The Addition of Chinese Herbal Medicines Is Effective as a Prophylactic Treatment Against Dental Diseases for Sjögren’s Syndrome Patients: Insight from Real-World Database
Abstract
1. Introduction
2. Methods
2.1. Data Source
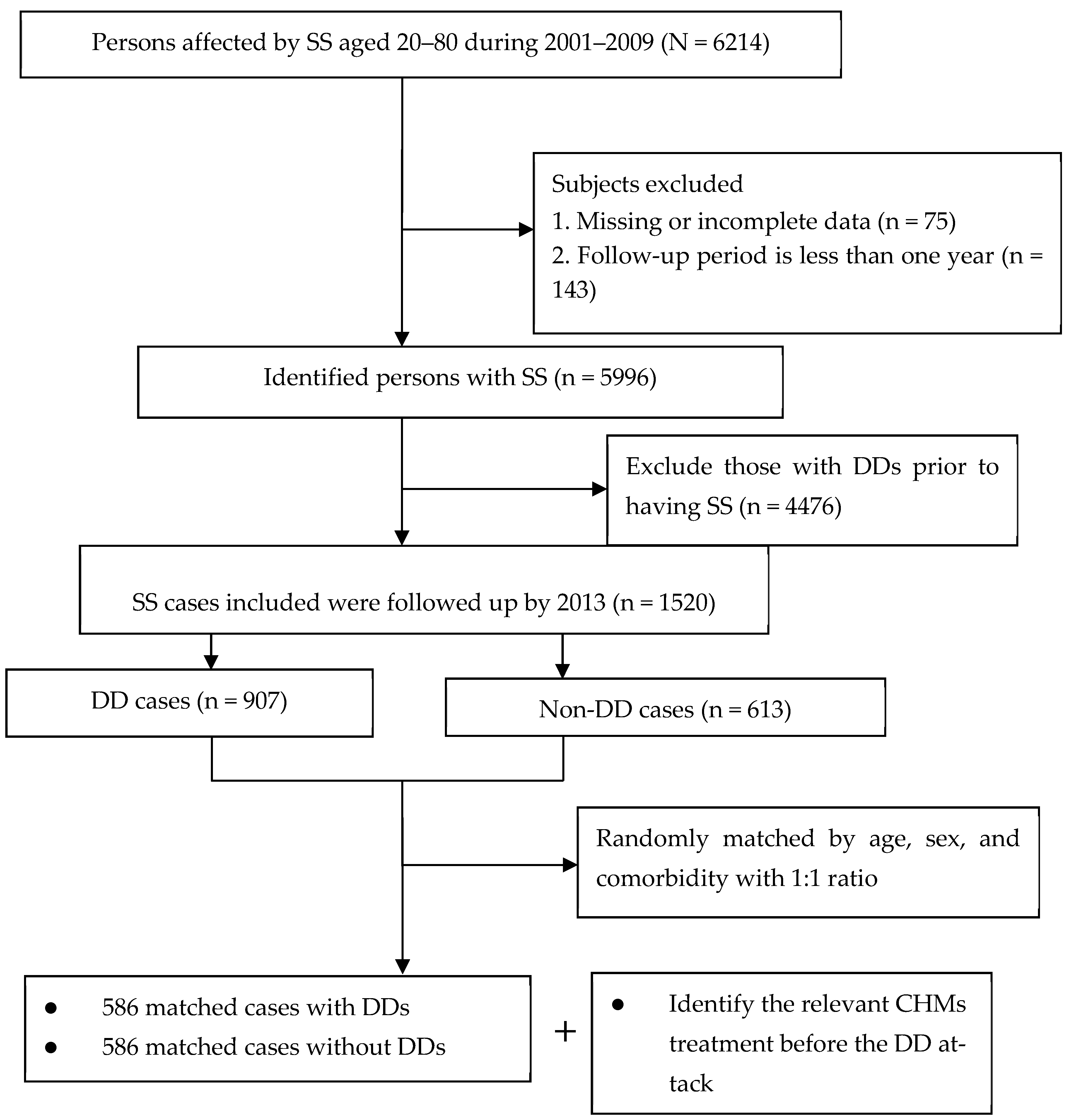
2.2. Study Cohort
2.3. Determination of Patient and Control Groups
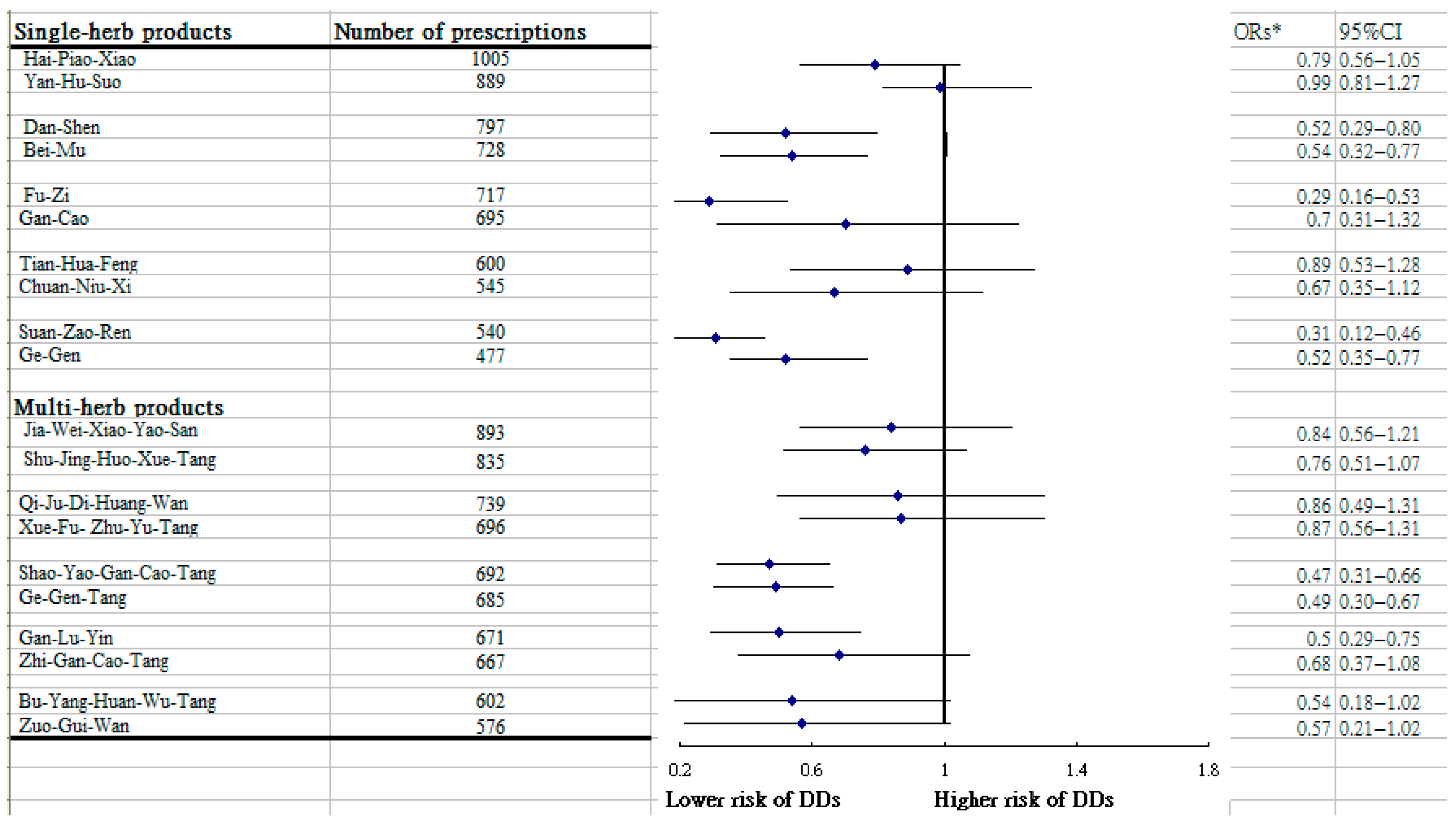
2.4. Identification of CHMs Use
2.5. Covariate Measures
2.6. Statistical Modeling
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, K.H.; See, L.C.; Kuo, C.F.; Chou, I.J.; Chou, M.J. Prevalence and incidence in patients with autoimmune rheumatic diseases: A nationwide population-based study in Taiwan. Arthritis Care Res. 2013, 65, 244–250. [Google Scholar] [CrossRef]
- Fox, R.I. Sjögren’s syndrome. Lancet 2005, 366, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Mariette, X.; Criswell, L.A. Primary Sjögren’s Syndrome. N. Engl. J. Med. 2018, 378, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Yura, Y.; Hamada, M. Outline of Salivary Gland Pathogenesis of Sjögren’s Syndrome and Current Therapeutic Approaches. Int. J. Mol. Sci. 2023, 24, 11179. [Google Scholar] [CrossRef] [PubMed]
- Tikhonova, S.; Booij, L.; D’Souza, V.; Crosara, K.T.B.; Siqueira, W.L.; Emami, E. Investigating the association between stress, saliva and dental caries: A scoping review. BMC Oral Health 2018, 18, 41. [Google Scholar] [CrossRef]
