A Single Bout of Foam Rolling After Nordic Hamstring Exercise Improves Flexibility but Has No Effect on Muscle Stiffness or Functional Muscle Parameters
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
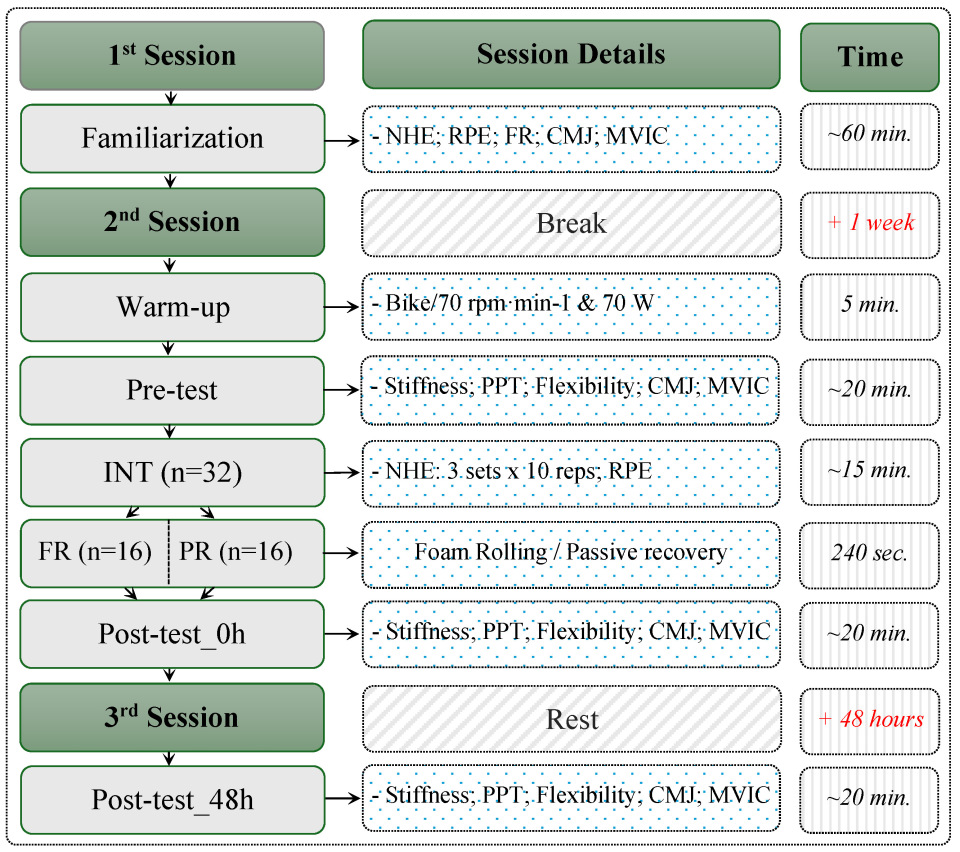
2.2. Study Design
2.3. Procedures
2.3.1. Tests
2.3.2. Nordic Hamstring Exercise (NHE)
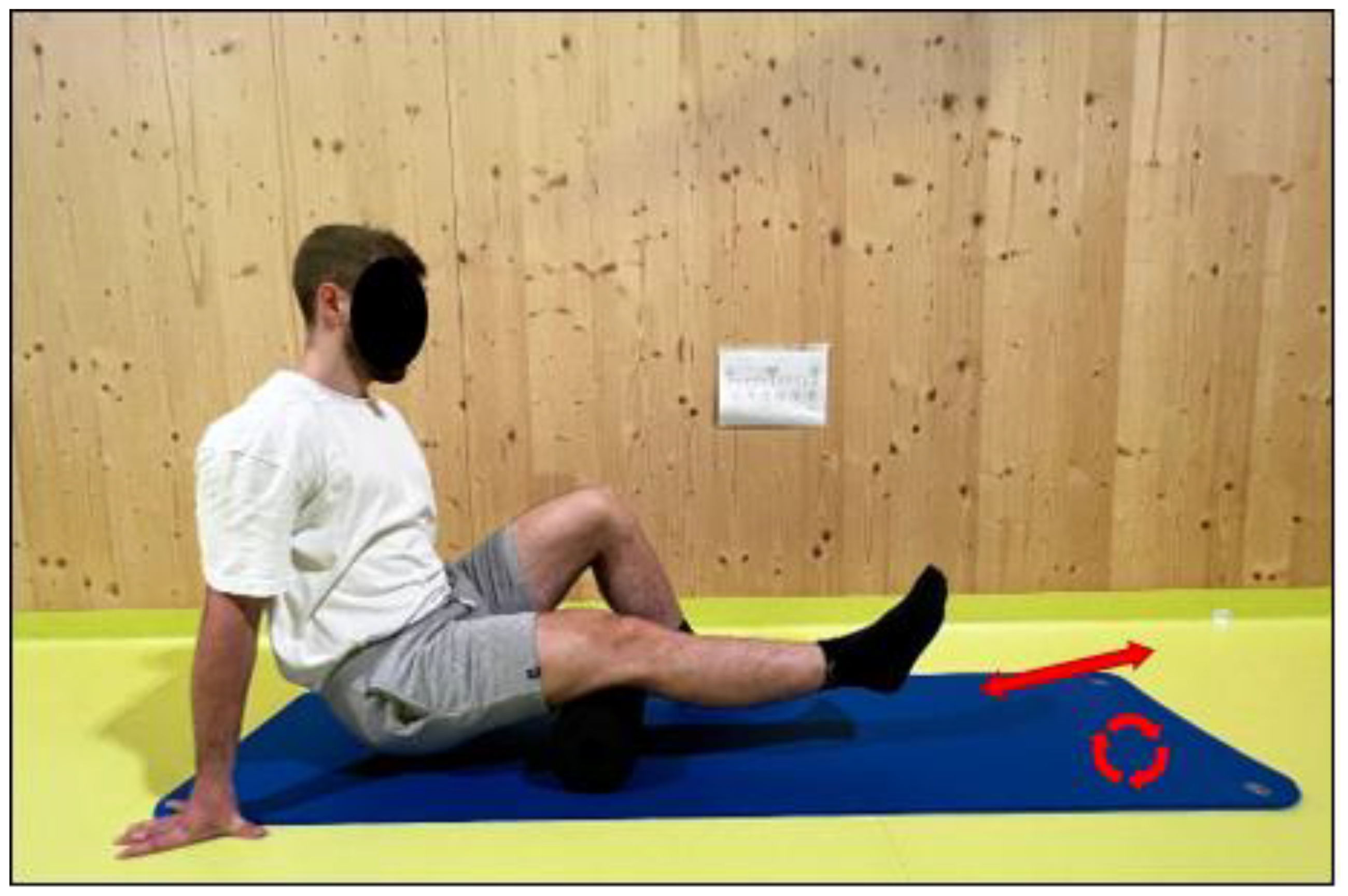
2.3.3. Recovery
2.4. Statistical Analyses
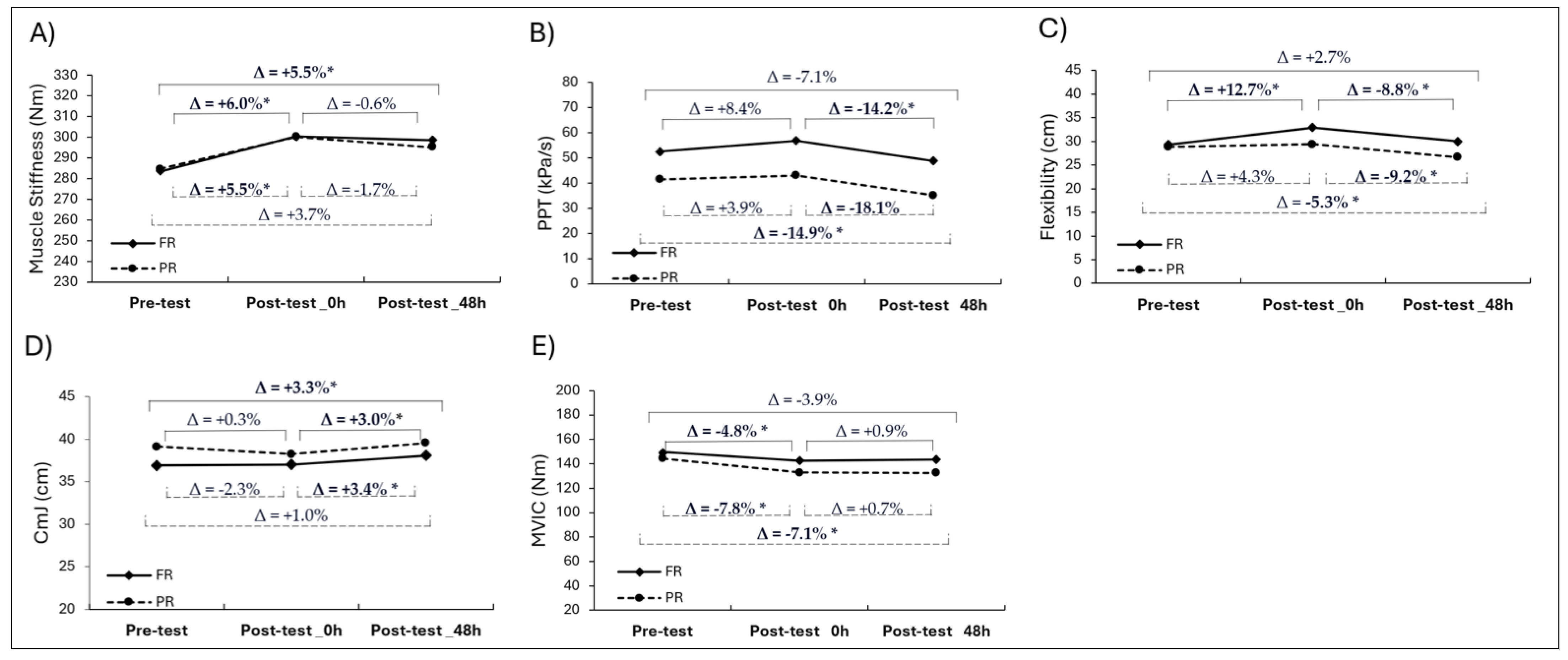
3. Results
3.1. Muscle Stiffness
3.2. Pain Pressure Threshold (PPT)
3.3. Flexibility
3.4. Countermovement Jump (CmJ) Height
3.5. Maximal Voluntary Isometric Contraction (MVIC) Peak Torque
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| CmJ | Countermovement jump |
| DOMS | Delayed onset muscle soreness |
| FR | Foam rolling |
| MVIC | Maximum voluntary isometric contraction |
| NHE | Nordic hamstring exercise |
| PPT | Pain pressure threshold |
| PR | Passive recovery |
| ROM | Range of motion |
| VAS | Visual analog scale |
References
- Petersen, J.; Thorborg, K.; Nielsen, M.B.; Budtz-Jørgensen, E.; Hölmich, P. Preventive Effect of Eccentric Training on Acute Hamstring Injuries in Men’s Soccer: A cluster-randomized controlled trial. Am. J. Sports Med. 2011, 39, 2296–2303. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Mampel, J.; Bautista, I.J.; Martín, F.; Maroto-Izquierdo, S.; Van Hooren, B.; Baraja-Vegas, L. Effects of ankle position during the Nordic Hamstring exercise on range of motion, heel contact force and hamstring muscle activation. Sports Biomech. 2022, 23, 2359–2371. [Google Scholar] [CrossRef] [PubMed]
