Selective Advantage of NACT in Advanced Ovarian Cancer: A Retrospective Single-Centre Analysis
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EOC | Epithelial ovarian cancer |
| NACT | Neoadjuvant chemotherapy |
| IDS | Interval debulking surgery |
| PDS | Primary debulking surgery |
| FIGO | International Federation of Gynecology and Obstetrics |
| SCS | Surgical Complexity Score |
| PCI | Peritoneal Cancer Index |
| PFS | Progression-free Survival |
| OS | Overall survival |
| HR | Hazard ratio |
| R0 | Complete cytoreduction |
| R1 | Incomplete cytoreduction |
| ECOG | Eastern Cooperative Oncology Group |
| CA-125 | Cancer antigen-125 |
| CT | Computed tomography |
| OR | Odds ratio |
| CI | Confidence interval |
| SMD | Standardized mean differences |
| RR | Rate ratio |
| IRR | Incidence rate ratios |
| BMI | Body mass index |
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Prat, J. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int. J. Gynaecol. Obstet. 2014, 124, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Berchuck, A.; Backes, F.J.; Cohen, J.; Grisham, R.; Leath, C.A.; Martin, L.; Matei, D.; Miller, D.S.; Robertson, S.; et al. NCCN Guidelines® Insights: Ovarian Cancer/Fallopian Tube Cancer/Primary Peritoneal Cancer, Version 3.2024. J. Natl. Compr. Canc. Netw. 2024, 22, 512–519. [Google Scholar] [CrossRef]
