.T.; Libby, P.; Ridker, P.M.; et al. Effects of IL-1β inhibition on anemia and clonal hematopoiesis in the randomized CANTOS trial. Blood Adv. 2023, 7, 7471–7484. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Hong, S.; Chen, T.; Liu, L.; Cao, C.; Lv, F.; Rabinowitz, J.D.; Huang, Y.; Chen, X. Live-cell imaging of NADPH production from specific pathways. CCS Chem. 2021, 3, 1642–1648. [Google Scholar] [CrossRef]
- Chandel, N.S. NADPH-The Forgotten Reducing Equivalent. Cold Spring Harb. Perspect. Biol. 2021, 13, a040550. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Z.; Hoshino, A.; Zheng, H.D.; Morley, M.; Arany, Z.; Rabinowitz, J.D. NADPH production by the oxidative pentose-phosphate pathway supports folate metabolism. Nat. Metab. 2019, 1, 404–415. [Google Scholar] [CrossRef]
- Jin, E.S.; Lee, M.H.; Murphy, R.E.; Malloy, C.R. Pentose phosphate pathway activity parallels lipogenesis but not antioxidant processes in rat liver. Am. J. Physiol.-Endocrinol. Metab. 2018, 314, E543–E551. [Google Scholar] [CrossRef]
- Chen, C.; Lai, X.; Zhang, Y.; Xie, L.; Yu, Z.; Dan, S.; Jiang, Y.; Chen, W.; Liu, L.; Yang, Y.; et al. NADPH metabolism determines the leukemogenic capacity and drug resistance of AML cells. Cell Rep. 2022, 39, 110607. [Google Scholar] [CrossRef]
- Bakker, S.T.; Passegué, E. Resilient and resourceful: Genome maintenance strategies in hematopoietic stem cells. Exp. Hematol. 2013, 41, 915–923. [Google Scholar] [CrossRef]
- Bhanot, H.; Weisberg, E.L.; Reddy, M.M.; Nonami, A.; Neuberg, D.; Stone, R.M.; Podar, K.; Salgia, R.; Griffin, J.D.; Sattler, M. Acute myeloid leukemia cells require 6-phosphogluconate dehydrogenase for cell growth and NADPH-dependent metabolic reprogramming. Oncotarget 2017, 8, 67639–67650. [Google Scholar] [CrossRef]
- Prieto-Bermejo, R.; Romo-González, M.; Pérez-Fernández, A.; Ijurko, C.; Hernández-Hernández, Á. Reactive oxygen species in haematopoiesis: Leukaemic cells take a walk on the wild side. J. Exp. Clin. Cancer Res. 2018, 37, 125. [Google Scholar] [CrossRef] [PubMed]
- Stanicka, J.; Russell, E.G.; Woolley, J.F.; Cotter, T.G. NADPH oxidase-generated hydrogen peroxide induces DNA damage in mutant FLT3-expressing leukemia cells. J. Biol. Chem. 2015, 290, 9348–9361. [Google Scholar] [CrossRef] [PubMed]
- Weiss, C.N.; Ito, K. DNA damage: A sensible mediator of the differentiation decision in hematopoietic stem cells and in leukemia. Int. J. Mol. Sci. 2015, 16, 6183–6201. [Google Scholar] [CrossRef]
- Uzun, S.; Pant, A.; Bartoszek, E.; Gueguen, P.; Frei, S.; Heusler, H.; Arborelli, I.; Zinner, C.P.; Soylu, N.K.; Terziroli Beretta-Piccoli, B. Analysis of Hyperexpanded T Cell Clones in SARS-CoV-2 Vaccine-Associated Liver Injury by Spatial Proteomics and Transcriptomics. Liver Int. 2025, 45, e70172. [Google Scholar] [CrossRef] [PubMed]
- Elumalai, K.; Srinivasan, S.; Shanmugam, A. Impact of COVID-19 vaccines on liver function: A state of the art and challenges for healthcare providers. Gastroenterol. Endosc. 2024, 2, 42–51. [Google Scholar] [CrossRef]
- Kamm, D.R.; McCommis, K.S. Hepatic stellate cells in physiology and pathology. J. Physiol. 2022, 600, 1825–1837. [Google Scholar] [CrossRef]
- Gupta, G.; Khadem, F.; Uzonna, J.E. Role of hepatic stellate cell (HSC)-derived cytokines in hepatic inflammation and immunity. Cytokine 2019, 124, 154542. [Google Scholar] [CrossRef]
- Gandhi, C.R. Oxidative Stress and Hepatic Stellate Cells: A PARADOXICAL RELATIONSHIP. Trends Cell Mol. Biol. 2012, 7, 1–10. [Google Scholar]
