Longer Health Resort Therapy Improves Outcomes in Long COVID: A Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Qualification Criteria
2.2. Data Collection
2.3. Individuals
2.4. Treatment Regimen
2.5. Methods of Assessment
2.6. Statistical Analysis
3. Results
4. Discussion
4.1. Functional Outcomes
4.2. Neuropsychiatric Long COVID Symptoms
4.3. Cardiopulmonary Long COVID Symptoms
4.4. Treatment Duration as a Predictor of Clinical Outcomes
4.5. Study Limitations
4.6. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chee, Y.J.; Fan, B.E.; Young, B.E.; Dalan, R.; Lye, D.C. Clinical trials on the pharmacological treatment of long COVID: A systematic review. J. Med. Virol. 2023, 95, e28289. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146, Erratum in: Nat. Rev. Microbiol. 2023, 21, 408. [Google Scholar] [CrossRef]
- Yong, S.J. Long COVID or post-COVID-19 syndrome: Putative pathophysiology, risk factors, and treatments. Infect. Dis. 2021, 53, 737–754. [Google Scholar] [CrossRef]
- Monje, M.; Iwasaki, A. The neurobiology of long COVID. Neuron 2022, 110, 3484–3496. [Google Scholar] [CrossRef] [PubMed]
- Líška, D.; Liptaková, E.; Babičová, A.; Batalik, L.; Baňárová, P.S.; Dobrodenková, S. What is the quality of life in patients with long COVID compared to a healthy control group? Front. Public Health 2022, 10, 975992. [Google Scholar] [CrossRef] [PubMed]
- Prazeres, F.; Romualdo, A.P.; Campos Pinto, I.; Silva, J.; Oliveira, A.M. The impact of long COVID on quality of life and work performance among healthcare workers in Portugal. PeerJ 2025, 13, e19089. [Google Scholar] [CrossRef] [PubMed]
- Gandjour, A. Long COVID: Costs for the German economy and health care and pension system. BMC Health Serv. Res. 2023, 14, 641. [Google Scholar] [CrossRef]
- Chuang, H.J.; Lin, C.W.; Hsiao, M.Y.; Wang, T.G.; Liang, H.W. Long COVID and rehabilitation. J. Formos. Med. Assoc. 2024, 123, 61–69. [Google Scholar] [CrossRef]
- Bigman, G.; Rusu, M.E.; Shelawala, N.; Sorkin, J.D.; Beamer, B.A.; Ryan, A.S. A Comprehensive Scoping Review on Diet and Nutrition in Relation to Long COVID-19 Symptoms and Recovery. Nutrients 2025, 17, 1802. [Google Scholar] [CrossRef]
- Barrea, L.; Grant, W.B.; Frias-Toral, E.; Vetrani, C.; Verde, L.; de Alteriis, G.; Docimo, A.; Savastano, S.; Colao, A.; Muscogiuri, G. Dietary Recommendations for Post-COVID-19 Syndrome. Nutrients 2022, 14, 1305. [Google Scholar] [CrossRef]
- Dietz, T.K.; Brondstater, K.N. Long COVID management: A mini review of current recommendations and underutilized modalities. Front. Med. 2024, 11, 1430444. [Google Scholar] [CrossRef]
- Al-Jabr, H.; Hawke, L.D.; Thompson, D.R.; Clifton, A.; Shenton, M.; Castle, D.J.; Ski, C.F. Interventions to support mental health in people with long COVID: A scoping review. BMC Public Health 2023, 23, 1186. [Google Scholar] [CrossRef]
- Hawkins, J.; Hires, C.; Keenan, L.; Dunne, E. Aromatherapy blend of thyme, orange, clove bud, and frankincense boosts energy levels in post-COVID-19 female patients: A randomized, double-blinded, placebo controlled clinical trial. Complement. Ther. Med. 2022, 67, 102823. [Google Scholar] [CrossRef] [PubMed]
