Transcatheter Aortic Valve Replacement in Older Adults: Integrating Cardiac Remodeling and Geriatric Syndromes—A Narrative Review
Abstract
1. Introduction
Search Strategy
2. Procedural Outcomes
2.1. TAVR-Associated Complications
2.2. Improvement of Symptoms and Functional Recovery
2.3. Structural Valve Durability
- TAVR is non-inferior to SAVR in terms of all-cause mortality in the short, medium, and long term.
- Concerning TAVR-associated complications, TAVR has lower atrial fibrillation rates, similar stroke rates, and higher PPI rates than SAVR in the short-, medium-, and long-term.
- TAVR offers rapid and sustained symptomatic improvement in terms of dyspnea and functional recovery, resulting in improved quality of life based on the KCCQ.
- Structural valve durability of TAVR is comparable to that of bioprosthetic valves in the short, medium, and long term.
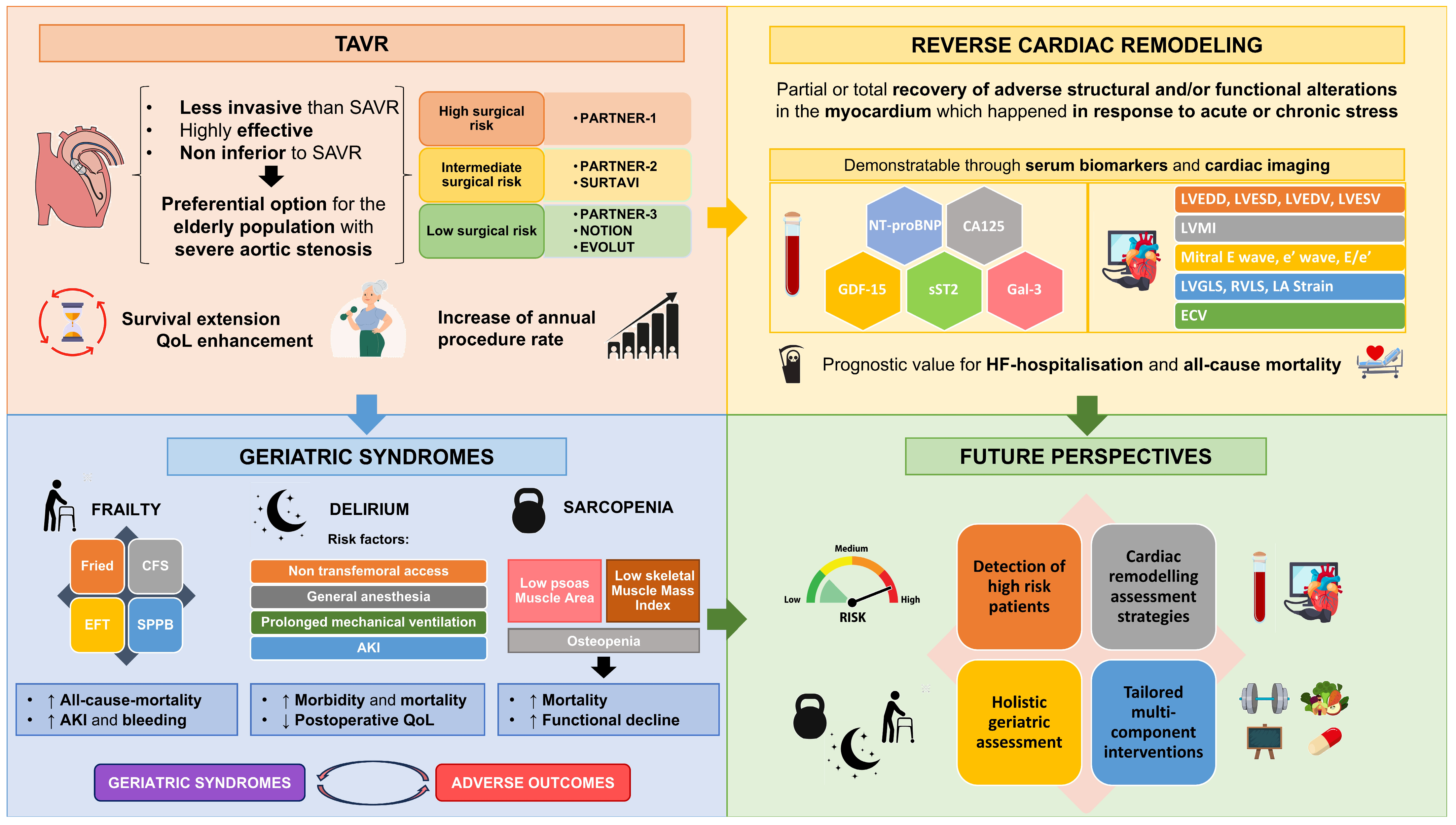
- TAVR is a less invasive and effective treatment for severe AS, improving patient outcomes, extending survival, and enhancing quality of life across diverse risk profiles.
- There is still a lack of evidence regarding the selection of patients who can benefit most from TAVR, especially in the elderly population.
- On the other hand, material and technical development and optimization of the patient before and after TAVR will enable even better results to be achieved.
3. Cardiac Remodeling
3.1. Cardiac Remodeling Assessment Through Serum Biomarkers
3.1.1. NT-proBNP
3.1.2. CA125
3.1.3. sST2
3.1.4. GDF-15
3.1.5. Galectin-3
3.1.6. Utility of Serum Biomarkers
3.2. Cardiac Remodeling Assessment Through Cardiac Imaging
- Baseline levels and post-TAVR evolution of serum biomarkers of cardiac remodelling, such as NT-proBNP, CA125, GDF-15, sST2, and Galectin-3 may play a key role due to their prognostic implications in terms of survival.
- Serum biomarkers can be combined with each other and with surgical risk scales, such as the STS score, to obtain better prognostic stratification of patients.
- Cardiac remodelling parameters obtained through cardiac imaging, such as LVEDD, LVESD, LVEDV, LVESV, LVMI, maximum mitral E wave velocity, medial or lateral e’ wave, E/e’ ratio, LVGLS, RVLS, LA deformation, and ECV, have been shown to have prognostic implications post-TAVR in terms of all-cause mortality and MACE.