- McHugh, M.P.; Connolly, D.A.J.; Eston, R.G.; Gleim, G.W. Exercise-Induced Muscle Damage and Potential Mechanisms for the Repeated Bout Effect. Sports Med. 1999, 27, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Seymore, K.D.; Domire, Z.J.; DeVita, P.; Rider, P.M.; Kulas, A.S. The effect of Nordic hamstring strength training on muscle architecture, stiffness, and strength. Eur. J. Appl. Physiol. 2017, 117, 943–953. [Google Scholar] [CrossRef]
- Nakamura, M.; Onuma, R.; Kiyono, R.; Yasaka, K.; Sato, S.; Yahata, K.; Fukaya, T.; Konrad, A. The Acute and Prolonged Effects of Different Durations of Foam Rolling on Range of Motion, Muscle Stiffness, and Muscle Strength. J. Sports Sci. Med. 2021, 20, 62–68. [Google Scholar] [CrossRef]
- Bestwick-Stevenson, T.; Toone, R.; Neupert, E.; Edwards, K.; Kluzek, S. Assessment of Fatigue and Recovery in Sport: Narrative Review. Int. J. Sports Med. 2022, 43, 1151–1162. [Google Scholar] [CrossRef]
- Reiner, M.M.; Glashüttner, C.; Bernsteiner, D.; Tilp, M.; Guilhem, G.; Morales-Artacho, A.; Konrad, A. A comparison of foam rolling and vibration foam rolling on the quadriceps muscle function and mechanical properties. Eur. J. Appl. Physiol. 2021, 121, 1461–1471. [Google Scholar] [CrossRef]
- Altarriba-Bartes, A.; Peña, J.; Vicens-Bordas, J.; Milà-Villaroel, R.; Calleja-González, J.; Marocolo, M. Post-competition recovery strategies in elite male soccer players. Effects on performance: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0240135. [Google Scholar] [CrossRef]