- Santana, K.; França, E.; Sato, J.; Silva, A.; Queiroz, M.; de Farias, J.; Rodrigues, D.; Souza, I.; Ribeiro, V.; Caparelli-Dáquer, E.; et al. Non-invasive brain stimulation for fatigue in post-acute sequelae of SARS-CoV-2 (PASC). Brain Stimul. 2023, 16, 100–107. [Google Scholar] [CrossRef]
- Zilberman-Itskovich, S.; Catalogna, M.; Sasson, E.; Elman-Shina, K.; Hadanny, A.; Lang, E.; Finci, S.; Polak, N.; Fishlev, G.; Korin, C.; et al. Hyperbaric oxygen therapy improves neurocognitive functions and symptoms of post-COVID condition: Randomised controlled trial. Sci. Rep. 2022, 12, 11252. [Google Scholar] [CrossRef] [PubMed]
- Onik, G.; Knapik, K.; Sieroń, K. Long COVID Cardiopulmonary Symptoms and Health Resort Treatment: A Retrospective Study. J. Clin. Med. 2024, 13, 5563. [Google Scholar] [CrossRef]
- Onik, G.; Knapik, K.; Dąbrowska-Galas, M.; Sieroń, K. Health Resort Treatment Improves Functioning and Physical Performance in Long COVID Patients: A Retrospective Study. Healthcare 2024, 12, 2344. [Google Scholar] [CrossRef] [PubMed]
- Onik, G.; Knapik, K.; Górka, D.; Sieroń, K. Health Resort Treatment Mitigates Neuropsychiatric Symptoms in Long COVID Patients: A Retrospective Study. Healthcare 2025, 13, 196. [Google Scholar] [CrossRef]
- Maccarone, M.C.; Masiero, S. Spa therapy interventions for post respiratory rehabilitation in COVID-19 subjects: Does the review of recent evidence suggest a role? Environ. Sci. Pollut. Res. Int. 2021, 28, 46063–46066. [Google Scholar] [CrossRef]
- Masiero, S.; Maccarone, M.C.; Agostini, F. Health resort medicine can be a suitable setting to recover disabilities in patients tested negative for COVID-19 discharged from hospital? A challenge for the future. Int. J. Biometeorol. 2020, 64, 1807–1809. [Google Scholar] [CrossRef]
- Gvozdjáková, A.; Sumbalová, Z.; Kucharská, J.; Rausová, Z.; Kovalčíková, E.; Takácsová, T.; Navas, P.; López-Lluch, G.; Mojto, V.; Palacka, P. Mountain spa rehabilitation improved health of patients with post-COVID-19 syndrome: Pilot study. Environ. Sci. Pollut. Res. Int. 2023, 30, 14200–14211. [Google Scholar] [CrossRef]
- Kędzierska, J. The role of health-resort treatment of children and adolescents in health care. J. Phys. Educ. Sport 2021, 21, 3040–3045. [Google Scholar] [CrossRef]
- Gutenbrunner, C.; Bender, T.; Cantista, P.; Karagülle, Z. A proposal for a worldwide definition of health resort medicine, balneology, medical hydrology and climatology. Int. J. Biometeorol. 2010, 54, 495–507. [Google Scholar] [CrossRef]
- European Physical and Rehabilitation Medicine Bodies Alliance. White Book on Physical and Rehabilitation Medicine in Europe. Introductions, Executive Summary, and Methodology. Eur. J. Phys. Rehabil. Med. 2018, 54, 125–155. [Google Scholar] [CrossRef]
- Castelli, L.; Galasso, L.; Mulè, A.; Ciorciari, A.; Fornasini, F.; Montaruli, A.; Roveda, E.; Esposito, F. Sleep and spa therapies: What is the role of balneotherapy associated with exercise? A systematic review. Front. Physiol. 2022, 13, 964232. [Google Scholar] [CrossRef]
- Masiero, S.; Maccarone, M.C.; Magro, G. Balneotherapy and human immune function in the era of COVID-19. Int. J. Biometeorol. 2020, 64, 1433–1434. [Google Scholar] [CrossRef]