- Colombo, N.; Sessa, C.; du Bois, A.; Ledermann, J.; McCluggage, W.G.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Vergote, I.; et al. ESMO-ESGO consensus conference recommendations on ovarian cancer. Ann. Oncol. 2019, 30, 672–705. [Google Scholar] [CrossRef]
- Kuusela, K.; Norppa, N.; Auranen, A.; Saarelainen, S. Maximal surgical effort increases the risk of postoperative complications in the treatment of advanced ovarian cancer. Eur. J. Surg. Oncol. 2022, 48, 2525–2530. [Google Scholar] [CrossRef]
- du Bois, A.; Reuss, A.; Pujade-Lauraine, E.; Harter, P.; Ray-Coquard, I.; Pfisterer, J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: A combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: By the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer 2009, 115, 1234–1244. [Google Scholar]
- Goff, B.A.; Matthews, B.J.; Larson, E.H.; Andrilla, C.H.; Wynn, M.; Lishner, D.M.; Baldwin, L.M. Predictors of comprehensive surgical treatment in patients with ovarian cancer. Cancer 2007, 109, 2031–2042. [Google Scholar] [CrossRef]
- Gaillard, S.; Lacchetti, C.; Armstrong, D.K.; Cliby, W.A.; Edelson, M.I.; Garcia, A.A.; Ghebre, R.G.; Gressel, G.M.; Lesnock, J.L.; Meyer, L.A.; et al. Neoadjuvant Chemotherapy for Newly Diagnosed, Advanced Ovarian Cancer: ASCO Guideline Update. J. Clin. Oncol. 2025, 43, 868–891. [Google Scholar] [CrossRef]
- Hou, J.; Kelly, M.; Yu, H.; Mcalpine, J.; Azodi, M.; Rutherford, T.; Schwartz, P. Neoadjuvant chemotherapy lessens surgical morbidity in advanced ovarian cancer and leads to improved survival in stage IV disease. Gynecol. Oncol. 2007, 105, 211–217. [Google Scholar] [CrossRef]