- Freiwald, J.; Baumgart, C.; Kühnemann, M.; Hoppe, M.W. Foam-Rolling in sport and therapy—Potential benefits and risks: Part 1—Definitions, Anatomy, Physiology, and Biomechanics. Sports Orthop. Traumatol. 2016, 32, 258–266. [Google Scholar] [CrossRef]
- Pablos, A.; Ceca, D.; Jorda, A.; Rivera, P.; Colmena, C.; Elvira, L.; Martínez-Arnau, F.M.; Valles, S.L. Protective Effects of Foam Rolling against Inflammation and Notexin Induced Muscle Damage in Rats. Int. J. Med. Sci. 2020, 17, 71–81. [Google Scholar] [CrossRef]
- Konrad, A.; Nakamura, M.; Paternoster, F.K.; Tilp, M.; Behm, D.G. A comparison of a single bout of stretching or foam rolling on range of motion in healthy adults. Eur. J. Appl. Physiol. 2022, 122, 1545–1557. [Google Scholar] [CrossRef]
- Behm, D.G.; Wilke, J. Do Self-Myofascial Release Devices Release Myofascia? Rolling Mechanisms: A Narrative Review. Sports Med. 2019, 49, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Yasaka, K.; Kiyono, R.; Onuma, R.; Yahata, K.; Sato, S.; Konrad, A. The Acute Effect of Foam Rolling on Eccentrically-Induced Muscle Damage. Int. J. Environ. Res. Public Health 2020, 18, 75. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, K.; Konrad, A.; Yoshida, R.; Murakami, Y.; Sato, S.; Aizawa, K.; Koizumi, R.; Thomas, E.; Nakamura, M. Comparison between 6-week foam rolling intervention program with and without vibration on rolling and non-rolling sides. Eur. J. Appl. Physiol. 2022, 122, 2061–2070. [Google Scholar] [CrossRef] [PubMed]