- Yan, M.; Cui, Y.; Xiang, Q. Metabolism of hepatic stellate cells in chronic liver diseases: Emerging molecular and therapeutic interventions. Theranostics 2025, 15, 1715–1740. [Google Scholar] [CrossRef]
- Ju, H.-Q.; Lin, J.-F.; Tian, T.; Xie, D.; Xu, R.-H. NADPH homeostasis in cancer: Functions, mechanisms and therapeutic implications. Signal Transduct. Target. Ther. 2020, 5, 231. [Google Scholar] [CrossRef]
- Vairetti, M.; Di Pasqua, L.G.; Cagna, M.; Richelmi, P.; Ferrigno, A.; Berardo, C. Changes in Glutathione Content in Liver Diseases: An Update. Antioxidants 2021, 10, 364. [Google Scholar] [CrossRef]
- Ahn, W.; Burnett, F.N.; Pandey, A.; Ghoshal, P.; Singla, B.; Simon, A.B.; Derella, C.C.; Addo, S.A.; Harris, R.A.; Lucas, R.; et al. SARS-CoV-2 Spike Protein Stimulates Macropinocytosis in Murine and Human Macrophages via PKC-NADPH Oxidase Signaling. Antioxidants 2024, 13, 175. [Google Scholar] [CrossRef] [PubMed]
- Suhail, S.; Zajac, J.; Fossum, C.; Lowater, H.; McCracken, C.; Severson, N.; Laatsch, B.; Narkiewicz-Jodko, A.; Johnson, B.; Liebau, J.; et al. Role of Oxidative Stress on SARS-CoV (SARS) and SARS-CoV-2 (COVID-19) Infection: A Review. Protein J. 2020, 39, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Conceicao, C.; Thakur, N.; Human, S.; Kelly, J.T.; Logan, L.; Bialy, D.; Bhat, S.; Stevenson-Leggett, P.; Zagrajek, A.K.; Hollinghurst, P. The SARS-CoV-2 Spike protein has a broad tropism for mammalian ACE2 proteins. PLoS Biol. 2020, 18, e3001016. [Google Scholar] [CrossRef]
- Kumar, P.; Osahon, O.; Vides, D.B.; Hanania, N.; Minard, C.G.; Sekhar, R.V. Severe Glutathione Deficiency, Oxidative Stress and Oxidant Damage in Adults Hospitalized with COVID-19: Implications for GlyNAC (Glycine and N-Acetylcysteine) Supplementation. Antioxidants 2022, 11, 50. [Google Scholar]
- Youn, J.Y.; Zhang, Y.; Wu, Y.; Cannesson, M.; Cai, H. Therapeutic application of estrogen for COVID-19: Attenuation of SARS-CoV-2 spike protein and IL-6 stimulated, ACE2-dependent NOX2 activation, ROS production and MCP-1 upregulation in endothelial cells. Redox Biol. 2021, 46, 102099. [Google Scholar] [CrossRef]
- Pramono, A.A.; Rather, G.M.; Herman, H.; Lestari, K.; Bertino, J.R. NAD- and NADPH-Contributing Enzymes as Therapeutic Targets in Cancer: An Overview. Biomolecules 2020, 10, 358. [Google Scholar] [CrossRef]
- Paolillo, R.; Boulanger, M.; Gâtel, P.; Gabellier, L.; De Toledo, M.; Tempé, D.; Hallal, R.; Akl, D.; Moreaux, J.; Baik, H.; et al. The NADPH oxidase NOX2 is a marker of adverse prognosis involved in chemoresistance of acute myeloid leukemias. Haematologica 2022, 107, 2562–2575. [Google Scholar] [CrossRef]
- Reed, S.C.; Croessmann, S.; Park, B.H. CHIP Happens: Clonal Hematopoiesis of Indeterminate Potential and Its Relationship to Solid Tumors. Clin. Cancer Res. 2023, 29, 1403–1411. [Google Scholar] [CrossRef]
- Keenan, C.; Kartika, T.; Chua, J.; Kaner, J.D. Folate Supplementation during Induction Chemotherapy for Acute Myeloid Leukemia (AML). Blood 2024, 144, 5958. [Google Scholar] [CrossRef]
- Mokhtari, V.; Afsharian, P.; Shahhoseini, M.; Kalantar, S.M.; Moini, A. A Review on Various Uses of N-Acetyl Cysteine. Cell J. 2017, 19, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, X.; Wang, L.; Ma, X.; Gong, Z.; Zhang, S.; Li, Y. Targeting the IDO1 pathway in cancer: From bench to bedside. J. Hematol. Oncol. 2018, 11, 100. [Google Scholar] [CrossRef]
- Kularatne, R.N.; Crist, R.M.; Stern, S.T. The Future of Tissue-Targeted Lipid Nanoparticle-Mediated Nucleic Acid Delivery. Pharmaceuticals 2022, 15, 897. [Google Scholar] [CrossRef] [PubMed]