- Gálvez, I.; Torres-Piles, S.; Ortega-Rincón, E. Balneotherapy, immune system, and stress response: A hormetic strategy? Int. J. Mol. Sci. 2018, 19, 1687. [Google Scholar] [CrossRef]
- Maraver, F.; Armijo, F.; Fernandez-Toran, M.A. Importance of the duration of treatment in Balneotherapy. Int. J. Biometeorol. 2021, 65, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Péter, I.; Jagicza, A.; Ajtay, Z.; Boncz, I.; Kiss, I.; Szendi, K.; Kustán, P.; Németh, B. Balneotherapy in Psoriasis Rehabilitation. In Vivo 2017, 31, 1163–1168. [Google Scholar] [CrossRef]
- Protano, C.; Vitali, M.; De Giorgi, A.; Marotta, D.; Crucianelli, S.; Fontana, M. Balneotherapy using thermal mineral water baths and dermatological diseases: A systematic review. Int. J. Biometeorol. 2024, 68, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Yolgösteren, E.; Külekçioğlu, S. The effectiveness of balneotherapy and thermal aquatic exercise in postoperative persistent lumbar pain syndrome. Int. J. Biometeorol. 2021, 65, 2137–2145. [Google Scholar] [CrossRef]
- Ferrara, E.; Scaramuzzino, M.; Murmura, G.; D’Addazio, G.; Sinjari, B. Emerging Evidence on Balneotherapy and Thermal Interventions in Post-COVID-19 Syndrome: A Systematic Review. Healthcare 2025, 13, 96. [Google Scholar] [CrossRef]
- Allam, N.M.; Koura, G.M.R.; Alrawaili, S.M.; Hamada, H.A.; Khater, H.A.; Balbaa, A.A. The effect of siwan therapy in management of patients with rheumatoid arthritis: A single blind randomized controlled trial. Biomed. Res. 2018, 29, 1400–1406. [Google Scholar] [CrossRef]
- Shchikota, A.M.; Pogonchenkova, I.V.; Rassulova, M.A.; Filippov, M.S.; Golubev, M.V.; Cherepanov, I.A. Use of hydrobalneotherapy in ambulatory rehabilitation of patients with post-COVID syndrome. Russ. J. Physiother. Balneol. Rehabil. 2023, 22, 141–150. [Google Scholar] [CrossRef]
- Ponikowska, I.; Adamczyk, P.; Wojciechowski, R.; Jarosz, K. Balneological treatment of patients with long COVID-19 syndrome in health-resort conditions. Acta. Balneol. 2023, 173, 75–81. [Google Scholar] [CrossRef]
- Ovejero, D.; Ribes, A.; Villar-García, J.; Trenchs-Rodriguez, M.; Lopez, D.; Nogués, X.; Güerri-Fernandez, R.; Garcia-Giralt, N. Balneotherapy for the treatment of post-COVID syndrome: A randomized controlled trial. BMC Complement. Med. Ther. 2025, 25, 37. [Google Scholar] [CrossRef]
- Vancea, A.; Iliescu, M.; Aivaz, K.A.; Popescu, M.N.; Beiu, C.; Spiru, L. Improving Functional Capacities and Well-Being in Older Adults: Strategies in Physical Medicine and Rehabilitation. Cureus 2024, 16, e66254. [Google Scholar] [CrossRef] [PubMed]
- Kanayama, H.; Kusaka, Y.; Hirai, T.; Inoue, H.; Agishi, Y.; Schuh, A. Climatotherapy in Japan: A pilot study. Int. J. Biometeorol. 2017, 61, 2141–2143. [Google Scholar] [CrossRef]
- Rapolienė, L.; Rapolis, D.; Bredelytė, A.; Taletavičienė, G.; Fioravanti, A.; Martinkėnas, A. Balneotherapy as a Complemen-tary Intervention for Stress and Cortisol Reduction: Findings from a Randomized Controlled Trial. Brain Sci. 2025, 15, 165. [Google Scholar] [CrossRef]