- Assessment of cardiac remodeling through serum biomarkers should be performed, as it is a non-invasive approach with prognostic implications.
- The combination of serum biomarkers (GDF-15, sST2, and NT-proBNP) and surgical risk scales (sST2 associated with the STS score) improves the ability to predict adverse events.
- The concomitant use of serological markers and cardiac imaging parameters together is reasonable for the assessment of cardiac remodeling.
- Although this is a promising field, further evidence is needed to support the use of serological biomarkers for cardiac remodelling assessment.
- Additional high-level evidence studies are needed to select the optimal assessment strategy for cardiac remodelling by combining serological markers and imaging techniques.
4. TAVR in Elderly Patients
4.1. Procedural Outcomes in Elderly Patients
4.2. Geriatric Syndromes and TAVR
4.2.1. Frailty
4.2.2. Delirium
4.2.3. Sarcopenia
4.3. Socioeconomic Impact of Geriatric Syndromes
4.4. Quality of Life
- Although the incidence of all-cause mortality and periprocedural complications may be higher in the very elderly, the prognostic and symptomatic benefits are superior to conservative treatment in well-selected patients.
- Geriatric syndromes like frailty, delirium, and sarcopenia are independent determinants of post-procedural outcomes, increasing morbidity and mortality in the short, medium, and long terms.
- Frailty and especially delirium significantly increase hospital length of stay and associated costs.
- TAVR in elderly patients clearly implies rapid and sustained benefits for HRQoL.
- In the elderly population, TAVR may be beneficial for carefully selected patients.
- Geriatric syndromes should be prevented, detected early, and addressed, as they lead to worse outcomes and higher overall costs.
- Evidence regarding procedural outcomes in very elderly patients (aged ≥90 years) is limited. More high-quality studies are needed to evaluate endpoints, such as all-cause mortality and periprocedural complications, in this population.
- Further research is needed to optimize non-invasive management in elderly patients deemed unsuitable for TAVR, with the goal of improving all-cause mortality, functional status, and quality of life.
5. Future Perspectives
- Research into strategies after TAVR to mitigate the onset of geriatric syndromes has recently increased. Two examples are the PERFORM-TAVI trial, which is evaluating the effect of exercise and nutritional approaches to reverse frailty, and the TELE-FRAIL TAVI study, which is testing the impact of telehealth-based multidisciplinary interventions (nutrition, exercise, education) on functional recovery in elderly patients after TAVR.
- Pharmacological management after TAVR is also a trending topic in research, with ongoing studies such as the DAPA-TAVI trial, which suggests that dapagliflozin may improve prognosis in elderly patients with severe AS, and the RASTAVI trial, which is currently investigating whether ACEIs could reduce adverse outcomes after TAVR.
- Artificial intelligence and machine learning models are emerging tools that enhance risk stratification, procedural planning, and personalized follow-up in TAVR, offering a forward-looking perspective for clinical integration.
- Multicomponent interventions after TAVR may improve functional recovery and quality of life in older adults.
- Pharmacological therapy after TAVR can improve procedural outcomes.
- AI-based predictive models can augment clinical decision-making and individualized patient management in TAVR care.
- Cost-efficiency of multicomponent interventions after TAVR should be investigated.
- Large-scale randomized clinical trials should be performed to integrate multidisciplinary management of older adults after TAVR, including exercise, nutrition, education, and pharmacological approaches.
- Validation and clinical integration of AI/ML tools require further prospective studies to confirm their predictive accuracy and clinical utility.
6. Conclusions
- TAVR has been proposed as the standard treatment for AS, especially in elderly patients, owing to its safety and efficacy profile.
- Cardiac remodeling must be assessed using both serum biomarkers and imaging parameters, as they can help optimize procedural planning and management after TAVR.
- Geriatric syndromes such as frailty, delirium, and sarcopenia increase adverse outcomes; therefore, prevention, early detection, and thorough management are imperative.
- Early identification of high-risk patients and multidisciplinary management are crucial for improving procedural outcomes, functional recovery, and long-term results.
- Evidence-based medicine is the cornerstone of obtaining maximum benefits for patients. Ongoing trials will yield tools for optimizing patient outcomes after TAVR.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Vekstein, A.M.; Wegermann, Z.K.; Manandhar, P.; Mack, M.J.; Cohen, D.J.; Hughes, G.C.; Harrison, J.K.; Kaneko, T.; Kapadia, S.R.; Stathogiannis, K.; et al. Outcomes of Transcatheter Aortic Valve Replacement in Low-Risk Patients in the United States: A Report from the STS/ACC TVT Registry. Circulation 2025, 151, 1134–1146. [Google Scholar] [CrossRef] [PubMed]