- Pearcey, G.E.P.; Bradbury-Squires, D.J.; Kawamoto, J.-E.; Drinkwater, E.J.; Behm, D.G.; Button, D.C. Foam Rolling for Delayed-Onset Muscle Soreness and Recovery of Dynamic Performance Measures. J. Athl. Train. 2015, 50, 5–13. [Google Scholar] [CrossRef]
- Kiyono, R.; Onuma, R.; Yasaka, K.; Sato, S.; Yahata, K.; Nakamura, M. Effects of 5-week foam rolling intervention on range of motion and muscle stiffness. J. Strength Cond. Res. 2022, 36, 1890–1895. [Google Scholar] [CrossRef]
- Cheatham, S.W.; Kolber, M.J.; Cain, M.; Lee, M. The effects of self-myofascial release using a foam roll or roller massager on joint range of motion, muscle recovery, and performance: A systematic review. Int. J. Sports Phys. Ther. 2015, 10, 827–838. [Google Scholar]
- Kasahara, K.; Yoshida, R.; Yahata, K.; Sato, S.; Murakami, Y.; Aizawa, K.; Konrad, A.; Nakamura, M. Comparison of the Acute Effects of Foam Rolling with High and Low Vibration Frequencies on Eccentrically Damaged Muscle. J. Sports Sci. Med. 2022, 21, 112–119. [Google Scholar] [CrossRef]
- Nakamura, M.; Kasahara, K.; Yoshida, R.; Yahata, K.; Sato, S.; Murakami, Y.; Aizawa, K.; Konrad, A. The Effect of Static Compression via Vibration Foam Rolling on Eccentrically Damaged Muscle. Int. J. Environ. Res. Public Health 2022, 19, 1823. [Google Scholar] [CrossRef]
- Wilke, J.; Müller, A.-L.; Giesche, F.; Power, G.; Ahmedi, H.; Behm, D.G. Acute Effects of Foam Rolling on Range of Motion in Healthy Adults: A Systematic Review with Multilevel Meta-analysis. Sports Med. 2020, 50, 387–402. [Google Scholar] [CrossRef]