- Liu, J.; Jiao, X.; Gao, Q. Neoadjuvant chemotherapy-related platinum resistance. Drug Discov. Today 2020, 25, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.; Tropé, C.G.; Amant, F.; Kristensen, G.B.; Ehlen, T.; Johnson, N.; Verheijen, R.H.M. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N. Engl. J. Med. 2010, 363, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Kehoe, S.; Hook, J.; Nankivell, M.; Jayson, G.C.; Kitchener, H.; Lopes, T.; Luesley, D.; Perren, T.; Bannoo, S.; Mascarenhas, M.; et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): An open-label, randomised, controlled, non-inferiority trial. Lancet 2015, 386, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Fagotti, A.; Ferrandina, M.G.; Vizzielli, G.; Pasciuto, T.; Fanfani, F.; Gallotta, V.; Margariti, P.A.; Chiantera, V.; Costantini, B.; Alletti, S.G.; et al. Randomized trial of primary debulking surgery versus neoadjuvant chemotherapy for advanced epithelial ovarian cancer (SCORPION-NCT01461850). Int. J. Gynecol. Cancer 2020, 30, 1657–1664. [Google Scholar] [CrossRef]
- Onda, T.; Satoh, T.; Ogawa, G.; Saito, T.; Kasamatsu, T.; Nakanishi, T.; Mizutani, T.; Takehara, K.; Okamoto, A.; Ushijima, K.; et al. Comparison of survival between primary debulking surgery and neoadjuvant chemotherapy for stage III/IV ovarian, tubal and peritoneal cancers in phase III randomised trial. Eur. J. Cancer 2020, 130, 114–125. [Google Scholar] [CrossRef]