- Forrest, J.K.; Yakubov, S.J.; Deeb, G.M.; Gada, H.; Mumtaz, M.A.; Ramlawi, B.; Bajwa, T.; Crouch, J.; Merhi, W.; Wai Sang, S.L.; et al. 5-Year outcomes after transcatheter or surgical aortic valve replacement in low-risk patients with aortic stenosis. J. Am. Coll. Cardiol. 2025, 85, 1523–1532. [Google Scholar] [CrossRef]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Pibarot, P.; Hahn, R.T.; Genereux, P.; Kodali, S.K.; Kapadia, S.R.; Cohen, D.J.; Pocock, S.J.; et al. Transcatheter aortic-valve replacement in low-risk patients at five years. N. Engl. J. Med. 2023, 389, 1949–1960. [Google Scholar] [CrossRef]
- Zhao, A.; Minhui, H.; Li, X.; Zhiyun, X. A meta-analysis of transfemoral versus transapical transcatheter aortic valve implantation on 30-day and 1-year outcomes. Heart Surg. Forum 2015, 18, 138–145. [Google Scholar] [CrossRef]
- Li, X.; Kong, M.; Jiang, D.; Dong, A. Comparison 30-day clinical complications between transfemoral versus transapical aortic valve replacement for aortic stenosis: A meta-analysis review. BMC Cardiovasc. Disord. 2021, 21, 123. [Google Scholar] [CrossRef]
- Tarantini, G.; Mojoli, M.; Urena, M.; Vahanian, A. Atrial fibrillation in patients undergoing transcatheter aortic valve implantation: Epidemiology, timing, predictors, and outcome. Eur. Heart J. 2017, 38, 1285–1293. [Google Scholar] [CrossRef]
- Siontis, G.C.M.; Praz, F.; Lanz, J.; Vollenbroich, R.; Roten, L.; Stortecky, S.; Räber, L.; Windecker, S.; Pilgrim, T. New-onset arrhythmias following transcatheter aortic valve implantation: A systematic review and meta-analysis. Heart 2018, 104, 1208–1215. [Google Scholar] [CrossRef]
- Barili, F.; Freemantle, N.; Musumeci, F.; Martin, B.; Anselmi, A.; Rinaldi, M.; Kaul, S.; Rodriguez-Roda, J.; Mauro, M.D.; Folliguet, T.; et al. Five-year outcomes in trials comparing transcatheter aortic valve implantation versus surgical aortic valve replacement: A pooled meta-analysis of reconstructed time-to-event data. Eur. J. Cardiothorac. Surg. 2022, 61, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Van Mieghem, N.M.; Deeb, G.M.; Søndergaard, L.; Grube, E.; Windecker, S.; Gada, H.; Mumtaz, M.; Olsen, P.S.; Heiser, J.C.; Merhi, W.; et al. Self-expanding transcatheter vs. surgical aortic valve replacement in intermediate-risk patients: 5-year outcomes of the SURTAVI randomized clinical trial. JAMA Cardiol. 2022, 7, 1000–1008. [Google Scholar] [CrossRef]
- Thyregod, H.G.H.; Jørgensen, T.H.; Ihlemann, N.; Steinbrüchel, D.A.; Nissen, H.; Kjeldsen, B.J.; Petursson, P.; Backer, O.D.; Olsen, P.S.; Søndergaard, L. Transcatheter or surgical aortic valve implantation: 10-year outcomes of the NOTION trial. Eur. Heart J. 2024, 45, 1116–1124. [Google Scholar] [CrossRef]
- Okuno, T.; Alaour, B.; Heg, D.; Tueller, D.; Pilgrim, T.; Muller, O.; Noble, S.; Jeger, R.; Reuthebuch, O.; Toggweiler, S.; et al. Long-term risk of stroke after transcatheter aortic valve replacement: Insights from the SwissTAVI Registry. JACC Cardiovasc. Interv. 2023, 16, 2986–2996. [Google Scholar] [CrossRef]
- Vlastra, W.; Jimenez-Quevedo, P.; Tchétché, D.; Chandrasekhar, J.; de Brito, F.S., Jr.; Barbanti, M.; Kornowski, R.; Latib, A.; D’Onofrio, A.; Ribichini, F.; et al. Predictors, incidence, and outcomes of patients undergoing transfemoral transcatheter aortic valve implantation complicated by stroke: A center-collaboration. Circ. Cardiovasc. Interv. 2019, 12, e007546. [Google Scholar] [CrossRef]
- Leone, A.; Castiello, D.S.; Angellotti, D.; Mariani, A.; Manzo, R.; Avvedimento, M.; Ilardi, F.; Piccolo, R.; Esposito, G.; Franzone, A. Incidence, predictors, and prognostic impact of temporary left bundle branch block after transcatheter aortic valve replacement. J. Electrocardiol. 2022, 74, 114–115. [Google Scholar] [CrossRef] [PubMed]
- Gonnah, A.R.; Abdelwahab, M.; Taylor, R.; Labib, A.; Masoud, O.; Debski, M.; Abdelaziz, H.K.; Roberts, D.H. Health-related quality of life following TAVI or cardiac surgery in patients at intermediate and low risk: A systematic review and meta-analysis. Clin. Med. 2023, 23, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Green, P.; Cohen, D.J.; Généreux, P.; McAndrew, T.; Arnold, S.V.; Alu, M.; Beohar, N.; Rihal, C.S.; Mack, M.J.; Kapadia, S.; et al. Relation between six-minute walk test performance and outcomes after transcatheter aortic valve implantation (from the PARTNER Trial). Am. J. Cardiol. 2013, 112, 700–706. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Richards, A.M.; de Boer, R.A.; Thum, T.; Arfsten, H.; Hülsmann, M.; Falcao-Pires, I.; Díez, J.; Foo, R.S.Y.; Chan, M.Y.; et al. Cardiac remodelling-Part 1: From cells and tissues to circulating biomarkers. A review from the Study Group on Biomarkers of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2022, 24, 927–943. [Google Scholar] [CrossRef]
- Aimo, A.; Vergaro, G.; González, A.; Barison, A.; Lupón, J.; Delgado, V.; Richards, A.M.; de Boer, R.A.; Thum, T.; Arfsten, H.; et al. Cardiac remodelling-Part 2: Clinical, imaging and laboratory findings. A review from the Study Group on Biomarkers of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2022, 24, 944–958. [Google Scholar] [CrossRef]
- Wernly, B.; Lichtenauer, M. Old dog, new tricks-CA125 for risk stratification in TAVI patients. Rev. Esp. Cardiol. 2019, 72, 892–895. [Google Scholar] [CrossRef]