- Couture, G.; Karlik, D.; Glass, S.C.; Hatzel, B.M. The Effect of Foam Rolling Duration on Hamstring Range of Motion. Open Orthop. J. 2015, 9, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Griefahn, A.; Oehlmann, J.; Zalpour, C.; von Piekartz, H. Do exercises with the Foam Roller have a short-term impact on the thoracolumbar fascia?—A randomized controlled trial. J. Bodyw. Mov. Ther. 2017, 21, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Junker, D.; Stöggl, T. The training effects of foam rolling on core strength endurance, balance, muscle performance, and range of motion: A randomized controlled trial. J. Sports Sci. Med. 2019, 8, 229–238. [Google Scholar] [PubMed]
- Konrad, A.; Nakamura, M.; Tilp, M.; Donti, O.; Behm, D.G. Foam Rolling Training Effects on Range of Motion: A Systematic Review and Meta-Analysis. Sports Med. 2022, 52, 2523–2535. [Google Scholar] [CrossRef]
- Pérez-Bellmunt, A.; Casasayas-Cos, O.; Ragazzi, P.; Rodríguez-Sanz, J.; Hidalgo-García, C.; Canet-Vintró, M.; Caballero-Martínez, I.; Pacheco, L.; López-De-Celis, C. Foam Rolling vs. Proprioceptive Neuromuscular Facilitation Stretching in the Hamstring Flexibility of Amateur Athletes: Control Trials. Int. J. Environ. Res. Public Health 2023, 20, 1439. [Google Scholar] [CrossRef]
- Skinner, B.; Dunn, L.; Moss, R. The Acute Effects of Theragun™ Percussive Therapy on Viscoelastic Tissue Dynamics and Hamstring Group Range of Motion. J. Sports Sci. Med. 2023, 22, 496–501. [Google Scholar] [CrossRef]
- Canadian Society for Exercise Physiology. Physical Activity and Readiness Questionnaire: PAR-q & You. 2002. Available online: https://lattimorefitness.com/wp-content/uploads/2023/09/ParQForm-1.pdf (accessed on 17 August 2025).
