The Effects of Hyaluronic Acid on Gait Parameters in Patients with Knee Osteoarthritis: A Systematic Literature Review
Abstract
1. Introduction
2. Materials and Methods
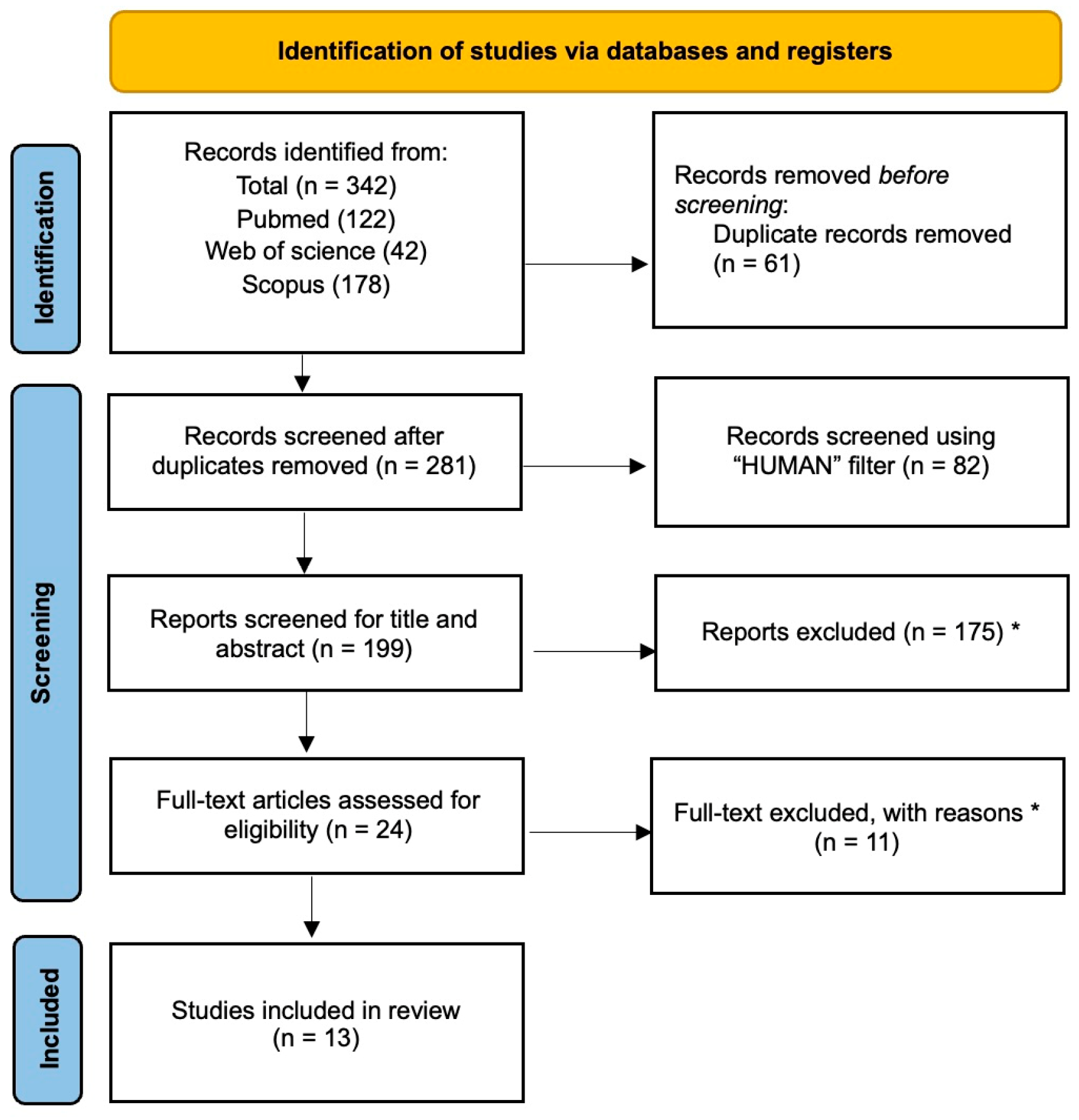
2.1. Selection of Articles
- Patients: patients with knee osteoarthritis (Grade I-II-III according to Kellgren–Lawrence and Ahlback);
- Intervention: IA injections of HA;
- Comparison: Healthy controls or individuals receiving corticosteroid injections or placebo;
- Outcomes: measurements of gait kinetics and functional assessment scores at baseline, and after follow-up.