- Bjørnstad, J.L.; Neverdal, N.O.; Vengen, O.A.; Knudsen, C.W.; Husebye, T.; Pepper, J.; Lie, M.; Christensen, G.; Tønnessen, T. Alterations in circulating activin A, GDF-15, TGF-beta3 and MMP-2, -3, and -9 during one year of left ventricular reverse remodelling in patients operated for severe aortic stenosis. Eur. J. Heart Fail. 2008, 10, 1201–1207. [Google Scholar] [CrossRef]
- Baldenhofer, G.; Zhang, K.; Spethmann, S.; Laule, M.; Eilers, B.; Leonhardt, F.; Sanad, W.; Dreger, H.; Sander, M.; Grubitzsch, H.; et al. Galectin-3 predicts short- and long-term outcome in patients undergoing transcatheter aortic valve implantation (TAVI). Int. J. Cardiol. 2014, 177, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Toutouzas, K.; Stathogiannis, K.; Latsios, G.; Synetos, A.; Drakopoulou, M.; Penesopoulou, V.; Michelongona, A.; Tsiamis, E.; Tousoulis, D. Biomarkers in aortic valve stenosis and their clinical significance in transcatheter aortic valve implantation. Curr. Med. Chem. 2019, 26, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, W. N-terminal pro-B-type natriuretic peptide is associated with clinical outcomes after transcatheter aortic valve replacement. J. Cardiothorac. Surg. 2023, 18, 286. [Google Scholar] [CrossRef]
- Ribeiro, H.B.; Urena, M.; Le Ven, F.; Nombela-Franco, L.; Allende, R.; Clavel, M.A.; Dahou, A.; Côté, M.; Laflamme, J.; Laflamme, L.; et al. Long-term prognostic value and serial changes of plasma N-terminal prohormone B-type natriuretic peptide in patients undergoing transcatheter aortic valve implantation. Am. J. Cardiol. 2014, 113, 851–859. [Google Scholar] [CrossRef]
- Koskinas, K.C.; O’Sullivan, C.J.; Heg, D.; Praz, F.; Stortecky, S.; Pilgrim, T.; Buellesfeld, L.; Jüni, P.; Windecker, S.; Wenaweser, P. Effect of B-type natriuretic peptides on long-term outcomes after transcatheter aortic valve implantation. Am. J. Cardiol. 2015, 116, 1560–1565. [Google Scholar] [CrossRef]
- Seoudy, H.; Frank, J.; Neu, M.; Güßefeld, N.; Klaus, Y.; Freitag-Wolf, S.; Lambers, M.; Lutter, G.; Dempfle, A.; Rangrez, A.Y.; et al. Periprocedural changes of NTproBNP are associated with survival after transcatheter aortic valve implantation. J. Am. Heart Assoc. 2019, 8, e010876. [Google Scholar] [CrossRef]
- Kaneko, H.; Hoelschermann, F.; Tambor, G. Impact of N-terminal pro-B-type natriuretic response on long term prognosis after transcatheter aortic valve implantation for severe aortic stenosis and heart failure. Heart Vessel. 2019, 34, 777–783. [Google Scholar] [CrossRef]
- Vale, N.C.; Campante, T.R.; Madeira, S.; Brito, J.; Sousa Almeida, M.; Nolasco, T.; Abecasis, J.; Rodrigues, G.; Carmo, J.; Furstenau, M.; et al. Post-procedural N-terminal pro-brain natriuretic peptide predicts one-year mortality after transcatheter aortic valve implantation. Rev. Port. Cardiol. 2018, 37, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Husser, O.; Núñez, J.; Núñez, E.; Holzamer, A.; Camboni, D.; Luchner, A.; Sanchis, J.; Bodi, V.; Riegger, G.A.; Schmid, C.; et al. Tumor marker carbohydrate antigen 125 predicts adverse outcome after transcatheter aortic valve implantation. JACC Cardiovasc. Interv. 2013, 6, 487–496. [Google Scholar] [CrossRef]
- Rheude, T.; Pellegrini, C.; Núñez, J.; Joner, M.; Trenkwalder, T.; Mayr, N.P.; Holdenrieder, S.; Bodi, V.; Koenig, W.; Kasel, A.M.; et al. Differential prognostic value of galectin-3 according to carbohydrate antigen 125 levels in transcatheter aortic valve implantation. Rev. Esp. Cardiol. 2019, 72, 907–915. [Google Scholar] [CrossRef]
- Wernly, B.; Lichtenauer, M.; Jirak, P. Soluble ST2 predicts 1-year outcome in patients undergoing transcatheter aortic valve implantation. Eur. J. Clin. Investig. 2017, 47, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Schmid, J.; Stojakovic, T.; Zweiker, D.; Scharnagl, H.; Maderthaner, R.D.; Scherr, D.; Maier, R.; Schmidt, A.; März, W.; Binder, J.S.; et al. ST2 predicts survival in patients undergoing transcatheter aortic valve implantation. Int. J. Cardiol. 2017, 244, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Oury, C.; Nchimi, A.; Lancellotti, P.; Bergler-Klein, J. Can blood biomarkers help predicting outcome in transcatheter aortic valve implantation? Front. Cardiovasc. Med. 2018, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Stundl, A.; Lünstedt, N.S.; Courtz, F.; Freitag-Wolf, S.; Frey, N.; Holdenrieder, S.; Zur, B.; Grube, E.; Nickenig, G.; Werner, N.; et al. Soluble ST2 for risk stratification and the prediction of mortality in patients undergoing transcatheter aortic valve implantation. Am. J. Cardiol. 2017, 120, 986–993. [Google Scholar] [CrossRef]
- Krau, N.C.; Lünstedt, N.S.; Freitag-Wolf, S.; Brehm, D.; Petzina, R.; Lutter, G.; Bramlage, P.; Dempfle, A.; Frey, N.; Frank, D. Elevated growth differentiation factor 15 levels predict outcome in patients undergoing transcatheter aortic valve implantation. Eur. J. Heart Fail. 2015, 17, 945–955. [Google Scholar] [CrossRef]
- Kim, J.B.; Kobayashi, Y.; Moneghetti, K.J.; Brenner, D.A.; O’Malley, R.; Schnittger, I.; Wu, J.C.; Murtagh, G.; Beshiri, A.; Fischbein, M.; et al. GDF-15 (growth differentiation factor 15) is associated with lack of ventricular recovery and mortality after transcatheter aortic valve replacement. Circ. Cardiovasc. Interv. 2017, 10, e005594. [Google Scholar] [CrossRef]