- Bolstad, A.I.; Skarstein, K. Epidemiology of Sjögren’s Syndrome-from an Oral Perspective. Curr. Oral Health Rep. 2016, 3, 328–336. [Google Scholar] [CrossRef]
- Kassan, S.S.; Moutsopoulos, H.M. Clinical Manifestations and Early Diagnosis of Sjögren Syndrome. Arch. Intern. Med. 2004, 164, 1275–1284. [Google Scholar] [CrossRef]
- Chuang, C.J.; Hsu, C.W.; Lu, M.C.; Koo, M. Increased risk of developing dental diseases in patients with primary Sjögren’s syndrome-A secondary cohort analysis of population-based claims data. PLoS ONE 2020, 15, e0239442. [Google Scholar] [CrossRef]
- Gheorghe, D.N.; Popescu, D.M.; Dinescu, S.C.; Silaghi, M.; Surlin, P.; Ciurea, P.L. Association between Sjögren’s Syndrome and Periodontitis: Epidemiological, Fundamental and Clinical Data: A Systematic Review. Diagnostics 2023, 13, 1401. [Google Scholar] [CrossRef]
- Xin, W.; Leung, K.C.; Lo, E.C.; Mok, M.Y.; Leung, M.H. A randomized, double-blind, placebo-controlled clinical trial of fluoride varnish in preventing dental caries of Sjögren’s syndrome patients. BMC Oral Health 2016, 16, 102. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Hung, K.C.; Lin, M.S.; Ko, C.H.; Lin, Y.S.; Chen, T.H.; Lin, C.Y.; Chen, Y.C. The effect of pilocarpine on dental caries in patients with primary Sjögren’s syndrome: A database prospective cohort study. Arthritis Res. Ther. 2019, 21, 251. [Google Scholar] [CrossRef]