- Tate, S.; Nishikimi, K.; Matsuoka, A.; Otsuka, S.; Shozu, M. Highly Aggressive Surgery Benefits in Patients with Advanced Ovarian Cancer. Anticancer. Res. 2022, 42, 3707–3716. [Google Scholar] [CrossRef]
- Aytekin, O.; Kerinc, S.K.; Tokalioglu, A.A.; Ucar, Y.O.; Kilic, F.; Comert, G.K. Does neoadjuvant chemotherapy reduce surgical complexity in patients with advanced-stage epithelial ovarian cancer? BMC Womens Health 2024, 24, 435. [Google Scholar] [CrossRef]
- Aletti, G.D.; Dowdy, S.C.; Podratz, K.C.; Cliby, W.A. Relationship among surgical complexity, short-term morbidity, and overall survival in primary surgery for advanced ovarian cancer. Am. J. Obstet. Gynecol. 2007, 197, 676.e1–676.e7. [Google Scholar] [CrossRef]
- Lampe, B.; Kroll, N.; Piso, P.; Forner, D.M.; Mallmann, P. Prognostic significance of Sugarbaker’s peritoneal cancer index for the operability of ovarian carcinoma. Int. J. Gynecol. Cancer 2015, 25, 135–144. [Google Scholar] [CrossRef]
- Bryant, A.M.; Johnson, E.M.; Grayling, M.; Hiu, S.M.; Elattar, A.; Gajjar, K.; Craig, D.M.; Vale, L.; Naik, R. Residual Disease Threshold After Primary Surgical Treatment for Advanced Epithelial Ovarian Cancer, Part 1: A Systematic Review and Network Meta-Analysis. Am. J. Ther. 2023, 30, e36–e55. [Google Scholar] [CrossRef]