- Sluka, M.; Hutyra, M.; Nykl, R.; Ostransky, J.; Furst, T.; Petrova, P.; Precek, J.; Hudec, S.; Taborsky, M. Risk stratification using growth differentiation factor 15 in patients undergoing transcatheter aortic valve implantation. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 2023, 167, 263–271. [Google Scholar] [CrossRef]
- Lindman, B.R.; Breyley, J.G.; Schilling, J.D.; Vatterott, A.M.; Zajarias, A.; Maniar, H.S.; Damiano, R.J., Jr.; Moon, M.R.; Lawton, J.S.; Gage, B.F.; et al. Prognostic utility of novel biomarkers of cardiovascular stress in patients with aortic stenosis undergoing valve replacement. Heart 2015, 101, 1382–1388. [Google Scholar] [CrossRef]
- Dahiya, G.; Kyvernitakis, A.; Joshi, A.A.; Lasorda, D.M.; Bailey, S.H.; Raina, A.; Biederman, R.W.W.; Kanwar, M.K. Impact of transcatheter aortic valve replacement on left ventricular hypertrophy, diastolic dysfunction and quality of life in patients with preserved left ventricular function. Int. J. Cardiovasc. Imaging 2021, 37, 485–492. [Google Scholar] [CrossRef]
- Fukui, M.; Hashimoto, G.; Lopes, B.B.C.; Stanberry, L.I.; Garcia, S.; Gössl, M.; Enriquez-Sarano, M.; Bapat, V.N.; Sorajja, P.; Lesser, J.R.; et al. Association of baseline and change in global longitudinal strain by computed tomography with post-transcatheter aortic valve replacement outcomes. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 476–484. [Google Scholar] [CrossRef]
- He, X.; Li, Y.; Wang, Y.; Tian, W.; Li, Z.; Ge, L.; Wang, G.; Chen, Z. Prognostic value of CT-derived myocardial biomarkers: Extracellular volume fraction and strain in patients with severe aortic stenosis undergoing transcatheter aortic valve replacement: A systematic review and meta-analysis. Acad. Radiol. 2024, 31, 4352–4364. [Google Scholar] [CrossRef]
- Xiao, Y.; Bi, W.; Qiao, W.; Wang, X.; Li, Y.; Ren, W. Prognostic value of ventricular longitudinal strain in patients undergoing transcatheter aortic valve replacement: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2022, 9, 965440. [Google Scholar] [CrossRef]
- Stens, N.A.; van Iersel, O.; Rooijakkers, M.J.P.; van Wely, M.H.; Nijveldt, R.; Bakker, E.A.; Rodwell, L.; Pedersen, A.L.D.; Poulsen, S.H.; Kjønås, D.; et al. Prognostic value of preprocedural LV global longitudinal strain for post-TAVR-related morbidity and mortality. JACC Cardiovasc. Imaging 2023, 16, 332–341. [Google Scholar] [CrossRef]
- Anastasius, M.; Ro, R.; Gavalas, M.; Patel, N.; Prandi, F.R.; Tang, G.H.L.; Krishnamoorthy, P.; Sharma, S.K.; Kini, A.; Lerakis, S. The effect of TAVR on left ventricular and left atrial mechanics in patients with aortic stenosis. J. Cardiovasc. Dev. Dis. 2022, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Asheghan, M.M.; Javadikasgari, H.; Attary, T.; Rouhollahi, A.; Straughan, R.; Willi, J.N.; Awal, R.; Sabe, A.; de la Cruz, K.I.; Nezami, F.R. Predicting one-year left ventricular mass index regression following transcatheter aortic valve replacement in patients with severe aortic stenosis: A new era is coming. Front. Cardiovasc. Med. 2023, 10, 1130152. [Google Scholar] [CrossRef]
- Meredith, T.; Mohammed, F.; Pomeroy, A.; Barbieri, S.; Meijering, E.; Jorm, L.; Roy, D.; Kovacic, J.; Feneley, M.; Hayward, C.; et al. Machine learning cluster analysis identifies increased 12-month mortality risk in transcatheter aortic valve replacement recipients. Front. Cardiovasc. Med. 2025, 12, 1444658. [Google Scholar] [CrossRef]
- Ramos, J.; Monteagudo, J.M.; González-Alujas, T.; Fuentes, M.E.; Sitges, M.; Peña, M.L.; Carrasco-Chinchilla, F.; Echeverría, T.; Bouzas, A.; Forteza Alberti, J.F.; et al. Large-scale assessment of aortic stenosis: Facing the next cardiac epidemic? Eur. Heart J. Cardiovasc. Imaging 2018, 19, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Osnabrugge, R.L.; Mylotte, D.; Head, S.J.; Van Mieghem, N.M.; Nkomo, V.T.; LeReun, C.M.; Bogers, A.J.; Piazza, N.; Kappetein, A.P. Aortic stenosis in the elderly: Disease prevalence and number of candidates for transcatheter aortic valve replacement: A meta-analysis and modeling study. J. Am. Coll. Cardiol. 2013, 62, 1002–1012. [Google Scholar] [CrossRef]
- Ramos, J.; Hernández, S.; Viéitez, A.; Abellas Sequeiros, M.; Alonso Salinas, G.L.; Zamorano Gómez, J.L. Cribado poblacional de estenosis aórtica: Prevalencia y perfil de riesgo. REC CardioClinics 2021, 56, 77–84. [Google Scholar] [CrossRef]
- Davidson, L.J.; Davidson, C.J. Transcatheter treatment of valvular heart disease: A review. JAMA 2021, 325, 2480–2494. [Google Scholar] [CrossRef]
- Stehli, J.; Koh, J.Q.S.; Duffy, S.J.; Zamani, J.; Yeong, C.C.; Paratz, E.; Martin, C.; Htun, N.M.; Stub, D.; Dick, R.; et al. Comparison of outcomes of transcatheter aortic valve implantation in patients ≥ 90 years versus <90 years. Am. J. Cardiol. 2015, 116, 1110–1115. [Google Scholar] [CrossRef]
- Zadrozny, M.; Hainzer, N.; Mehilli, J.; Jochheim, D.; Gschwendtner, S.; Steffen, J.; Theiss, H.; Braun, D.; Hagl, C.; Sadoni, S. TAVR in nonagenarians: An analysis investigating safety, efficacy, symptomatic improvement, and long-term survival. J. Cardiol. 2021, 78, 44–50. [Google Scholar] [CrossRef]
- Liu, Y.; Du, Y.; Fu, M.; Ma, Y.; Wang, D.; Zhang, J.; Liu, W.; Zhao, Y.; Zhou, Y. Clinical outcomes of transcatheter aortic valve replacement in nonagenarians: A systematic review and meta-analysis. J. Interv. Cardiol. 2019, 2019, 5819232. [Google Scholar] [CrossRef] [PubMed]