- Konrad, A.; Seiberl, W.; Tilp, M.; Holzer, D.; Paternoster, F.K. What to stretch?—Isolated proprioceptive neuromuscular facilitation stretching of either quadriceps or triceps surae followed by post-stretching activities alters tissue stiffness and jump performance. Sports Biomech. 2022, 23, 2798–2815. [Google Scholar] [CrossRef]
- Konrad, A.; Paternoster, F.K. No Association between Jump Parameters and Tissue Stiffness in the Quadriceps and Triceps Surae Muscles in Recreationally Active Young Adult Males. Appl. Sci. 2022, 12, 1596. [Google Scholar] [CrossRef]
- Dellalana, L.E.; Chen, F.; Vain, A.; Gandelman, J.S.; Põldemaa, M.; Chen, H.; Tkaczyk, E.R. Reproducibility of the durometer and myoton devices for skin stiffness measurement in healthy subjects. Skin Res. Technol. 2018, 25, 289–293. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Hopkinson, L.D.; Nolet, P.S.; Srbely, J. The reliability of pressure pain threshold in individuals with low back or neck pain: A systematic review. Br. J. Pain 2023, 17, 579–591. [Google Scholar] [CrossRef]
- Reiner, M.M.; Tilp, M.; Guilhem, G.; Morales-Artacho, A.; Konrad, A. Comparison of A Single Vibration Foam Rolling and Static Stretching Exercise on the Muscle Function and Mechanical Properties of the Hamstring Muscles. J. Sports Sci. Med. 2022, 21, 287–297. [Google Scholar] [CrossRef]
- Konrad, A.; Bernsteiner, D.; Budini, F.; Reiner, M.M.; Glashüttner, C.; Berger, C.; Tilp, M. Tissue flossing of the thigh increases isometric strength acutely but has no effects on flexibility or jump height. Eur. J. Sport Sci. 2020, 21, 1648–1658. [Google Scholar] [CrossRef]
- Konrad, A.; Reiner, M.; Bernsteiner, D.; Glashüttner, C.; Thaller, S.; Tilp, M. Joint Flexibility and Isometric Strength Parameters Are Not Relevant Determinants for Countermovement Jump Performance. Int. J. Environ. Res. Public Health 2021, 18, 2510. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Kim, S.-H.; Park, D.-J. Effect of slope angle on muscle activity during variations of the Nordic exercise. J. Exerc. Rehabilitation 2019, 15, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Behm, D.G.; Alizadeh, S.; Anvar, S.H.; Mahmoud, M.M.I.; Ramsay, E.; Hanlon, C.; Cheatham, S. Foam Rolling Prescription: A Clinical Commentary. J. Strength Cond. Res. 2020, 34, 3301–3308. [Google Scholar] [CrossRef] [PubMed]
- Sulowska-Daszyk, I.; Skiba, A. The Influence of Self-Myofascial Release on Muscle Flexibility in Long-Distance Runners. Int. J. Environ. Res. Public Health 2022, 19, 457. [Google Scholar] [CrossRef]
- Konrad, A.; Tilp, M. Increased range of motion after static stretching is not due to changes in muscle and tendon structures. Clin. Biomech. 2014, 29, 636–642. [Google Scholar] [CrossRef]
- Hedley, G. Notes on visceral adhesions as fascial pathology. J. Bodyw. Mov. Ther. 2010, 14, 255–261. [Google Scholar] [CrossRef]