- Mueller, J.J.; Zhou, Q.C.; Iasonos, A.; O’CEarbhaill, R.E.; Alvi, F.A.; El Haraki, A.; Eriksson, A.G.Z.; Gardner, G.J.; Sonoda, Y.; Levine, D.A.; et al. Neoadjuvant chemotherapy and primary debulking surgery utilization for advanced-stage ovarian cancer at a comprehensive cancer center. Gynecol. Oncol. 2016, 140, 436–442. [Google Scholar] [CrossRef]
- Tozzi, R.; Giannice, R.; Cianci, S.; Tardino, S.; Campanile, R.G.; Gubbala, K.; Fachechi, G.; Ferrari, F.; Martinek, I.; Majd, H.S. Neo-adjuvant chemotherapy does not increase the rate of complete resection and does not significantly reduce the morbidity of Visceral-Peritoneal Debulking (VPD) in patients with stage IIIC-IV ovarian cancer. Gynecol. Oncol. 2015, 138, 252–258. [Google Scholar] [CrossRef]
- Ghirardi, V.; Moruzzi, M.; Bizzarri, N.; Vargiu, V.; D’INdinosante, M.; Garganese, G.; Pasciuto, T.; Loverro, M.; Scambia, G.; Fagotti, A. Minimal residual disease at primary debulking surgery versus complete tumor resection at interval debulking surgery in advanced epithelial ovarian cancer: A survival analysis. Gynecol. Oncol. 2020, 157, 209–213. [Google Scholar] [CrossRef]
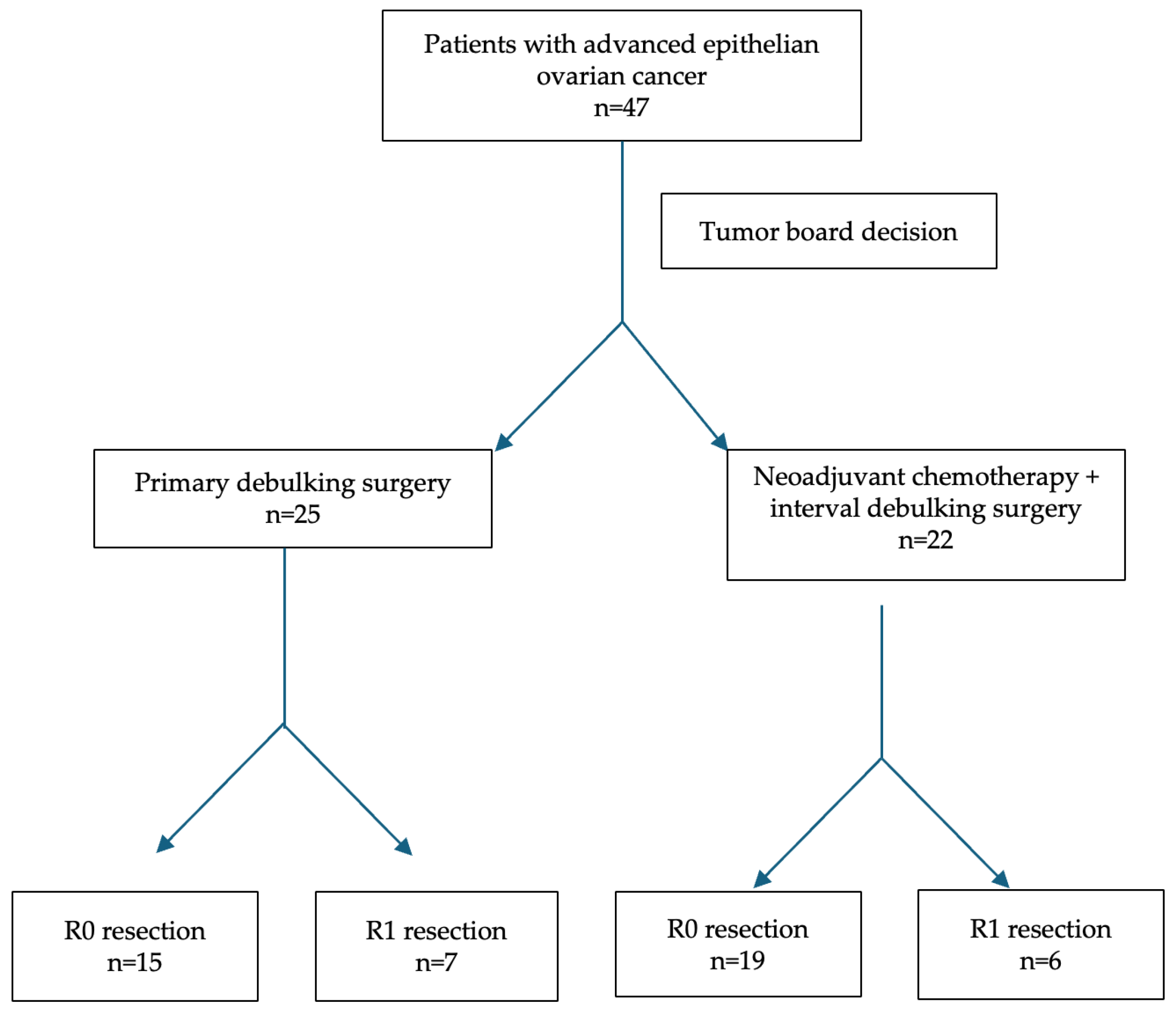
| Variable | NACT + IDS (n = 25) | PDS (n = 22) | p-Value |
|---|---|---|---|
| Mean age, years | 61.4 ± 12.4 | 57.8 ± 13.1 | 0.34 |
| BMI (kg/m2) | 23.6 ± 3.5 | 24.8 ± 5.4 | 0.37 |
| CA-125 (U/mL), pre-treatment | 1329.8 ± 1025.7 | 590.4 ± 1235.5 | 0.03 |
| CA-125 (U/mL), post-treatment | 111.2 ± 218.3 | 132.4 ± 486.2 | 0.85 |