- Demir, O.M.; Curio, J.; Pagnesi, M.; Rahman, H.; Mitomo, S.; Colombo, A.; Chau, M.; Prendergast, B.; Latib, A. Transcatheter aortic valve replacement in nonagenarians: A systematic review and meta-analysis. J. Invasive Cardiol. 2022, 34, E226–E236. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Afilalo, J.; Lauck, S.; Kim, D.H.; Lefèvre, T.; Piazza, N.; Lachapelle, K.; Martucci, G.; Lamy, A.; Labinaz, M.; Peterson, M.D.; et al. Frailty in older adults undergoing aortic valve replacement: The FRAILTY-AVR study. J. Am. Coll. Cardiol. 2017, 70, 689–700. [Google Scholar] [CrossRef]
- Thongprayoon, C.; Cheungpasitporn, W.; Thamcharoen, N.; Ungprasert, P.; Kittanamongkolchai, W.; Mao, M.A.; Sakhuja, A.; Greason, K.L.; Kashani, K. Association of frailty status with acute kidney injury and mortality after transcatheter aortic valve replacement: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0177157. [Google Scholar] [CrossRef]
- Huang, L.; Zhou, X.; Yang, X.; Yu, H. The impact of preoperative frailty status on outcomes after transcatheter aortic valve replacement: An update of systematic review and meta-analysis. Medicine 2018, 97, e13475. [Google Scholar] [CrossRef]
- Katz, S.; Ford, A.B.; Moskowitz, R.W.; Jackson, B.A.; Jaffe, M.W. Studies of illness in the aged. The index of ADL: A standardized measure of biological and psychosocial function. JAMA 1963, 185, 914–919. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef] [PubMed]
- Ochani, S.; Adnan, A.; Siddiqui, A.; Kalwar, A.; Kukreja, S.; Ahmad, M.; Ashraf, M.H.; Asghar, M.A. Postoperative delirium in 47 379 individuals undergoing transcatheter aortic valve replacement: A systematic review and meta-analysis. Ann. Med. Surg. 2023, 85, 4476–4490. [Google Scholar] [CrossRef]
- Ma, X.; Chu, H.; Han, K.; Shao, Q.; Yu, Y.; Jia, S.; Wang, D.; Wang, Z.; Zhou, Y. Postoperative delirium after transcatheter aortic valve replacement: An updated systematic review and meta-analysis. J. Am. Geriatr. Soc. 2023, 71, 646–660. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Soud, M.; Alahdab, F.; Ho, G.; Kuku, K.O.; Cejudo-Tejeda, M.; Hideo-Kajita, A.; de Araujo Gonçalves, P.; Teles, R.C.; Waksman, R.; Garcia-Garcia, H.M. Usefulness of skeletal muscle area detected by computed tomography to predict mortality in patients undergoing transcatheter aortic valve replacement: A meta-analysis study. Int. J. Cardiovasc. Imaging 2019, 35, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Solla-Suarez, P.; Arif, S.G.; Ahmad, F.; Rastogi, N.; Meng, A.; Cohen, J.M.; Rodighiero, J.; Piazza, N.; Martucci, G.; Lauck, S.; et al. Osteosarcopenia and mortality in older adults undergoing transcatheter aortic valve replacement. JAMA Cardiol. 2024, 9, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Damluji, A.A.; Rodriguez, G.; Noel, T.; Davis, L.; Dahya, V.; Tehrani, B.; Epps, K.; Sherwood, M.; Sarin, E.; Walston, J.; et al. Sarcopenia and health-related quality of life in older adults after transcatheter aortic valve replacement. Am. Heart J. 2020, 224, 171–181. [Google Scholar] [CrossRef]
- Kwak, M.J.; Rasu, R.; Morgan, R.O.; Lee, J.; Rianon, N.J.; Holmes, H.M.; Dhoble, A.; Kim, D.H. The association of economic outcome and geriatric syndromes among older adults with transcatheter aortic valve replacement (TAVR). J. Health Econ. Outcomes Res. 2020, 7, 175–181. [Google Scholar] [CrossRef]
- Moreines, L.T.; David, D.; Murali, K.P.; Dickson, V.V.; Brody, A. The perspectives of older adults related to transcatheter aortic valve replacement: An integrative review. Heart Lung 2024, 68, 23–36. [Google Scholar] [CrossRef]
- Arnold, S.V.; Petrossian, G.; Reardon, M.J.; Kleiman, N.S.; Yakubov, S.J.; Wang, K.; Hermiller, J., Jr.; Harrison, J.K.; Deeb, G.M.; Huang, J.; et al. Five-year clinical and quality of life outcomes from the CoreValve US pivotal extreme risk trial. Circ. Cardiovasc. Interv. 2021, 14, e010258. [Google Scholar] [CrossRef]
- Fountotos, R.; Lauck, S.; Piazza, N.; Martucci, G.; Arora, R.; Asgar, A.; Forcillo, J.; Kouz, R.; Labinaz, M.; Lamy, A.; et al. Protein and exercise to reverse frailty in older men and women undergoing transcatheter aortic valve replacement: Design of the PERFORM-TAVR trial. Can. J. Cardiol. 2024, 40, 267–274. [Google Scholar] [CrossRef]
- Bernal-Labrador, E.; Romaguera, R.; García-Blas, S.; Regueiro, A.; Serra, V.; Asmarats, L.; Tizón-Marcos, H.; Agudelo, V.; Scardino, C.; Casanova-Sandoval, J.M.; et al. Telematic Intervention on Frailty in Patients Undergoing TAVI. Design of the TELE-FRAIL TAVI Clinical Trial. REC Interv. Cardiol. 2025, in press. [Google Scholar] [CrossRef]
- Raposeiras-Roubin, S.; Amat-Santos, I.J.; Rossello, X.; González Ferreiro, R.; González Bermúdez, I.; Lopez Otero, D.; Nombela-Franco, L.; Gheorghe, L.; Diez, J.L.; Baladrón Zorita, C.; et al. Dapagliflozin in patients undergoing transcatheter aortic-valve implantation. N. Engl. J. Med. 2025, 392, 1396–1405. [Google Scholar] [CrossRef]
- Amat-Santos, I.J.; Catalá, P.; Diez Del Hoyo, F.; Fernandez-Diaz, J.A.; Alonso-Briales, J.H.; Del Trigo, M.; Regueiro, A.; Juan-Salvadores, P.; Serra, V.; Gutierrez-Ibanes, E.; et al. Rationale and design of the RASTAVI trial: Ramipril in patients with aortic stenosis after TAVI. J. Am. Heart Assoc. 2020, 9, e016911. [Google Scholar] [CrossRef]