- Monte, A.; Zignoli, A. Muscle and tendon stiffness and belly gearing positively correlate with rate of torque development during explosive fixed end contractions. J. Biomech. 2021, 114, 110110. [Google Scholar] [CrossRef]
- Hotfiel, T.; Freiwald, J.; Hoppe, M.W.; Lutter, C.; Forst, R.; Grim, C.; Bloch, W.; Hüttel, M.; Heiss, R. Advances in Delayed-Onset Muscle Soreness (DOMS): Part I: Pathogenesis and Diagnostics. Sportverletz. Sportschaden 2018, 32, 243–250. [Google Scholar] [CrossRef]
- Hosseinzadeh, M.; Andersen, O.K.; Arendt-Nielsen, L.; Madeleine, P. Pain sensitivity is normalized after a repeated bout of eccentric exercise. Eur. J. Appl. Physiol. 2013, 113, 2595–2602. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, G.Z.; Button, D.C.; Drinkwater, E.J.; Behm, D.G. Foam Rolling as a Recovery Tool after an Intense Bout of Physical Activity. Med. Sci. Sports Exerc. 2014, 46, 131–142. [Google Scholar] [CrossRef]
- Connolly, D.A.; Sayers, S.P.; McHugh, M.P. Treatment and prevention of delayed onset muscle soreness. J. Strength Cond. Res. 2003, 17, 197–208. [Google Scholar] [CrossRef]
- Rolli, F.; Vitale, J.A.; Pugliese, L.; Boccia, G.; LA Torre, A.; Pollitt, L. The impact of foot angle on lower limb muscles activity during the back squat and counter movement jump. J. Sports Med. Phys. Fit. 2021, 62, 890–897. [Google Scholar] [CrossRef]
- Silva, A.F.; Nobari, H.; Badicu, G.; Ceylan, H.I.; Lima, R.; Lagoa, M.J.; Luz, C.; Clemente, F.M. Reliability levels of motor competence in youth athletes. BMC Pediatr. 2022, 22, 430. [Google Scholar] [CrossRef]
- Proske, U.; Morgan, D.L. Muscle damage from eccentric exercise: Mechanism, mechanical signs, adaptation and clinical applications. J. Physiol. 2001, 537 Pt 2, 333–345. [Google Scholar] [CrossRef]
- Ducrocq, G.P.; AL Assad, S.H.; Kouzkouz, N.; Hureau, T.J. The Role of Contraction Mode in Determining Exercise Tolerance, Torque–Duration Relationship, and Neuromuscular Fatigue. Med. Sci. Sports Exerc. 2023, 55, 1218–1231. [Google Scholar] [CrossRef]
- Sullivan, K.M.; Silvey, D.B.J.; Button, D.C.; Behm, D.G. Roller-massager application to the hamstrings increases sit-and-reach range of motion within five to ten seconds without performance impairments. Int. J. Sports Phys. Ther. 2013, 8, 228–236. [Google Scholar] [PubMed Central]
- Bobbert, M.F.; Van Soest, A.J. Why Do People Jump the Way They Do? Exerc. Sport Sci. Rev. 2001, 29, 95–102. [Google Scholar] [CrossRef]

| Variable | FR | PR | ||||
|---|---|---|---|---|---|---|
| Mean (SD) | Min | Max | Mean (SD) | Min | Max | |
| Age (years) | 23.7 (3.7) | 18 | 29 | 22.8 (3.5) | 18 | 29 |
| Height (cm) | 178.2 (5.3) | 170 | 192 | 179.7 (6.7) | 168 | 195 |
| Weight (kg) | 75.0 (10.3) | 52 | 91 | 75.7 (13.1) | 60 | 114 |