2.2. Data Extraction
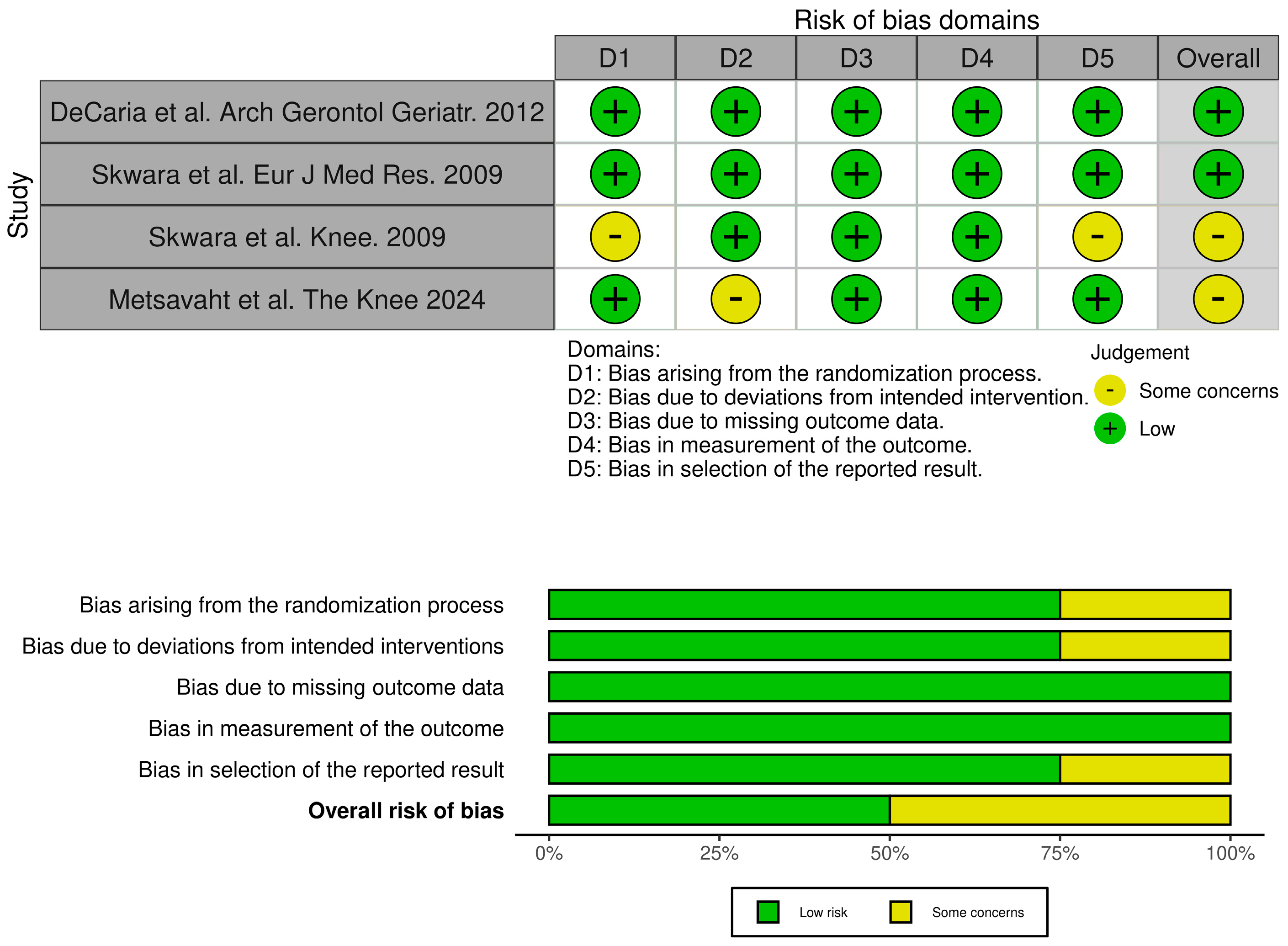
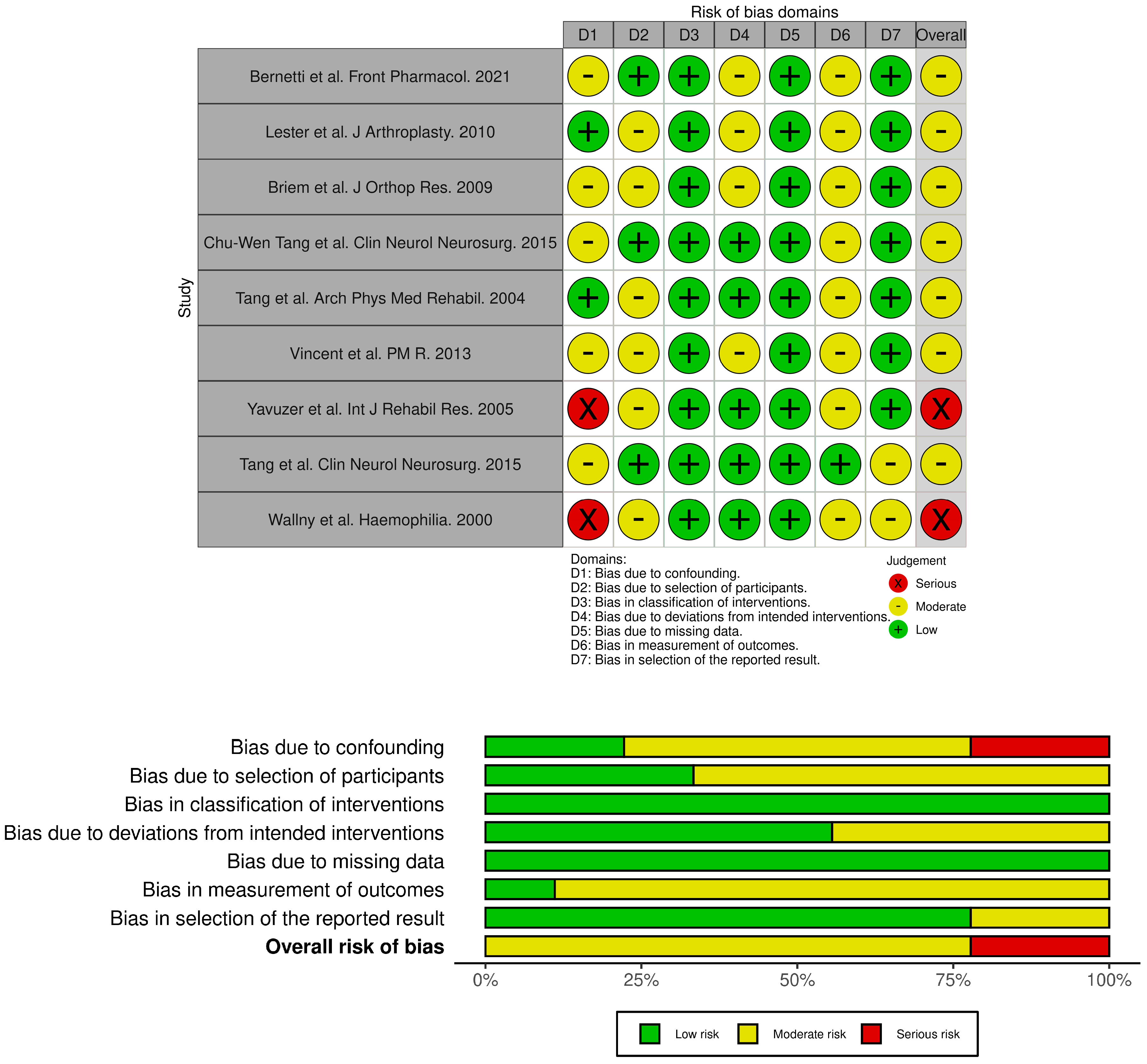
2.3. Quality Assessment
| Article | Study Design | Nationality | Study Group | Control Group | Type of HA | Intervention | Comparison | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Bernetti et al. Front Pharmacol. 2021 [30] | QUASI-EXPERIMENTAL | Italy | n = 31; 8 M/23 F, professional or regular player (at least 2–3 times per week) KOA grade I-III | None | Hymovis MO.RE | One shot | with the baseline | WOMAC A-C KOOS VAS GAIT data collection using the ELITE system |
| Skwara et al. Knee. 2009 [31] | RCT | Germany | n = 21; 8 M/13 F KOA grade II–III | n = 21; 9 M/12 F grade II–III 15 participants included in the final analysis. | Linear, medium-to-high | once a week for 5 weeks | Triamcinolone acetonide | KSS Lequesne Score, VAS SF-36 Gait analysis and EMG (Helen–Hayes marker set) |
| Lester et al. J.Arthroplasty. 2010 [32] | QUASI-EXPERIMENTAL | USA | n = 53; 25 M/28 F KOA grade III–IV | none | Supartz High weight | once a week for 5 weeks | with the baseline | Gait analysis with Intelligent Device for Energy Expenditure and Activity (IDEEA; MiniSun, Fresno, CA) |
| Briem et al. J Orthop Res. 2009 [33] | QUASI-EXPERIMENTAL | Iceland | n = 27; 17 M/10 F, KOA grade I–IV | none | Hyalgan | once a week for 5 weeks | with the baseline | KOS ADL KOOS Gait analysis with Eight camera system (VICON) and two Bertec force plates |
| DeCaria et al. Arch Gerontol Geriatr. 2012 [34] | RCT | Canada | n = 15; 8 M/7 F; KOA grade II–III | n = 15; 8 M/7 F; KOA grade II–III | Medium-to-high | Once a week for 3 weeks + Home exercises | Placebo + Home exercises | WOMAC LK3.1 Gait analysis with GAITRite system. |