- Rapolienė, L.; Razbadauskas, A.; Sąlyga, J.; Martinkėnas, A. Stress and Fatigue Management Using Balneotherapy in a Short-Time Randomized Controlled Trial. Evid. Based Complement. Alternat. Med. 2016, 2016, 9631684. [Google Scholar] [CrossRef]
- Rapolienė, L.; Taletavičienė, G.; Balčius, A.; Martinkėnas, A.; Kontautienė, V.; Fioravanti, A. Short and Long-term effects of balneotherapy on musculoskeletal pain and fatigue associated with stress. Int. J. Biometeorol. 2025. [Google Scholar] [CrossRef]
- Bestaş, E.; Dündar, Ü.; Köken, T.; Koca, B.; Yeşil, H. The comparison of effects of balneotherapy, water-based and land-based exercises on disease activity, symptoms, sleep quality, quality of life and serum sclerostin level in patients with ankylosing spondylitis: A prospective, randomized study. Arch. Rheumatol. 2021, 37, 159–168. [Google Scholar] [CrossRef]
- García-López, H.; García-Giménez, M.T.; Obrero-Gaitán, E.; Lara-Palomo, I.C.; Castro-Sánchez, A.M.; Rey, R.R.; Cor-tés-Pérez, I. Effectiveness of balneotherapy in reducing pain, disability, and depression in patients with Fibromyalgia syn-drome: A systematic review with meta-analysis. Int. J. Biometeorol. 2024, 68, 1935–1951. [Google Scholar] [CrossRef]
- Karaarslan, F.; Ozkuk, K.; Seringec Karabulut, S.; Bekpinar, S.; Karagulle, M.Z.; Erdogan, N. How does spa treatment affect cardiovascular function and vascular endothelium in patients with generalized osteoarthritis? A pilot study through plasma asymmetric di-methyl arginine (ADMA) and L-arginine/ADMA ratio. Int. J. Biometeorol. 2018, 62, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Pagourelias, E.D.; Zorou, P.G.; Tsaligopoulos, M.; Athyros, V.G.; Karagiannis, A.; Efthimiadis, G.K. Carbon dioxide balneotherapy and cardiovascular disease. Int. J. Biometeorol. 2011, 55, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Zapolski, T.; Kornecki, W.; Jaroszyński, A. The Influence of Balneotherapy Using Salty Sulfide–Hydrogen Sulfide Water on Selected Markers of the Cardiovascular System: A Prospective Study. J. Clin. Med. 2024, 13, 3526. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.C.; Song, Q.C.; Chen, C.Y.; Su, T.C. Cardiovascular physiological effects of balneotherapy: Focused on seasonal differences. Hypertens. Res. 2023, 46, 1650–1661. [Google Scholar] [CrossRef]
- Khaltaev, N.; Solimene, U.; Vitale, F.; Zanasi, A. Balneotherapy and hydrotherapy in chronic respiratory disease. J. Thorac. Dis. 2020, 12, 4459–4468. [Google Scholar] [CrossRef]
- Jia, G.; Su, C.-H. Tailored Physical Activity Interventions for Long COVID: Current Approaches and Benefits—A Narrative Review. Healthcare 2024, 12, 1539. [Google Scholar] [CrossRef]
- Bernard, P.L.; de la Tribonniere, X.; Pellecchia, A.; Gamon, L.; Herman, F.; Picot, M.C.; Raffort, N.J.; Paillard, T.; Robiaud, J.B.; Ninot, G.; et al. Effects on Physical Functioning and Fear of Falling of a 3-Week Balneotherapy Pro-gram Alone or Associated with a Physical Activity and Educational Program in Older Adult Fallers: A Randomized-Controlled Trial. Clin. Interv. Aging 2024, 19, 1753–1763. [Google Scholar] [CrossRef]