- Amat-Santos, I.J.; Santos-Martínez, S.; Julca, F.; Catalá, P.; Rodríguez-Gabella, T.; Redondo-Diéguez, A.; Hinojosa, W.; Veras, C.; Campo, A.; Serrador Frutos, A.; et al. Impact of renin-angiotensin system inhibitors on outcomes after surgical or transcatheter aortic valve replacement. A meta-analysis. Rev. Esp. Cardiol. 2021, 74, 421–426. [Google Scholar] [CrossRef]
- Fischer-Rasokat, U.; Bänsch, C.; Renker, M.; Rolf, A.; Charitos, E.I.; Weferling, M.; Liebetrau, C.; Herrmann, E.; Choi, Y.H.; Hamm, C.W.; et al. Effects of renin-angiotensin system inhibitor type and dosage on survival after transcatheter aortic valve implantation. Eur. Heart J. Cardiovasc. Pharmacother. 2022, 8, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Gabella, T.; Catalá, P.; Muñoz-García, A.J.; Nombela-Franco, L.; Del Valle, R.; Gutiérrez, E.; Regueiro, A.; Jimenez-Diaz, V.A.; Ribeiro, H.B.; Rivero, F.; et al. Renin-Angiotensin System Inhibition Following Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2019, 74, 631–641. [Google Scholar] [CrossRef]
- Okuno, T.; Overtchouk, P.; Asami, M.; Tomii, D.; Stortecky, S.; Praz, F.; Lanz, J.; Siontis, G.C.M.; Gräni, C.; Windecker, S.; et al. Deep learning-based prediction of early cerebrovascular events after transcatheter aortic valve replacement. Sci. Rep. 2021, 11, 18754. [Google Scholar] [CrossRef] [PubMed]
- Tahir, A.M.; Mutlu, O.; Bensaali, F.; Ward, R.; Ghareeb, A.N.; Helmy, S.M.H.A.; Othman, K.T.; Al-Hashemi, M.A.; Abujalala, S.; Chowdhury, M.E.H.; et al. Latest Developments in Adapting Deep Learning for Assessing TAVR Procedures and Outcomes. J. Clin. Med. 2023, 12, 4774. [Google Scholar] [CrossRef]
- Penso, M.; Pepi, M.; Fusini, L.; Muratori, M.; Cefalù, C.; Mantegazza, V.; Gripari, P.; Ali, S.G.; Fabbiocchi, F.; Bartorelli, A.L.; et al. Predicting Long-Term Mortality in TAVI Patients Using Machine Learning Techniques. J. Cardiovasc. Dev. Dis. 2021, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Sazzad, F.; Ler, A.A.L.; Furqan, M.S.; Tan, L.K.Z.; Leo, H.L.; Kuntjoro, I.; Tay, E.; Kofidis, T. Harnessing the power of artificial intelligence in predicting all-cause mortality in transcatheter aortic valve replacement: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2024, 11, 1343210. [Google Scholar] [CrossRef] [PubMed]
- Dey, D.; Slomka, P.J.; Leeson, P.; Comaniciu, D.; Shrestha, S.; Sengupta, P.P.; Marwick, T.H. Artificial Intelligence in Cardiovascular Imaging: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 1317–1335. [Google Scholar] [CrossRef] [PubMed]
| Biomarker | Origin | Function | Prognostic Value |
|---|---|---|---|
| NTpro-BNP | Released by ventricular cardiomyocytes in response to parietal stress | Inhibition of RAS and SNS for natriuresis and arteriolar vasodilation. Reduction of adverse cardiac remodeling and/or increase of reverse cardiac remodeling | Elevated baseline levels: Not clear if predictor of worse prognosis in terms of HF hospitalization or all-cause mortality No reduction of levels after TAVR: Increase in all-cause mortality. |
| CA125 | Glycoprotein mainly released by mesothelial cells of the peritoneum, pericardium, and pleura. | Non-specific tissue irritation marker. Useful in oncological processes and widely used in heart failure decompensation, especially if pleural effusion or ascites | Elevated baseline levels: Increase in all-cause mortality or MACE. No reduction of levels after TAVR: Increase in all-cause mortality or MACE. |
| SST2 | Released by immune cells in inflammatory processes and by cardiomyocytes in response to parietal stress | Inhibits IL-33/ST2 signaling system to prevent myocardial fibrosis and hypertrophic adverse remodeling | Elevated baseline levels: Increase in all-cause mortality. No reduction of levels after TAVR: Increase in all-cause mortality. * Its addition to the STS-score in patients undergoing TAVR could improve prognostic value for all-cause mortality and/or MACE. |
| GDF-15 | Cytokine of the transforming growth factor-B family, released by macrophages, adipocytes, and cardiomyocytes, among others. | Activates MAP-K to regulate TGF-β1/SMAD3 axis, reducing inflammation and oxidative and myocardial stress. In myocardial tissue, it has anti-hypertrophic and antifibrotic effect. | Elevated baseline levels: Lower reversal remodeling after TAVR and an increase in all-cause mortality and MACE. No reduction of levels after TAVR: Increased all-cause mortality and MACE. |
| Galectin-3 | Lectin released by monocytes, macrophages, epithelial cells, and cardiomyocytes, among others. | Regulation of signaling pathways related to cell adhesion, cell proliferation, and inflammation. In the myocardial tissue, related to inflammation, fibrosis, and hypertrophy. | Elevated baseline levels: It might be a predictor of lower reversal remodeling after TAVR. Predictor of increased all-cause mortality and MACE only in the presence of elevated NTproBNP values. |
| Imaging Feature | Description | Measured | Prognostic Value |
|---|---|---|---|
| LVEDD LVESD LVEDV LVESV | Left ventricular end-diastolic diameter (LVEDD) Left ventricular end-systolic diameter (LVESD) Left ventricular end diastolic volume (LVEDV) Left ventricular end systolic volume (LVESD) | Transthoracic echocardiography:
Cardiac magnetic resonance | Lowering of the parameters 1 month–1 year after TAVR favors cardiac reverse remodeling, and improvement of HF-related quality of life assessed by KCCQ. |
| LVMI | Left Ventricular mass index: estimation of the weight of the left ventricle LV mass formula: 0.8 × (1.04 × (((LVEDD + IVS +PW)3 − LVEDD3))) + 0.6 LV mass index = LV Mass/Body surface area | Transthoracic echocardiography: IVS: interventricular septum diameter (diastolic) PW: diameter of the posterior wall of the left ventricle (diastolic) | |
| Peak velocity of mitral E wave | Peak velocity of rapid passive left ventricular diastolic filling | Pulsed-wave doppler through the mitral valve, positioned at the level of the junction of the leaflets | |
| Peak velocity of mitral e’ medial or lateral wave | Peak velocity of the medial or lateral mitral annulus during rapid left ventricular diastolic filling | Tissue doppler waveform placed immediately above the lateral or medial section of the mitral annulus | |
| E/e’ ratio | Ratio of the peak velocities of the previously described waveforms | Ratio of the peak velocities of the previously described waveforms | |
| LVGLS | Left Ventricular Global Longitudinal Strain Measurement of left ventricular cardiac muscle deformation from basal to apical using the speckle tracking technique. | Transthoracic echocardiography, using 4-chamber, 2-chamber, and 3-chamber echocardiographic apical views. Cardiac Computed Tomography. | Decreased LVGLS prior to TAVR: Increased all-cause mortality and MACE. No increase in LVGLS after TAVR: Increased all-cause mortality and MACE. |
| ECV | Extracellular volume of the myocardium. Representation of the myocardial interstitial space may be an indicator of cardiac fibrosis. It has been associated in the field of HF with cardiac events such as mortality or admission for HF. | Cardiac Magnetic Resonance (T1 mapping) Cardiac Computed Tomography | Increased ECV prior to TAVR: Increased all-cause mortality and MACE. |
| LA strain | Left atrial deformation during the cardiac cycle measured by speckle tracking technology | Transthoracic echocardiography, using a 4-chamber apical view. | No increase in LA strain after TAVR: Increased all-cause mortality and MACE. |
| RVLS | Right ventricle myocardial deformation during the cardiac cycle measured by speckle tracking technology | Transthoracic echocardiography, using a 4-chamber apical view. | No increase in RVLS strain after TAVR: Increased all-cause mortality and MACE. |
| Author, Journal, Year | Population (Comparative Groups) | Endpoints Explored | Main Findings |
|---|---|---|---|
| Abramowitz et al., Am J Cardiol, 2015 [52] | 136 patients ≥ 90 years (mean age 92.4) vs. 598 < 90 years (mean age 79.7) | Procedural success, 30-day and 1-year mortality. Major and minor complications | Similar success, mortality, and major complications. Minor vascular complications were more frequent in nonagenarians. |
| Zadrozny et al., J Cardiol, 2021 [53] | 2336 patients (153 ≥ 90 years; 6.6%) vs. younger patients | Stroke, PPI, AKI, anemia, major bleeding, residual aortic insufficiency | No significant differences in periprocedural complications. |
| Liu et al., J Interv Cardiol, 2019 [54] | Meta-analysis of 5 studies: 25,371 ≥ 90 vs. 21,442 < 90 years | 30-day and 1-year mortality. Vascular complications, bleeding, stroke, PPI, AKI | Higher mortality, bleeding, stroke, and vascular events in the ≥90 group; Similar PPI and AKI. TAVR still superior to conservative treatment. |
| Demir et al., J Invasive Cardiol, 2022 [55] | Meta-analysis of 23 studies (2012–2019), 78,858 total; 16,094 ≥ 90 (20.4%) | Perioperative and 1-year mortality | Perioperative mortality 6.1% (lower than the STS-predicted 10%). One-year mortality 20.5% (better than 40% with conservative care). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-González, A.; Molero-de-Ávila, R.; Cedeño-Veloz, B.A.; Fernández-Jarne, E.; Lozano-Vicario, L.; Ramallal Martínez, R.; Martínez-Velilla, N.; Alonso Salinas, G.L. Transcatheter Aortic Valve Replacement in Older Adults: Integrating Cardiac Remodeling and Geriatric Syndromes—A Narrative Review. Medicina 2025, 61, 1515. https://doi.org/10.3390/medicina61091515
Fernández-González A, Molero-de-Ávila R, Cedeño-Veloz BA, Fernández-Jarne E, Lozano-Vicario L, Ramallal Martínez R, Martínez-Velilla N, Alonso Salinas GL. Transcatheter Aortic Valve Replacement in Older Adults: Integrating Cardiac Remodeling and Geriatric Syndromes—A Narrative Review. Medicina. 2025; 61(9):1515. https://doi.org/10.3390/medicina61091515
Chicago/Turabian StyleFernández-González, Andoni, Rodrigo Molero-de-Ávila, Bernardo Abel Cedeño-Veloz, Elena Fernández-Jarne, Lucia Lozano-Vicario, Raúl Ramallal Martínez, Nicolas Martínez-Velilla, and Gonzalo Luis Alonso Salinas. 2025. "Transcatheter Aortic Valve Replacement in Older Adults: Integrating Cardiac Remodeling and Geriatric Syndromes—A Narrative Review" Medicina 61, no. 9: 1515. https://doi.org/10.3390/medicina61091515
APA StyleFernández-González, A., Molero-de-Ávila, R., Cedeño-Veloz, B. A., Fernández-Jarne, E., Lozano-Vicario, L., Ramallal Martínez, R., Martínez-Velilla, N., & Alonso Salinas, G. L. (2025). Transcatheter Aortic Valve Replacement in Older Adults: Integrating Cardiac Remodeling and Geriatric Syndromes—A Narrative Review. Medicina, 61(9), 1515. https://doi.org/10.3390/medicina61091515