- Nayar, G.; Gauna, A.; Chukkapalli, S.; Velsko, I.; Kesavalu, L.; Cha, S. Polymicrobial infection alter inflammatory microRNA in rat salivary glands during periodontal disease. Anaerobe 2016, 38, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.J.; He, Q.M.; Zhang, Q.; Fu, K.H.; Li, R.L.; Peng, W.; Gao, Y.X. Traditional Chinese medicine is a useful and promising alternative strategy for treatment of Sjogren’s syndrome: A review. J. Integr. Med. 2021, 19, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, H.; Li, D.; Fan, Y.; Liu, M.; Ren, H.; Liu, L. Botanical characterization, phytochemistry, biosynthesis, pharmacology clinical application, and breeding techniques of the Chinese herbal medicine Fritillaria unibracteata. Front. Pharmacol. 2024, 15, 1428037. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, Y.; Kido, J.I.; Nishikawa, Y.; Kido, R.; Sakamoto, E.; Bando, M.; Naruishi, K.; Nagata, T.; Yumoto, H. Gan-Lu-Yin (Kanroin), Traditional Chinese Herbal Extracts, Reduces Osteoclast Differentiation In Vitro and Prevents Alveolar Bone Resorption in Rat Experimental Periodontitis. J. Clin. Med. 2021, 10, 386. [Google Scholar] [CrossRef]
- Abu-Amer, Y. NF-κB signaling and bone resorption. Osteoporos. Int. 2013, 24, 2377–2386. [Google Scholar] [CrossRef]
- National Health Insurance Administration, Ministry of Health and Welfare. The Introduction of National Helath Insurance Program. Available online: https://dep.mohw.gov.tw/DOS/cp-5301-62356-113.html (accessed on 21 January 2025).