- Dogaru, G.; Ciubean, A.D.; Marinescu, L.; Pop, B.M.; Pașca, G.S.; Ciumărnean, L. The effectiveness of balneotherapy on pain, walking, and function in patients with diabetic neuropathy: A prospective observational study. Int. J. Biometeorol. 2025, 69, 319–329. [Google Scholar] [CrossRef]
- Silva, J.; Martins, J.; Nicomédio, C.; Gonçalves, C.; Palito, C.; Gonçalves, R.; Fernandes, P.O.; Nunes, A.; Alves, M.J. A Novel Approach to Assess Balneotherapy Effects on Musculoskeletal Diseases—An Open Interventional Trial Combining Physiological Indicators, Biomarkers, and Patients’ Health Perception. Geriatrics 2023, 8, 55. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, X.; Li, H.; Zhang, H.; Xu, J. Mechanisms of long COVID: An updated review. Chin. Med. J. Pulm. Crit. Care Med. 2023, 1, 231–240. [Google Scholar] [CrossRef]
- Cheleschi, S.; Tenti, S.; Seccafico, I.; Gálvez, I.; Fioravanti, A.; Ortega, E. Balneotherapy year in review 2021: Focus on the mechanisms of action of balneotherapy in rheumatic diseases. Environ. Sci. Pollut. Res. Int. 2022, 29, 8054–8073. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.Y.; Kwak, H.B.; Kim, A.H.; Park, S.H.; Heo, J.W.; Kim, H.K.; Ko, J.R.; Lee, S.J.; Bang, H.S.; Sim, J.W.; et al. Cardiac adaptation to exercise training in health and disease. Pflugers Arch. 2020, 472, 155–168. [Google Scholar] [CrossRef]
- Lippi, G.; Sanchis-Gomar, F.; Henry, B.M. COVID-19 and its long-term sequelae: What do we know in 2023? Pol. Arch. Intern. Med. 2023, 133, 16402. [Google Scholar] [CrossRef] [PubMed]
- Aiyegbusi, O.L.; Hughes, S.E.; Turner, G.; Rivera, S.C.; McMullan, C.; Chandan, J.S.; Haroon, S.; Price, G.; Davies, E.H.; Niran-tharakumar, K.; et al. Symptoms, complications and management of long COVID: A review. J. R. Soc. Med. 2021, 114, 428–442. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Wang, F.; Duan, Q.; Zhao, X.; Yang, F. Prevalence of fatigue and perceived fatigability in older adults: A systematic review and meta-analysis. Sci. Rep. 2025, 15, 4818. [Google Scholar] [CrossRef]
- Moreh, E.; Jacobs, J.M.; Stessman, J. Fatigue, function, and mortality in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 887–895. [Google Scholar] [CrossRef]
- Pomatto, L.C.D.; Davies, K.J.A. The role of declining adaptive homeostasis in ageing. J. Physiol. 2017, 595, 7275–7309. [Google Scholar] [CrossRef]
- Latorre-Román, P.Á.; Rentero-Blanco, M.; Laredo-Aguilera, J.A.; García-Pinillos, F. Effect of a 12-day balneotherapy programme on pain, mood, sleep, and depression in healthy elderly people. Psychogeriatrics 2015, 15, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Nopp, S.; Moik, F.; Klok, F.A.; Gattinger, D.; Petrovic, M.; Vonbank, K.; Koczulla, A.R.; Ay, C.; Zwick, R.H. Outpatient Pulmonary Rehabilitation in Patients with Long COVID Improves Exercise Capacity, Functional Status, Dyspnea, Fatigue, and Quality of Life. Respiration 2022, 101, 593–601. [Google Scholar] [CrossRef]
- Jimeno-Almazán, A.; Franco-López, F.; Buendía-Romero, Á.; Martínez-Cava, A.; Sánchez-Agar, J.A.; Sánchez-Alcaraz Martínez, B.J.; Courel-Ibáñez, J.; Pallarés, J.G. Rehabilitation for post-COVID-19 condition through a supervised exercise intervention: A randomised controlled trial. Scand. J. Med. Sci. Sports 2022, 32, 1791–1801. [Google Scholar] [CrossRef] [PubMed]