| Chu-Wen Tang et al. Clin Neurol Neurosurg. 2015 [35] | QUASI-EXPERIMENTAL | Taiwan | n = 23; 7 M/16 F; KOA I–II | n = 14; 5M/9F non-affected | Low-to-medium (ARTZ) | one a week for 5 weeks (bilateral) | not affected patients | Analysis of gait cycle by Vicon 370 and two AMTI force plates EMG |
| Skwara et al. Eur J Med Res. 2009 [36] | RCT | Germany | n = 24; 12 M/12 F; grade II–III KOA | n = 30; 15M/15F; grade II–III KOA | Durolane Cross-linked High weight | One shot | Triamcinolone acetonide | VAS Lequesne score KSS Gait analysis by Helen–Hayes markers EMG |
| Tang et al. Arch Phys Med Rehabil. 2004 [37] | QUASI-EXPERIMENTAL | Taiwan | n = 15, only women grade I–II KOA | n = 15, only women. non-affected | Medium weight | one per week for 5 weeks (bilateral) | not affected patients | Gait patterns with a Vicon 370 optoelectronic motion analysis system and 2 AMTI force plates |
| Vincent et al. PM R. 2013 [38] | Prospective cohort | USA | n = 34, 11 M/23 D grade II KOA | n = 19, 7 M/12 D grade II KOA | Medium-to-high | one a week for 3 weeks | not injected patients | NRS WOMAC SF-36 Gait analysis on 8 meter walkway |
| Yavuzer et al. Int J Rehabil Res. 2005 [39] | QUASI-EXPERIMENTAL | Turkyie | n = 12, 4 M/8 F KOA grade II–III. | none | Hylan G-F 20 | once a week for 3 weeks (bilateral) | with the baseline | WOMAC pain, stiffness, physical functioning subscores Gait analysis with Vicon 370 system and two Bertec force plates. |
| Chu-Wen Tang et al. Clin Neurol Neurosurg. 2015 [40] | QUASI-EXPERIMENTAL | Taiwan | n = 25, 9 M/16 F KOA grade I–II | n = 15, 5 M/10 F healthy subjects | Medium weight (Artz) | once a week for 5 weeks (bilateral) | not affected patients | VAS Lequesne score Gait analysis with Vicon 370 and two AMTI force plates |