| Surgical Procedure | NACT + IDS (n = 25), n (%) | PDS (n = 22), n (%) |
|---|---|---|
| Wedge resection (liver) | 0 (0%) | 1 (5%) |
| Stripping diaphragm/ falciform ligament | 5 (20%) | 5 (23%) |
| Splenectomy | 0 (0%) | 1 (5%) |
| Mesenteric resection | 5 (20%) | 3 (14%) |
| Large bowel resection | 2 (8%) | 4 (18%) |
| Small bowel resection | 1 (4%) | 2 (9%) |
| Ileocecal resection/ right hemicolectomy | 1 (4%) | 0 (0%) |
| Appendectomy | 2 (8%) | 7 (32%) |
| Stoma formation | 1 (4%) | 1 (5%) |
| Lesser omentum/ stomach resection | 4 (16%) | 1 (5%) |
| Cholecystectomy | 1 (4%) | 0 (0%) |
| Pelvic peritonectomy | 17 (68%) | 15 (62%) |
| Bladder peritonectomy | 9 (36%) | 9 (41%) |
| Upper abdominal peritonectomy | 9 (36%) | 5 (22%) |
| Retroperitoneal wall resection | 2 (8%) | 1 (5%) |
| Para-aortic lymph node dissection | 3 (12%) | 3 (14%) |
| Pelvic lymph node dissection | 6 (24%) | 5 (23%) |
| Ureter resection | 1 (4%) | 0 (0%) |
| Total number of procedures performed | 69 | 63 |
| Variable | NACT + IDS (n = 25) | PDS (n = 22) | p-Value | SMD | Effect Size |
|---|---|---|---|---|---|
| Oncologic characteristics | |||||
| R0, n (%) | 19 (76) | 15 (68) | 0.75 | +0.17 | OR 1.48 (0.41–5.33) |
| R1, n (%) | 6 (24) | 7 (32) | 0.75 | −0.17 | OR 0.68 (0.19–2.44) |
| FIGO stage III, n (%) | 17 (68) | 20 (90) | 0.08 | −0.56 | OR 0.21 (0.04–1.14) |
| FIGO stage IV, n (%) | 8 (32) | 2 (9) | 0.08 | +0.56 | OR 4.71 (0.88–25.22) |
| Surgical parameters | |||||