- Shih, C.C.; Liao, C.C.; Su, Y.C.; Tsai, C.C.; Lin, J.G. Gender differences in traditional Chinese medicine use among adults in Taiwan. PLoS ONE 2012, 7, e32540. [Google Scholar] [CrossRef]
- Lu, M.C.; Livneh, H.; Chiu, L.M.; Lai, N.S.; Yeh, C.C.; Tsai, T.Y. A survey of traditional Chinese medicine use among rheumatoid arthritis patients: A claims data–based cohort study. Clin. Rheumatol. 2019, 38, 1393–1400. [Google Scholar] [CrossRef]
- Liu, C.Y.; Hung, Y.T.; Chuang, Y.L.; Chen, Y.J.; Weng, W.S.; Liu, J.S.; Liang, K.Y. Incorporating development stratification of Taiwan townships into sampling design of large scale health interview survey. J. Health Manag. 2006, 4, 1–22. [Google Scholar] [CrossRef]
- Deyo, R.A.; Cherkin, D.C.; Ciol, M.A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol. 1992, 45, 613–619. [Google Scholar] [CrossRef]
- Li, H.; Hung, A.; Li, M.; Yang, A.W.H. Fritillariae thunbergii bulbus: Traditional uses, phytochemistry, pharmacodynamics, pharmacokinetics and toxicity. Int. J. Mol. Sci. 2019, 20, 1667. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Chavakis, T.; Lambris, J.D. Current understanding of periodontal disease pathogenesis and targets for host-modulation therapy. Periodontology 2000 2020, 84, 14–34. [Google Scholar] [CrossRef]
- Herbert, B.A.; Steinkamp, H.M.; Gaestel, M.; Kirkwood, K.L. Mitogen-Activated Protein Kinase 2 Signaling Shapes Macrophage Plasticity in Aggregatibacter actinomycetemcomitans-Induced Bone Loss. Infect. Immun. 2017, 85, e00552-16. [Google Scholar] [CrossRef] [PubMed]
- Shivers, K.Y.; Amador, N.; Abrams, L.; Hunter, D.; Jenab, S.; Quiñones-Jenab, V. Estrogen alters baseline and inflammatory-induced cytokine levels independent from hypothalamic–pituitary–adrenal axis activity. Cytokine 2015, 72, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yin, J.; Song, X.; Lai, S.; Zhong, S.; Jia, Y. The effect of exogenous estrogen on depressive mood in women: A systematic review and meta-analysis of randomized controlled trials. J. Psychiatr. Res. 2023, 162, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.; Lai, J.N.; Yu, M.C.; Chen, C.Y.; Hsieh, Y.T.; Hsu, Y.F.; Wei, J.C.C. Traditional Chinese Medicine in patients with primary sjogren’s syndrome: A randomized, double-blind, placebo-controlled clinical trial. Front. Med. 2021, 8, 744194. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.-P.; Deng, G.-F.; Shao, Y.-Y.; Xu, D.; Zhao, Y.-N.; Sun, Y.-F.; Zhang, S.-Q.; Hou, R.-G.; Liu, J.-J. Shaoyao-Gancao Decoction Ameliorates the Inflammation State in Polycystic Ovary Syndrome Rats via Remodeling Gut Microbiota and Suppressing the TLR4/NF-κB Pathway. Front. Pharmacol. 2021, 12, 670054. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, H.; Wang, J.; Zhang, Z.; Li, C. Puerarin decreases bone loss and collagen destruction in rats with ligature-induced periodontitis. J. Periodontal Res. 2015, 50, 748–757. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Li, Z.; Cheng, F.-E.; Nan, Y.; Li, W.-Q. Radix Codonopsis: A review of anticancer pharmacological activities. Front. Pharmacol. 2025, 15, 1498707. [Google Scholar] [CrossRef]