| Wallny et al. Haemophilia. 2000 [41] | PROSPECTIVE OBSERVATIONAL CASE SERIES | Germany | n = 20; hemophilic arthropathy of the knee | none | Hyalart® | once a week for 5 weeks | with the baseline | WFH advisory committee score Aichroth score VAS RX and MRI Biomechanical motion analysis by ultrasound topometry |
| Metsavaht et al. The Knee 2024 [42] | RCT | Brazil | n = 21; 5 M/16 F; KOA grade III–IV | n = 21; 5 M/16 F; KOA grade III–IV | Synolis (HA + sorbitol) | one-shot | Placebo | Gait analysis VICON motion analysis |
3. Results
3.1. Descriptive Analysis
3.2. Primary Outcomes
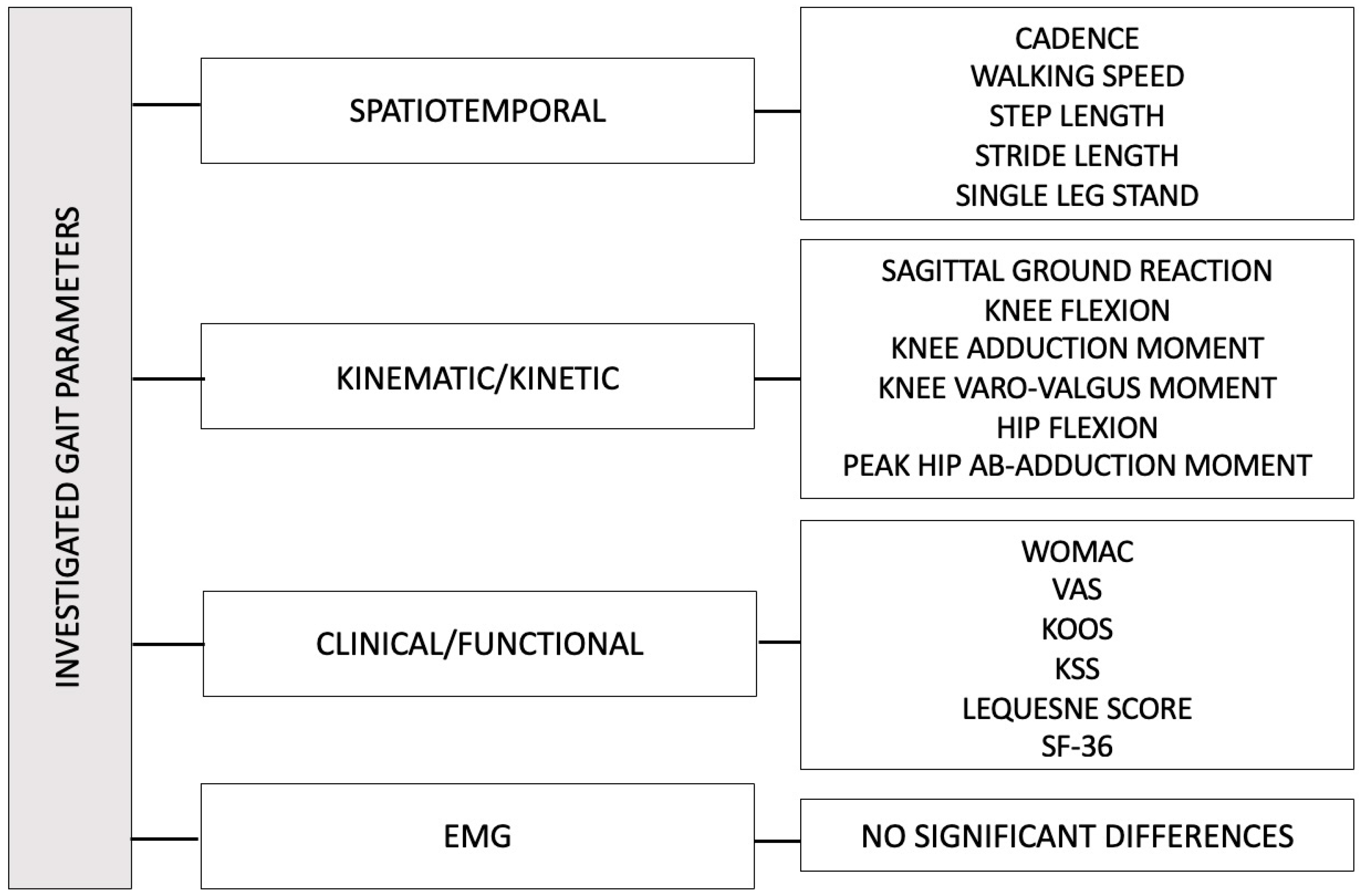
3.2.1. Gait Parameters
3.2.2. EMGs
3.3. Secondary Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Carozzo, S.; Vatrano, M.; Coschignano, F.; Battaglia, R.; Calabrò, R.S.; Pignolo, L.; Contrada, M.; Tonin, P.; Cerasa, A.; Demeco, A. Efficacy of Visual Feedback Training for Motor Recovery in Post-Operative Subjects with Knee Replacement: A Randomized Controlled Trial. J. Clin. Med. 2022, 11, 7355. [Google Scholar] [CrossRef]
- Perruccio, A.V.; Young, J.J.; Wilfong, J.M.; Power, J.D.; Canizares, M.; Badley, E.M. Osteoarthritis year in review 2023: Epidemiology & therapy. Osteoarthr. Cartil. 2024, 32, 159–165. [Google Scholar]
- Allen, K.; Thoma, L.; Golightly, Y. Epidemiology of osteoarthritis. Osteoarthr. Cartil. 2022, 30, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Lee, K.; Ju, J.H. Recent Updates of Diagnosis, Pathophysiology, and Treatment on Osteoarthritis of the Knee. Int. J. Mol. Sci. 2021, 22, 2619. [Google Scholar] [CrossRef] [PubMed]