| Surgical Complexity Score (mean ± SD) | 5.0 ± 1.3 | 6.2 ± 2.4 | 0.04 | −0.62 | Cohen’s d = −0.63 |
| Low SCS (1–3), n (%) | 5 (20) | 1 (4.5) | |||
| Intermediate SCS (4–7), n (%) | 16 (64) | 7 (31.8) | |||
| High SCS (≥8), n (%) | 4 (16) | 14 (63.6) | 0.001 | – | OR 0.11 (0.03–0.43) |
| Peritoneal Cancer Index (mean ± SD) | 6.1 ± 3.4 | 7.7 ± 3.7 | 0.13 | −0.45 | Cohen’s d = −0.45 |
| Operating time, min (mean ± SD) | 140 ± 41.8 | 196.6 ± 63.3 | 0.001 | −1.06 | Cohen’s d = −1.06 |
| Estimated blood loss, mL (mean ± SD) | 242 ± 126.4 | 256.8 ± 114.7 | 0.68 | −0.12 | Cohen’s d = −0.12 |
| Perioperative outcomes | |||||
| Hospital stay, days (mean ± SD) | 6.8 ± 4.5 | 7.4 ± 1.9 | 0.55 | −0.17 | Cohen’s d = −0.17 |
| Clavien–Dindo score, mean ± SD | 1.8 ± 0.7 | 1.9 ± 0.7 | 0.63 | −0.14 | Cohen’s d = −0.14 |
| Clavien–Dindo I–II/III–IV, n/n | 23/2 | 20/2 | 1.00 | – | OR 1.15 (0.15–8.93) |
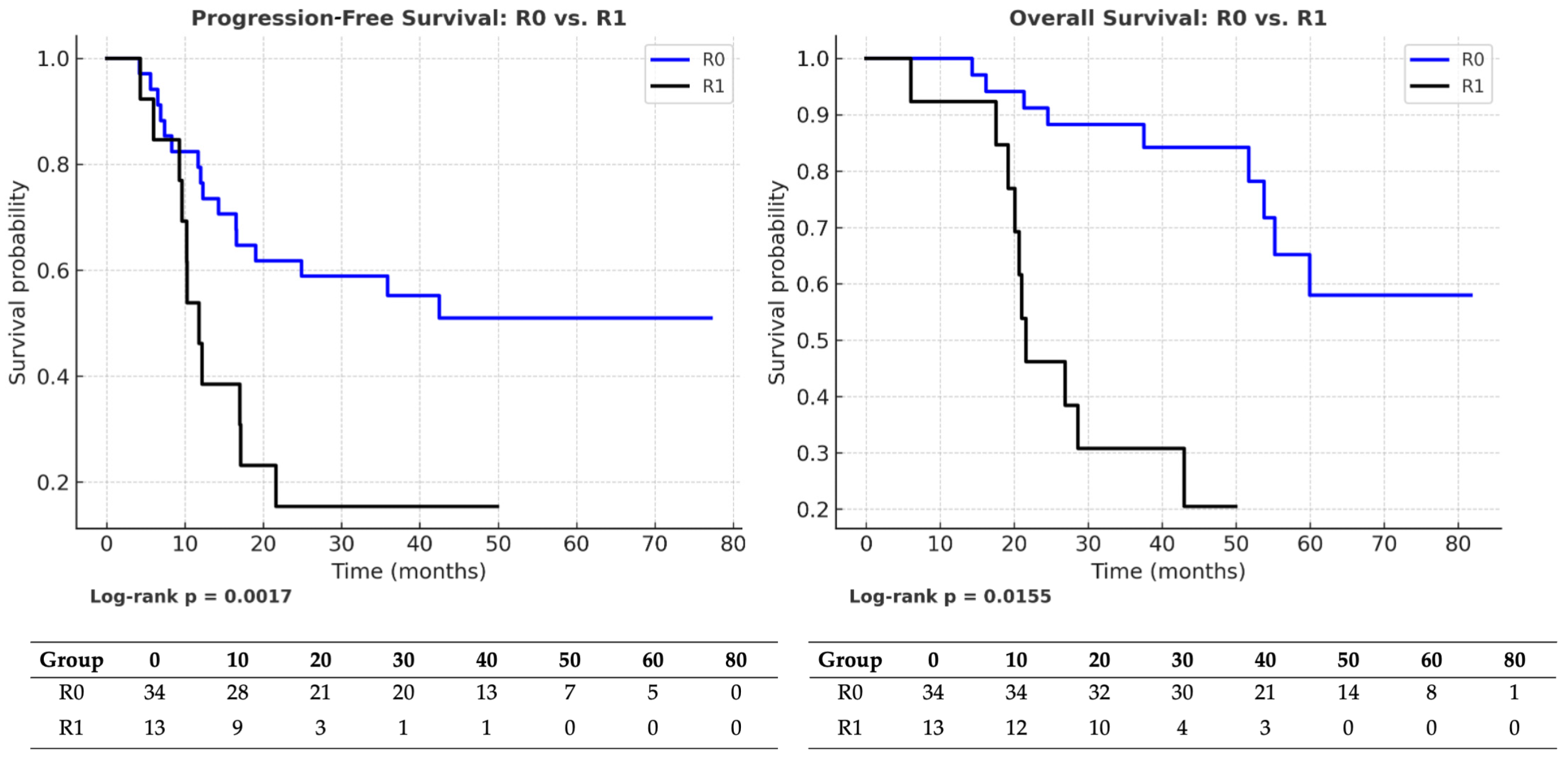
| Comparison | Group/Outcome | HR (95% CI) | p-Value | Median (Months) | Events/Total (Censoring %) |
|---|---|---|---|---|---|
| R0 → R1 | PFS (entire cohort) | 0.30 (0.14–0.64) | 0.0017 | R0: 36.5 vs. R1: 12 | R0: 18/34 (47%) vs. R1: 11/13 (15%) |
| OS (entire cohort) | 0.39 (0.18–0.84) | 0.0155 | R0: 40.5 vs. R1: 22 | R0: 17/34 (50%) vs. R1: 11/13 (15%) | |
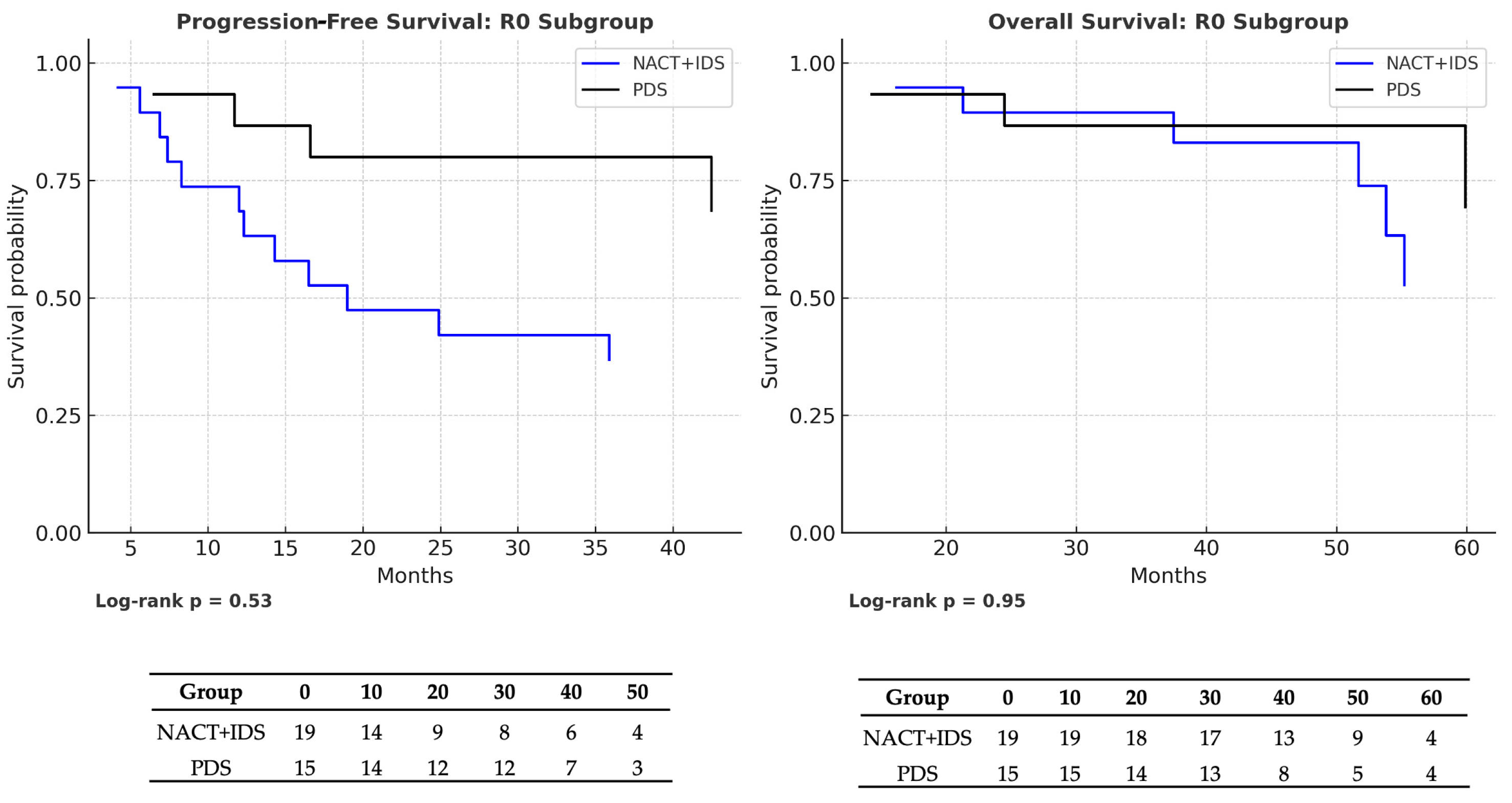
| NACT → PDS (R0 subgroup) | PFS | 1.17 (0.45–3.01) | 0.53 | NACT: 39 vs. PDS: 38 | NACT: 10/19 (47.4%) vs. PDS: 8/15 (46.7%) |