- Jin, X.; Xu, H.; Huang, C.; Ma, H.; Xiong, X.; Cheng, L.; Wang, F.; Feng, Y.; Zhang, G. A Traditional Chinese Medicine Formula Danshen Baibixiao Ameliorates Imiquimod-Induced Psoriasis-Like Inflammation in Mice. Front. Pharmacol. 2021, 12, 749626. [Google Scholar] [CrossRef]
- Guo, S.; Wu, J.; Ni, M.; Jia, S.; Zhang, J.; Zhou, W.; Liu, X.; Wang, M.; Zhang, X. Comparative Efficacy of Danshen Class Injections for Treating Acute Coronary Syndrome: A Multidimensional Bayesian Network Meta-Analysis of Randomized Controlled Trials. Front. Pharmacol. 2020, 11, 1260. [Google Scholar] [CrossRef]
- Belibasakis, G.N.; Emingil, G.; Saygan, B.; Turkoglu, O.; Atilla, G.; Bostanci, N. Gene expression of transcription factor NFATc1 in periodontal diseases. APMIS Acta Pathol. Microbiol. Immunol. Scand. 2011, 119, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Jeon, H.; Kwon, J.E.; Suh, H.; Kim, B.H.; Yun, M.K.; Lim, Y.J.; Kang, S.C. Anti-osteoporotic effects of Salvia miltiorrhiza Bunge EtOH extract both in ovariectomized and naturally menopausal mouse models. J. Ethnopharmacol. 2020, 258, 112874. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.R.; Kim, H.Y.; Park, J.K.; Park, S.K.; Chang, M.S. Aconiti Lateralis Preparata Radix Activates the Proliferation of Mouse Bone Marrow Mesenchymal Stem Cells and Induces Osteogenic Lineage Differentiation through the Bone Morphogenetic Protein-2/Smad-Dependent Runx2 Pathway. Evid.-Based Complement. Altern. Med. eCAM 2013, 2013, 586741. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Mai, Z.; Cui, L.; Zhao, X. Engineering exosomes and biomaterial-assisted exosomes as therapeutic carriers for bone regeneration. Stem Cell Res. Ther. 2023, 14, 55. [Google Scholar] [CrossRef]
- Du, Y.; Yan, T.; Wu, B.; He, B.; Jia, Y. Research on the mechanism of antidepressive effect of Suanzaoren Decoction through TLR4/MyD88/NF-κB pathway and Wnt/β-catenin pathway. J. Ethnopharmacol. 2024, 319, 117190. [Google Scholar] [CrossRef]
- Sadek, K.M.; El Moshy, S.; Radwan, I.A.; Rady, D.; Abbass, M.M.S.; El-Rashidy, A.A.; Dörfer, C.E.; Fawzy El-Sayed, K.M. Molecular Basis beyond Interrelated Bone Resorption/Regeneration in Periodontal Diseases: A Concise Review. Int. J. Mol. Sci. 2023, 24, 4599. [Google Scholar] [CrossRef]
- Jayakumar, T.; Lin, K.C.; Chang, C.C.; Hsia, C.W.; Manubolu, M.; Huang, W.C.; Sheu, J.R.; Hsia, C.H. Targeting MAPK/NF-κB Pathways in Anti-Inflammatory Potential of Rutaecarpine: Impact on Src/FAK-Mediated Macrophage Migration. Int. J. Mol. Sci. 2021, 23, 92. [Google Scholar] [CrossRef]
| Variables | Numbers (%) | DDs Cases | Non-DDs Cases | p |