- Jimeno-Almazán, A.; Buendía-Romero, Á.; Martínez-Cava, A.; Franco-López, F.; Sánchez-Alcaraz, B.J.; Courel-Ibáñez, J.; Pallarés, J.G. Effects of a concurrent training, respiratory muscle exercise, and self-management recommendations on recovery from post-COVID-19 conditions: The RECOVE trial. J. Appl. Physiol. 2023, 134, 95–104. [Google Scholar] [CrossRef] [PubMed]
| Neuropsychiatric Disorders | Cardiovascular Disorders | Respiratory Disorders | Rheumatic Disorders |
|---|---|---|---|
| Parkinson’s disease, multiple sclerosis, epilepsy, a history of stroke, depression, blindness | Coronary artery disease, heart failure, a history of myocardial infarction and/or endarterectomy, previous percutaneous coronary interventions and/or coronary artery bypass grafting, arrhythmias (atrioventricular or bundle branch blocks, and atrial fibrillation), pacemaker implantation, peripheral artery disease | Chronic obstructive pulmonary disease, emphysema, pneumoconiosis, asthma | Rheumatoid arthritis, ankylosing spondylitis |
| Modality | n | % of Patients |
|---|---|---|
| Pneumatic massage | 78 | 65.5% |
| Low-level laser therapy | 73 | 61.3% |
| Local cryotherapy | 61 | 51.3% |
| Infrared light therapy | 47 | 39.4% |
| Whirlpool baths | 47 | 39.5% |
| Magnetic fields | 46 | 38.7% |
| Electrotherapy | 39 | 32.8% |
| Pearl baths | 27 | 22.7% |
| Peloids | 15 | 12.6% |
| Classical massage | 7 | 5.9% |
| Ultrasound therapy | 7 | 5.9% |
| Lymphatic drainage | 1 | 0.8% |
| Group 1 (n = 73) | Group 2 (n = 46) | p Value | |
|---|---|---|---|
| Age [years] | 63.66 ± 8.92 | 65.32 ± 8.08 | 0.31 |
| Body mass [kg] | 85.61 ± 14.24 | 84.69 ± 15.49 | 0.74 |
| Height [m] | 1.67 ± 0.08 | 1.67 ± 0.09 | 0.75 |
| BMI [kg/m2] | 30.59 ± 4.93 | 30.31 ± 4.29 | 0.75 |
| Systolic blood pressure [mmHg] | 140.61 ± 14.33 | 138.37 ± 11.12 | 0.37 |
| Diastolic blood pressure [mmHg] | 80.94 ± 8.02 | 78.80 ± 6.90 | 0.14 |
| Treatment duration [days] | 20.89 ± 0.54 | 29.67 ± 7.23 | <0.0001 |
| Comorbidities | Group 1 (% of individuals) | Group 2 (% of individuals) | p value |
| Osteoarthritis | 12% | 35% | 0.15 |
| Diabetes mellitus + hypertension | 14% | 15% | |
| Hypertension | 45% | 26% | |
| Diabetes mellitus | 1% | 4% | |
| Diabetes mellitus + hypertension + hypothyroidism | 1% | 0% | |
| Diabetes mellitus + hypothyroidism | 0% | 2% | |
| Hypertension + hypothyroidism | 6% | 7% | |
| Hypertension + gout | 4% | 2% | |
| Hypothyroidism | 6% | 7% | |
| Benign prostatic hyperplasia | 1% | 0% |
| Fatigue [Points] | Malaise [Points] | Rest Dyspnea [Points] | Exertional Dyspnea [Points] | Cough [Points] | ||
|---|---|---|---|---|---|---|
| Group 1 | Pre-treatment | 4.4 ± 2.76 | 4.32 ± 2.87 | 0.76 ± 1.47 | 4.11 ± 2.83 | 0.83 ± 1.49 |
| Post-treatment | 2.04 ± 1.43 | 1.86 ± 1.44 | 0.23 ± 0.54 | 1.71 ± 1.26 | 0.21 ± 0.49 | |
| p value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Mean ∆ | 2.35 ± 1.67 | 2.45 ± 1.75 | 0.53 ± 1.07 | 2.40 ± 1.94 | 0.63 ± 1.29 | |
| Group 2 | Pre-treatment | 5.39 ± 0.46 | 5.01 ± 1.57 | 1.72 ± 1.5 | 1.96 ± 1.96 | 1.17 ± 1.31 |