| OS | 0.99 (0.38–2.60) | 0.95 | NACT: 54 vs. PDS: 48 | NACT: 9/19 (52.6%) vs. PDS: 8/15 (46.7%) | |
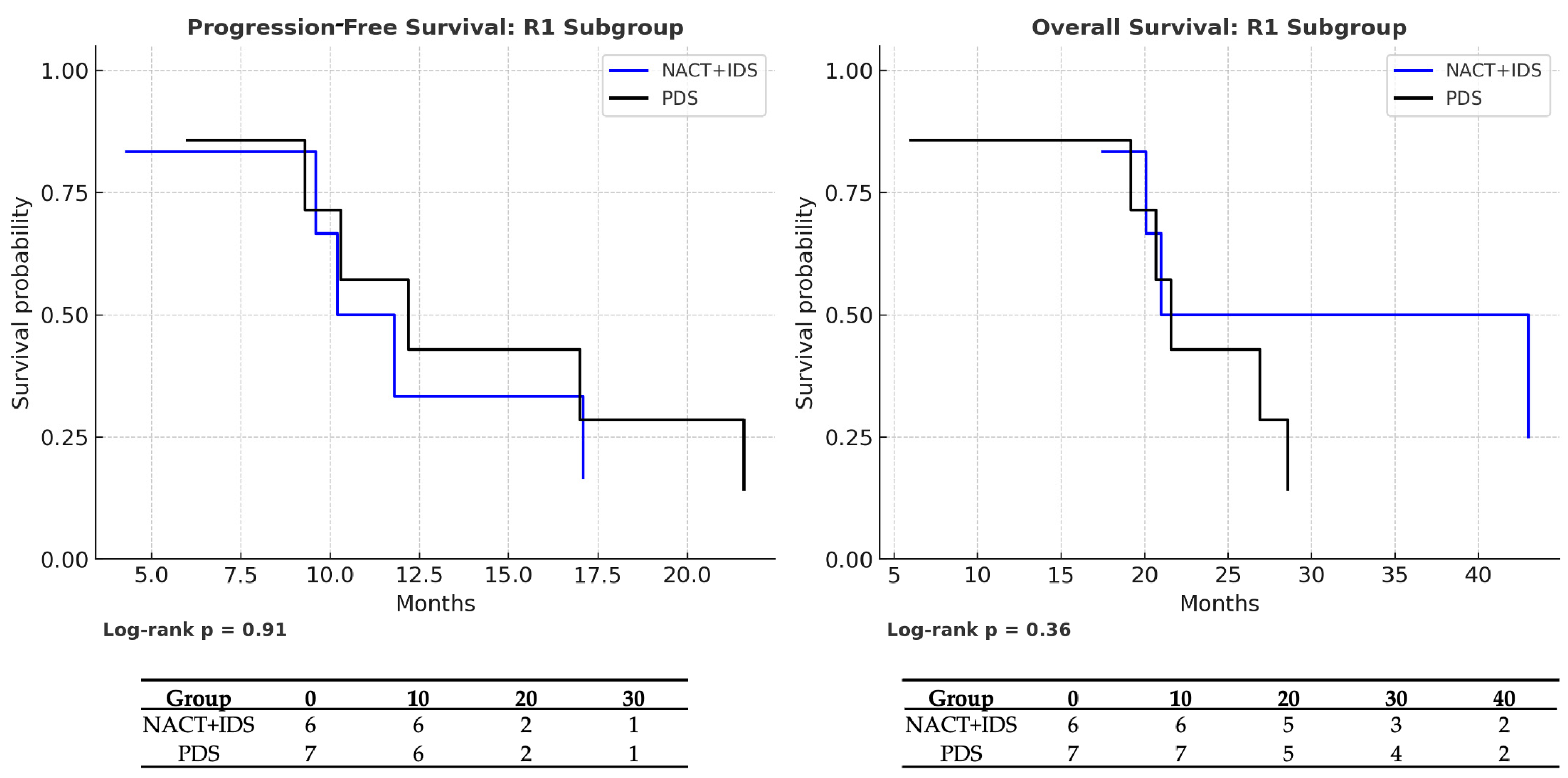
| NACT → PDS (R1 subgroup) | PFS | 0.85 (0.26–2.78) | 0.91 | NACT: 10 vs. PDS: 12 | NACT: 5/6 (16.7%) vs. PDS: 6/7 (14.3%) |
| OS | 0.53 (0.15–1.81) | 0.36 | NACT: 21 vs. PDS: 22 | NACT: 5/6 (16.7%) vs. PDS: 6/7 (14.3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berczi, A.S.; Lampé, O.; Krasznai, Z.T.; Orosz, M.; Fábián, L.; Lampé, R. Selective Advantage of NACT in Advanced Ovarian Cancer: A Retrospective Single-Centre Analysis. Medicina 2025, 61, 1493. https://doi.org/10.3390/medicina61081493
Berczi AS, Lampé O, Krasznai ZT, Orosz M, Fábián L, Lampé R. Selective Advantage of NACT in Advanced Ovarian Cancer: A Retrospective Single-Centre Analysis. Medicina. 2025; 61(8):1493. https://doi.org/10.3390/medicina61081493
Chicago/Turabian StyleBerczi, Adrienne Szilvia, Olivér Lampé, Zoárd Tibor Krasznai, Mónika Orosz, Lili Fábián, and Rudolf Lampé. 2025. "Selective Advantage of NACT in Advanced Ovarian Cancer: A Retrospective Single-Centre Analysis" Medicina 61, no. 8: 1493. https://doi.org/10.3390/medicina61081493
APA StyleBerczi, A. S., Lampé, O., Krasznai, Z. T., Orosz, M., Fábián, L., & Lampé, R. (2025). Selective Advantage of NACT in Advanced Ovarian Cancer: A Retrospective Single-Centre Analysis. Medicina, 61(8), 1493. https://doi.org/10.3390/medicina61081493