- Heidari, B.; Hajian-Tilaki, K.; Babaei, M. Determinants of pain in patients with symptomatic knee osteoarthritis. Casp. J. Intern Med. 2016, 7, 153–161. [Google Scholar]
- Geng, R.; Li, J.; Yu, C.; Zhang, C.; Chen, F.; Chen, J.; Ni, H.; Wang, J.; Kang, K.; Wei, Z.; et al. Knee osteoarthritis: Current status and research progress in treatment (Review). Exp. Ther. Med. 2023, 26, 481. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, C.; Lin, F. Efficacy and safety of mesenchymal stem cell injections for knee osteoarthritis: A systematic review and meta-analysis. J. Res. Med. Sci. 2024, 29, 55. [Google Scholar] [CrossRef]
- Faucher, M.; Poiraudeau, S.; Lefevre-Colau, M.M.; Rannou, F.; Fermanian, J.; Revel, M. Algo-functional assessment of knee osteoarthritis: Comparison of the test-retest reliability and construct validity of the WOMAC and Lequesne indexes. Osteoarthr. Cartil. 2002, 10, 602–610. [Google Scholar] [CrossRef]
- Huskisson, E.C. Measurement of pain. Lancet 1974, 304, 1127–1131. [Google Scholar] [CrossRef]
- White, D.K.; Master, H. Patient Reported Measures of Physical Function in Knee Osteoarthritis. Rheum. Dis. Clin. N. Am. 2016, 42, 239–252. [Google Scholar] [CrossRef]
- Andriacchi, T.P.; Lang, P.L.; Alexander, E.J.; Hurwitz, D.E. Methods for evaluating the progression of osteoarthritis. J. Rehabil. Res. Dev. 2000, 37, 163–170. [Google Scholar]
- Favre, J.; Jolles, B.M. Gait analysis of patients with knee osteoarthritis highlights a pathological mechanical pathway and provides a basis for therapeutic interventions. EFORT Open Rev. 2016, 1, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Demeco, A.; Frizziero, A.; Nuresi, C.; Buccino, G.; Pisani, F.; Martini, C.; Foresti, R.; Costantino, C. Gait Alteration in Individual with Limb Loss: The Role of Inertial Sensors. Sensors 2023, 23, 1880. [Google Scholar] [CrossRef] [PubMed]