| Post-treatment | 2.33 ± 1.48 | 1.59 ± 1.00 | 0.46 ± 0.72 | 0.48 ± 0.84 | 0.15 ± 0.42 | |
| p value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Mean ∆ | 3.07 ± 1.32 | 3.44 ± 1.50 | 1.26 ± 1.32 | 1.48 ± 1.50 | 1.02 ± 1.13 | |
| Cohen’s d | −0.46 | −0.59 | −0.62 | 0.52 | −0.32 | |
| [95% CI] | −0.83; −0.08 | −0.97; −0.22 | −1.00; −0.24 | 0.14; 0.89 | −0.69; 0.05 | |
| Sputum [points] | Chest tightness [points] | Chest pain [points] | Palpitations [points] | Fast heart rate [points] | ||
| Group 1 | Pre-treatment | 0.37 ± 0.99 | 0.88 ± 1.74 | 0.29 ± 0.83 | 0.22 ± 0.71 | 0.22 ± 0.77 |
| Post-treatment | 0.11 ± 0.4 | 0.18 ± 0.61 | 0.04 ± 0.2 | 0.06 ± 0.28 | 0.06 ± 0.23 | |
| p value | 0.0009 | <0.0001 | 0.004 | 0.02 | 0.06 | |
| Mean ∆ | 0.26 ± 0.69 | 0.69 ± 1.52 | 0.25 ± 0.70 | 0.16 ± 0.55 | 0.16 ± 0.73 | |
| Group 2 | Pre-treatment | 1.17 ± 1.22 | 0.52 ± 0.94 | 0.44 ± 1.17 | 0.24 ± 0.87 | 0.28 ± 0.91 |
| Post-treatment | 0.17 ± 0.53 | 0.24 ± 0.6 | 0.12 ± 0.4 | 0.11 ± 0.48 | 0.13 ± 0.54 | |
| p value | 0.001 | 0.03 | 0.08 | 0.28 | 0.22 | |
| Mean ∆ | 1.00 ± 1.01 | 0.28 ± 0.81 | 0.30 ± 1.09 | 0.13 ± 0.62 | 0.15 ± 0.67 | |
| Cohen’s d | −0.89 | 0.32 | −0.07 | 0.06 | 0.02 | |
| [95% CI] | −1.28; −0.51 | −0.05; 0.69 | −0.44; 0.30 | −0.31; 0.43 | −0.35; 0.39 | |
| Concentration Disorders [Points] | Memory Disorders [Points] | Headaches [Points] | Dizziness [Points] | ||
|---|---|---|---|---|---|
| Group 1 | Pre-treatment | 2.19 ± 1.39 | 2.56 ± 2.67 | 0.85 ± 1.76 | 0.74 ± 1.54 |
| Post-treatment | 1.41 ± 1.38 | 1.41 ± 1.61 | 0.44 ± 1.01 | 0.36 ± 0.89 | |
| p value | <0.0001 | <0.0001 | 0.002 | 0.0005 | |
| Mean ∆ | 1.08 ± 1.64 | 1.15 ± 1.69 | 0.41 ± 1.07 | 0.38 ± 0.95 | |
| Group 2 | Pre-treatment | 2.96 ± 2.26 | 2.89 ± 2.23 | 2.41 ± 2.06 | 2.35 ± 2.1 |
| Post-treatment | 0.52 ± 0.81 | 0.46 ± 0.75 | 0.39 ± 0.68 | 0.33 ± 0.52 | |
| p value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Mean ∆ | 2.43 ± 2.17 | 2.43 ± 2.08 | 2.02 ± 1.89 | 2.02 ± 1.96 | |
| Cohen’s d | −0.73 | −0.69 | −1.12 | −1.15 | |
| [95% CI] | −1.11; −0.35 | −1.07; −0.31 | −1.51; −0.72 | −1.54; −0.75 | |
| Sleep disorders [points] | Paresthesia [points] | Depression [points] | Anxiety [points] | ||
| Group 1 | Pre-treatment | 2.22 ± 2.31 | 2.37 ± 2.38 | 1.08 ± 1.91 | 0.89 ± 1.6 |
| Post-treatment | 1.15 ± 1.43 | 0.75 ± 0.97 | 0.59 ± 1.31 | 0.39 ± 0.76 | |
| p value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Mean ∆ | 1.07 ± 1.58 | 1.61 ± 1.79 | 0.49 ± 1.02 | 0.51 ± 1.07 | |
| Group 2 | Pre-treatment | 2.98 ± 1.98 | 2.65 ± 1.98 | 2.09 ± 1.79 | 1.52 ± 1.57 |
| Post-treatment | 0.39 ± 0.68 | 0.46 ± 0.72 | 0.30 ± 0.59 | 0.28 ± 0. 54 | |
| p value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Mean ∆ | 2.59 ± 2.01 | 2.20 ± 1.82 | 1.78 ± 1.65 | 1.24 ± 1.55 | |
| Cohen’s d | −0.86 | −0.32 | −1.00 | −0.57 | |
| [95% CI] | −1.25; −0.48 | −0.69; 0.05 | −1.39; −0.6 | −0.95; −0.19 | |
| cons | β | SE | t | F(6, 110) | 95% CI | R2 | Adj. R2 | p Value | ||
|---|---|---|---|---|---|---|---|---|---|---|
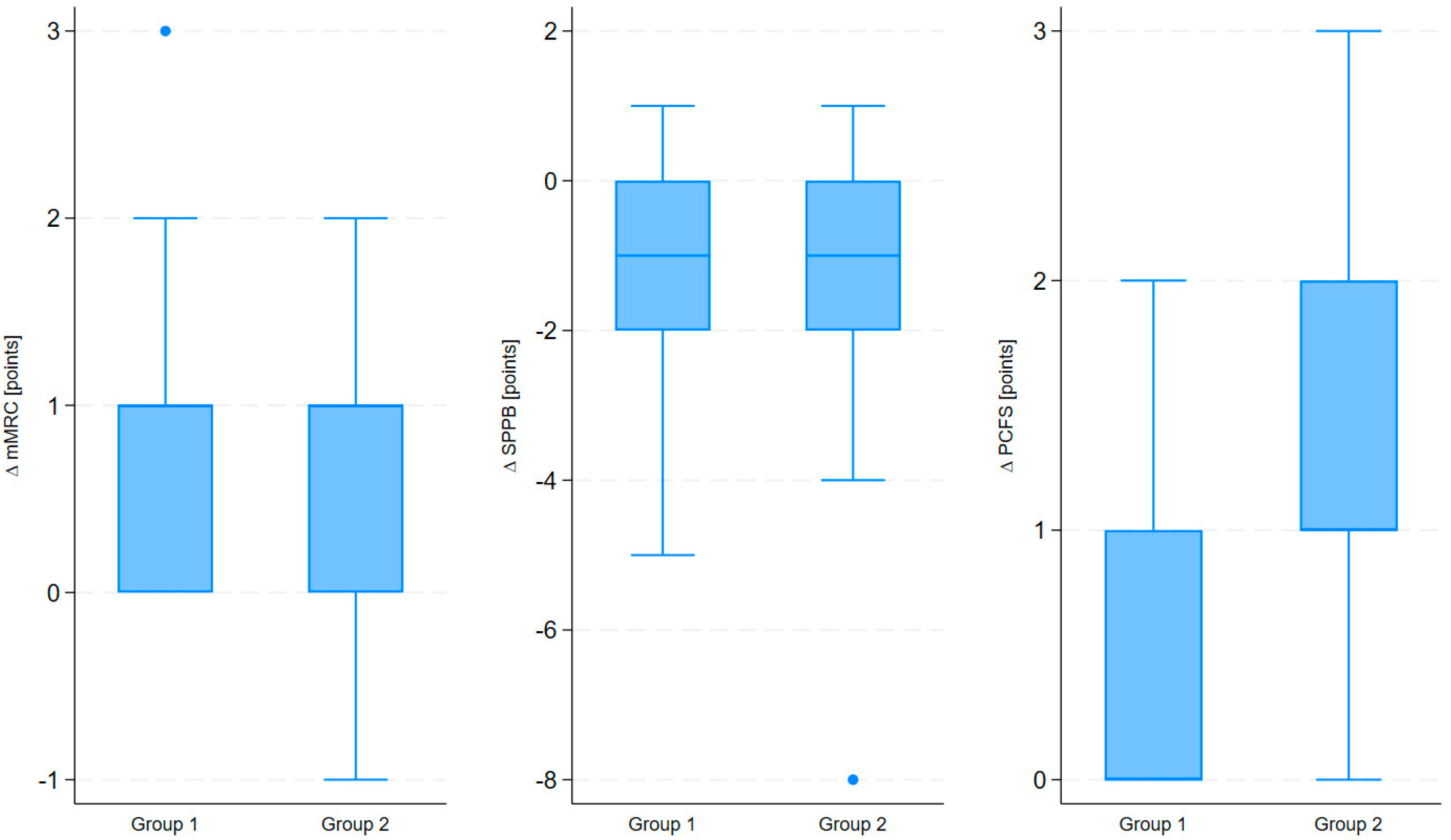