| BMI (kg/m2) | 21.0 (2.7) | 15 | 24 | 21.0 (3.2) | 16 | 30 |
| RPE | 18.0 (0.8) | 17 | 19 | 18.2 (0.8) | 17 | 20 |
| PA (MET-min/wk) | 3997.3 (2070.1) | 1272 | 9378 | 4462.8 (2332.7) | 1775 | 10.344 |
| Variable | Time | FR | PR | ||
|---|---|---|---|---|---|
| Mean (SD) | [95% CI] | Mean (SD) | [95% CI] | ||
| Stiffness (Nm) | Pre-test | 283.5 (32.1) ab | [266.4–300.1] | 284.6 (37.3) a | [264.7–304.6] |
| Post-test_0h | 300.4 (29.6) | [284.6–316.2] | 300.2 (49.2) | [274.0–326.4] | |
| Post-test_48h | 298.6 (27.3) | [284.0–313.1] | 295.2 (39.8) | [274.0–316.4] | |
| PPT (kPa/s) | Pre-test | 52.5 (19.1) | [42.3–62.7] | 41.5 (14.0) b | [34.0–49.0] |
| Post-test_0h | 56.9 (17.5) b | [47.6–66.2] | 43.1 (15.2) b | [35.0–51.2] | |
| Post-test_48h | 48.8 (17.7) | [39.4–58.2] | 35.3 (13.7) | [28.0–42.6] | |
| Flexibility (cm) | Pre-test | 29.2 (8.9) a | [24.5–34.0] | 28.2 (8.2) b | [24.4–33.2] |
| Post-test_0h | 32.9 (8.2) b | [28.6–37.3] | 29.4 (8.1) b | [25.1–33.6] | |
| Post-test_48h | 30.0 (8.8) | [25.3–34.6] | 26.7 (8.6) | [22.1–31.2] | |
| CmJ (cm) | Pre-test | 36.9 (4.3) b | [34.4–39.4] | 39.1 (5.4) | [36.6–41.6] |
| Post-test_0h | 37.0 (4.1) b | [34.7–39.3] | 38.2 (4.7) b | [36.0–40.6] | |
| Post-test_48h | 38.1 (4.0) | [35.7–40.2] | 39.5 (5.1) | [37.2–41.9] | |
| MVIC (Nm) | Pre-test | 149.6 (30.6) | [133.3–166.0] | 144.2 (32.5) ab | [129.5–159.2] |
| Post-test_0h | 142.4 (29.0) | [127.0–157.9] | 133.0 (33.7) | [117.0–148.8] | |
| Post-test_48h | 143.7 (30.6) | [127.2–160.0] | 134.0 (30.1) | [120.6–148.1] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodoplu, C.; Burger, C.; Fischer, J.; Manieu Seguel, J.; Arabacı, R.; Konrad, A. A Single Bout of Foam Rolling After Nordic Hamstring Exercise Improves Flexibility but Has No Effect on Muscle Stiffness or Functional Muscle Parameters. Medicina 2025, 61, 1486. https://doi.org/10.3390/medicina61081486
Rodoplu C, Burger C, Fischer J, Manieu Seguel J, Arabacı R, Konrad A. A Single Bout of Foam Rolling After Nordic Hamstring Exercise Improves Flexibility but Has No Effect on Muscle Stiffness or Functional Muscle Parameters. Medicina. 2025; 61(8):1486. https://doi.org/10.3390/medicina61081486
Chicago/Turabian StyleRodoplu, Coşkun, Christian Burger, Josef Fischer, Josefina Manieu Seguel, Ramiz Arabacı, and Andreas Konrad. 2025. "A Single Bout of Foam Rolling After Nordic Hamstring Exercise Improves Flexibility but Has No Effect on Muscle Stiffness or Functional Muscle Parameters" Medicina 61, no. 8: 1486. https://doi.org/10.3390/medicina61081486
APA StyleRodoplu, C., Burger, C., Fischer, J., Manieu Seguel, J., Arabacı, R., & Konrad, A. (2025). A Single Bout of Foam Rolling After Nordic Hamstring Exercise Improves Flexibility but Has No Effect on Muscle Stiffness or Functional Muscle Parameters. Medicina, 61(8), 1486. https://doi.org/10.3390/medicina61081486