|---|---|---|---|---|
| n = 586 (%) | n = 586 (%) | |||
| Age (years) | 0.06 | |||
| ≤50 | 291 (24.8) | 160 (27.3) | 131 (22.4) | |
| >50 | 881 (75.2) | 426 (72.7) | 455 (77.6) | |
| Mean | 59.9 (13.6) | 59.1 (13.7) | 60.6 (13.5) | 0.07 |
| Sex | 0.90 | |||
| Male | 394 (33.6) | 196 (33.4) | 198 (33.8) | |
| Female | 778 (66.4) | 390 (66.6) | 388 (66.2) | |
| Monthly income | 0.16 | |||
| 25th percentile | 498 (42.5) | 249 (42.5) | 249 (42.5) | |
| 50th percentile | 646 (55.2) | 318 (54.3) | 328 (56.0) | |
| 75th percentile | 27 (2.3) | 19 (3.2) | 9 (1.5) | |
| Residential area | 0.26 | |||
| Urban | 560 (47.7) | 275 (46.9) | 285 (48.6) | |
| Suburban | 201 (17.1) | 111 (18.9) | 90 (15.4) | |
| Rural | 411 (35.1) | 200 (34.2) | 211 (36.0) | |
| CCI | 5.9 (9.8) | 6.4 (10.3) | 5.5 (9.3) | 0.13 |
| CHMs Exposure | Patients | Crude ORs (95% CI) | Adjusted ORs * (95% CI) | |||
|---|---|---|---|---|---|---|
| DDs Cases n = 586 | Non-DDs Cases n = 586 | |||||
| Non-CHMs users | 461 | 78.7 | 420 | 71.7 | 1 | 1 |
| CHMs users | 125 | 21.3 | 166 | 28.3 | 0.69 (0.53–0.89) | 0.68 (0.52–0.90) |
| Group 1 (31 days–364 days) | 96 | 16.4 | 116 | 19.8 | 0.72 (0.54–0.98) | 0.73 (0.54–0.98) |
| Group 2 (365 days or longer) | 29 | 4.9 | 50 | 8.5 | 0.57 (0.36–0.94) | 0.56 (0.35–0.92) |
| Crude ORs (95% CI) | Adjusted ORs * (95% CI) | |
|---|---|---|
| Female | ||
| ≤50 | 0.44 (023–0.80) | 0.45 (0.25–0.82) |
| >50 | 0.65 (0.20–0.95) | 0.64 (0.20–0.98) |
| Male | ||
| ≤50 | 0.72 (0.49–1.20) | 0.71 (0.48–1.18) |
| >50 | 0.94 (0.52–1.64) | 0.93 (0.53–1.62) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juan, C.-Y.; Chen, W.-J.; Livneh, H.; Lu, M.-C.; Tsai, T.-Y. The Addition of Chinese Herbal Medicines Is Effective as a Prophylactic Treatment Against Dental Diseases for Sjögren’s Syndrome Patients: Insight from Real-World Database. Medicina 2025, 61, 1537. https://doi.org/10.3390/medicina61091537
Juan C-Y, Chen W-J, Livneh H, Lu M-C, Tsai T-Y. The Addition of Chinese Herbal Medicines Is Effective as a Prophylactic Treatment Against Dental Diseases for Sjögren’s Syndrome Patients: Insight from Real-World Database. Medicina. 2025; 61(9):1537. https://doi.org/10.3390/medicina61091537
Chicago/Turabian StyleJuan, Ching-Ya, Wei-Jen Chen, Hanoch Livneh, Ming-Chi Lu, and Tzung-Yi Tsai. 2025. "The Addition of Chinese Herbal Medicines Is Effective as a Prophylactic Treatment Against Dental Diseases for Sjögren’s Syndrome Patients: Insight from Real-World Database" Medicina 61, no. 9: 1537. https://doi.org/10.3390/medicina61091537
APA StyleJuan, C.-Y., Chen, W.-J., Livneh, H., Lu, M.-C., & Tsai, T.-Y. (2025). The Addition of Chinese Herbal Medicines Is Effective as a Prophylactic Treatment Against Dental Diseases for Sjögren’s Syndrome Patients: Insight from Real-World Database. Medicina, 61(9), 1537. https://doi.org/10.3390/medicina61091537