| Treatment duration | ∆ PCFS | 3.19 | 0.033 | 0.011 | 3.00 | 3.74 | 0.011; 0.054 | 0.169 | 0.124 | 0.003 |
| ∆ SPPB | −2.28 | −0.036 | 0.021 | −1.74 | 2.85 | −0.077; 0.005 | 0.135 | 0.088 | 0.085 | |
| ∆ mMRC | 2.61 | −0.011 | 0.011 | −1.04 | 1.61 | −0.033; 0.010 | 0.081 | 0.031 | 0.301 | |
| cons | β | SE | t | F(6, 110) | 95% CI | R2 | Adj. R2 | p Value | ||
|---|---|---|---|---|---|---|---|---|---|---|
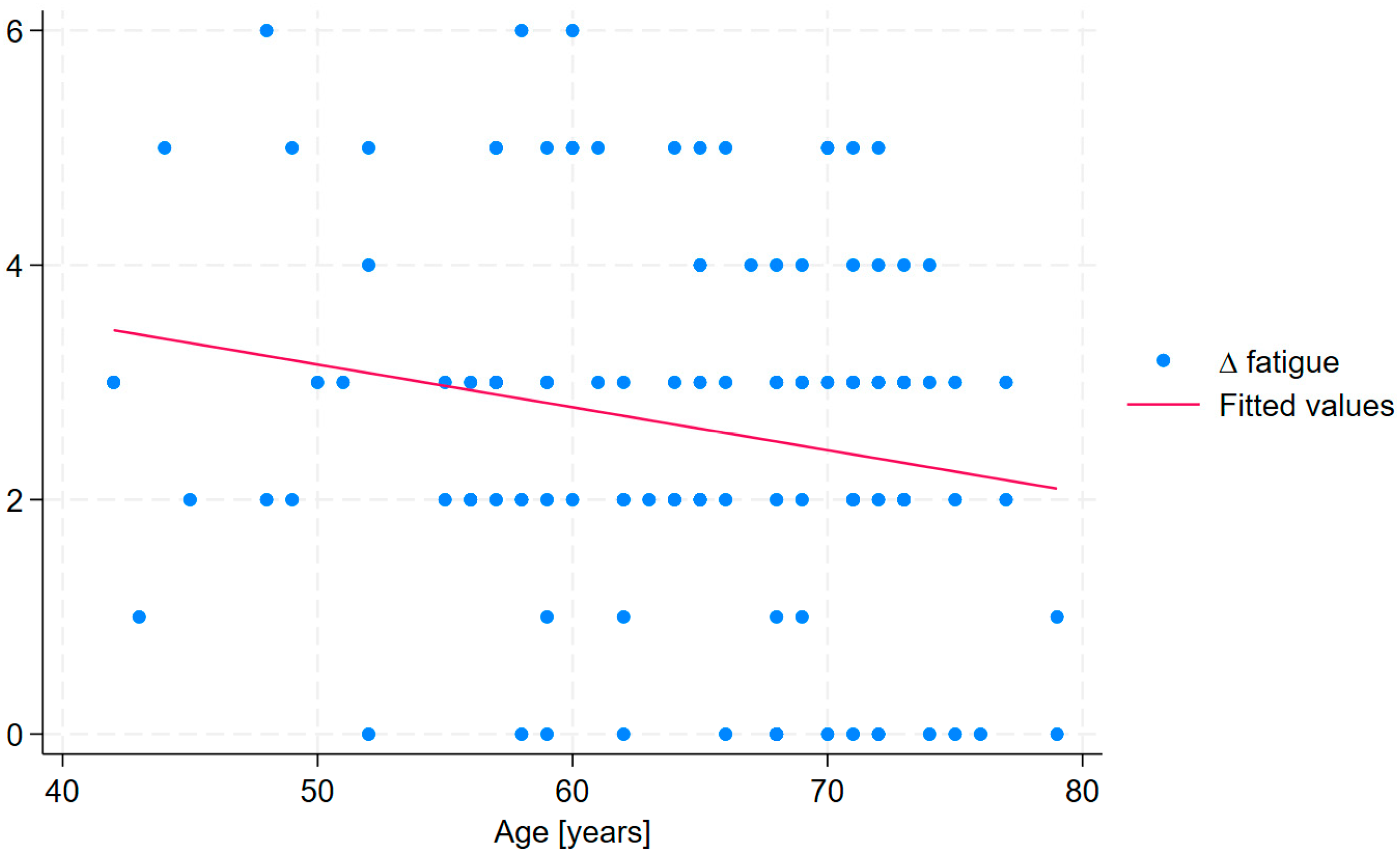
| Treatment duration | ∆ fatigue * | 6.49 | 0.057 | 0.023 | 2.49 | 2.61 | 0.012; 0.102 | 0.125 | 0.077 | 0.014 |
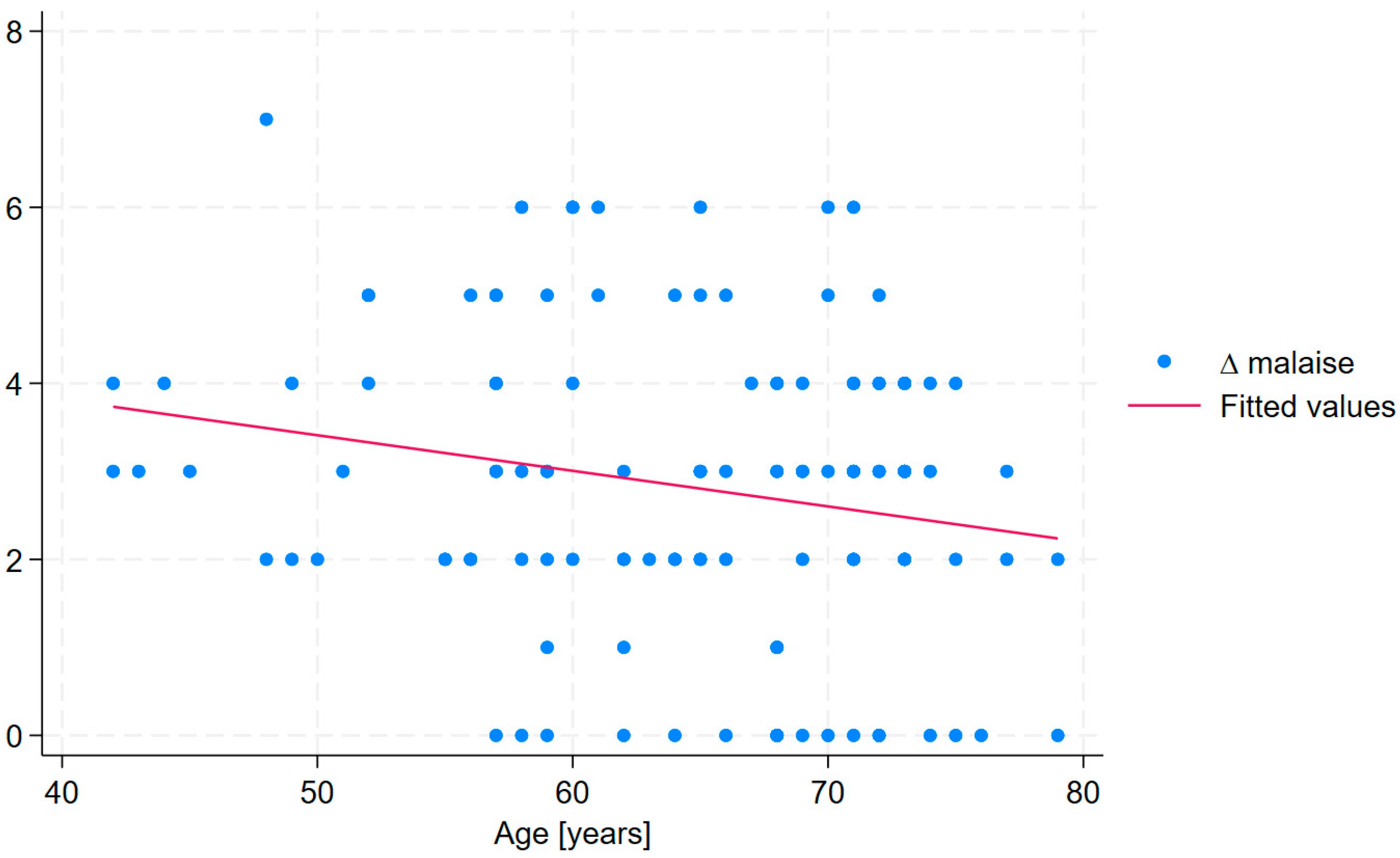
| ∆ malaise * | 7.31 | 0.063 | 0.024 | 2.59 | 3.33 | 0.015; 0.112 | 0.154 | 0.108 | 0.011 | |
| ∆ rest dyspnea | 1.46 | 0.037 | 0.018 | 2.01 | 1.01 | 0.0004; 0.016 | 0.052 | 0.0003 | 0.047 | |
| ∆ exertional dyspnea | 2.46 | −0.043 | 0.027 | −1.61 | 2.48 | −0.096; 0.01 | 0.119 | 0.071 | 0.110 | |
| ∆ cough | 2.41 | 0.014 | 0.019 | 0.74 | 1.24 | −0.023; 0.051 | 0.063 | 0.012 | 0.460 | |
| ∆ sputum | 0.48 | 0.036 | 0.013 | 2.67 | 1.61 | 0.009; 0.062 | 0.081 | 0.031 | 0.009 | |
| ∆ chest tightness | 4.13 | −0.015 | 0.019 | −0.75 | 1.77 | −0.053; 0.024 | 0.088 | 0.038 | 0.458 | |
| ∆ chest pain | 0.02 | 0.005 | 0.013 | 0.40 | 0.62 | −0.021; 0.032 | 0.033 | −0.02 | 0.689 | |
| ∆ palpitations | 0.62 | −0.001 | 0.009 | −0.15 | 0.73 | −0.019; 0.016 | 0.039 | −0.014 | 0.879 | |
| ∆ fast heart rate | 0.44 | 0.006 | 0.011 | 0.56 | 1.19 | −0.015; 0.027 | 0.061 | 0.01 | 0.578 | |
| cons | β | SE | t | F(6, 110) | 95% CI | R2 | Adj. R2 | p Value | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment duration | ∆ concentration disorders | 4.43 | 0.089 | 0.028 | 3.14 | 2.78 | 0.033; 0.145 | 0.132 | 0.084 | 0.002 |
| ∆ memory disorders | 3.02 | 0.104 | 0.027 | 3.79 | 4.17 | 0.05; 0.16 | 0.185 | 0.141 | <0.0001 | |
| ∆ headaches | 0.24 | 0.103 | 0.023 | 4.53 | 4.54 | 0.058; 0.148 | 0.198 | 0.155 | 0.0004 | |
| ∆ dizziness | −0.31 | 0.096 | 0.022 | 4.30 | 5.14 | 0.052; 0.141 | 0.219 | 0.176 | <0.0001 | |
| ∆ sleep disorders | −1.88 | 0.112 | 0.027 | 4.37 | 3.91 | 0.064; 0.170 | 0.176 | 0.131 | <0.0001 | |
| ∆ paresthesia | 3.96 | 0.035 | 0.27 | 1.27 | 1.117 | −0.2; 0.09 | 0.06 | 0.009 | 0.21 | |
| ∆ depression | 1.05 | 0.072 | 0.021 | 3.48 | 2.88 | 0.031; 0.113 | 0.136 | 0.089 | 0.001 | |
| ∆ anxiety | 2.56 | 0.047 | 0.019 | 2.44 | 2.03 | 0.009; 0.086 | 0.099 | 0.051 | 0.02 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onik, G.; Sieroń, K. Longer Health Resort Therapy Improves Outcomes in Long COVID: A Retrospective Study. Medicina 2025, 61, 1686. https://doi.org/10.3390/medicina61091686
Onik G, Sieroń K. Longer Health Resort Therapy Improves Outcomes in Long COVID: A Retrospective Study. Medicina. 2025; 61(9):1686. https://doi.org/10.3390/medicina61091686
Chicago/Turabian StyleOnik, Grzegorz, and Karolina Sieroń. 2025. "Longer Health Resort Therapy Improves Outcomes in Long COVID: A Retrospective Study" Medicina 61, no. 9: 1686. https://doi.org/10.3390/medicina61091686
APA StyleOnik, G., & Sieroń, K. (2025). Longer Health Resort Therapy Improves Outcomes in Long COVID: A Retrospective Study. Medicina, 61(9), 1686. https://doi.org/10.3390/medicina61091686