- Efe, C.; Kulkarni, A.V.; Terziroli Beretta-Piccoli, B.; Magro, B.; Stättermayer, A.; Cengiz, M.; Clayton-Chubb, D.; Lammert, C.; Bernsmeier, C.; Gül, Ö. Liver injury after SARS-CoV-2 vaccination: Features of immune-mediated hepatitis, role of corticosteroid therapy and outcome. Hepatology 2022, 76, 1576–1586. [Google Scholar] [CrossRef] [PubMed]
- Blank, M.; Israeli, E.; Gertel, S.; Amital, H.; Shoenfeld, Y. Molecular mimicry in autoimmunity and vaccinations. In Autoimmunity: From Bench to Bedside [Internet]; El Rosario University Press: Bogotá, Colombia, 2013. [Google Scholar]
- Segal, Y.; Shoenfeld, Y. Vaccine-induced autoimmunity: The role of molecular mimicry and immune crossreaction. Cell. Mol. Immunol. 2018, 15, 586–594. [Google Scholar] [CrossRef]
- Safary, A.; Akbarzadeh-Khiavi, M.; Barar, J.; Omidi, Y. SARS-CoV-2 vaccine-triggered autoimmunity: Molecular mimicry and/or bystander activation of the immune system. Bioimpacts 2023, 13, 269–273. [Google Scholar] [CrossRef]
| Mechanism | Testable Prediction | Measurable Outcome |
|---|---|---|
| Folate/One-Carbon Metabolism | Transient folate depletion following vaccination | Plasma and bone marrow tetrahydrofolate levels; lymphocyte proliferation and NK cell activity |
| Hepatic Lipid Processing/Lipid Overload | Alterations in sphingolipid and phospholipid profiles; lipid raft composition | Plasma and liver metabolomics; hepatocyte lipid droplet morphology; ER stress markers |
| Tryptophan Catabolism/IDO Activation | Increased kynurenine pathway activity mediated by cytokine induction | Kynurenine-to-tryptophan ratio; T-cell proliferation and effector function assays |
| Iron Homeostasis/Hepcidin Induction | Redistribution of iron between liver, macrophages, and bone marrow | Serum hepcidin, ferritin, transferrin saturation; bone marrow erythropoietic activity |
| NADPH/Redox Homeostasis | Elevated NADPH consumption and transient oxidative stress | NADPH/NADP+ ratio; reactive oxygen species (ROS) levels; antioxidant enzyme activity |
| Spike Protein-Specific Effects | Direct modulation of host metabolic pathways, including folate, lipid, and redox metabolism | Hepatocyte or cardiomyocyte lipid accumulation; ferroptotic sensitivity; NADPH utilization |
| Clinical Susceptibility | Individuals with pre-existing metabolic or genetic vulnerabilities exhibit exaggerated responses | Correlation of metabolomic changes with MTHFR polymorphisms, dyslipidemia, chronic inflammation; hematopoietic clonality |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erdoğdu, B.; Kaplan, O.; Çelebier, M.; Malkan, Ü.Y.; Haznedaroğlu, İ.C. Hepatic Metabolic Dysregulation as a Potential Amplifier of Leukemogenesis Following mRNA Vaccination: A Novel Mechanistic Hypothesis. Medicina 2025, 61, 1687. https://doi.org/10.3390/medicina61091687
Erdoğdu B, Kaplan O, Çelebier M, Malkan ÜY, Haznedaroğlu İC. Hepatic Metabolic Dysregulation as a Potential Amplifier of Leukemogenesis Following mRNA Vaccination: A Novel Mechanistic Hypothesis. Medicina. 2025; 61(9):1687. https://doi.org/10.3390/medicina61091687
Chicago/Turabian StyleErdoğdu, Batuhan, Ozan Kaplan, Mustafa Çelebier, Ümit Yavuz Malkan, and İbrahim Celalettin Haznedaroğlu. 2025. "Hepatic Metabolic Dysregulation as a Potential Amplifier of Leukemogenesis Following mRNA Vaccination: A Novel Mechanistic Hypothesis" Medicina 61, no. 9: 1687. https://doi.org/10.3390/medicina61091687
APA StyleErdoğdu, B., Kaplan, O., Çelebier, M., Malkan, Ü. Y., & Haznedaroğlu, İ. C. (2025). Hepatic Metabolic Dysregulation as a Potential Amplifier of Leukemogenesis Following mRNA Vaccination: A Novel Mechanistic Hypothesis. Medicina, 61(9), 1687. https://doi.org/10.3390/medicina61091687