- de Sire, A.; Demeco, A.; Marotta, N.; Spanò, R.; Curci, C.; Farì, G.; Fortunato, F.; Iona, T.; Lippi, L.; Paolucci, T.; et al. Neuromuscular Impairment of Knee Stabilizer Muscles in a COVID-19 Cluster of Female Volleyball Players: Which Role for Rehabilitation in the Post-COVID-19 Return-to-Play? Appl. Sci. 2022, 12, 557. [Google Scholar] [CrossRef]
- Hussain, S.M.; Neilly, D.W.; Baliga, S.; Patil, S.; Meek, R.M.D. Knee osteoarthritis: A review of management options. Scott. Med. J. 2016, 61, 7–16. [Google Scholar] [CrossRef]
- Lim, W.B.; Al-Dadah, O. Conservative treatment of knee osteoarthritis: A review of the literature. World J. Orthop. 2022, 13, 212–229. [Google Scholar] [CrossRef]
- Indelli, P.F.; Giuntoli, M. Early osteoarthritis of the knee: From conservative to surgical management. Ann. Transl. Med. 2018, 6, 398. [Google Scholar] [CrossRef]
- Qiao, X.; Yan, L.; Feng, Y.; Li, X.; Zhang, K.; Lv, Z.; Xu, C.; Zhao, S.; Liu, F.; Yang, X.; et al. Efficacy and safety of corticosteroids, hyaluronic acid, and PRP and combination therapy for knee osteoarthritis: A systematic review and network meta-analysis. BMC Musculoskelet. Disord. 2023, 24, 926. [Google Scholar] [CrossRef]
- Utamawatin, K.; Phruetthiphat, O.A.; Apinyankul, R.; Chaiamnuay, S. The efficacy of intra-articular triamcinolone acetonide 10 mg vs. 40 mg in patients with knee osteoarthritis: A non-inferiority, randomized, controlled, double-blind, multicenter study. BMC Musculoskelet. Disord. 2023, 24, 92. [Google Scholar] [CrossRef]
- Fransen, M.; McConnell, S.; Harmer, A.R.; Esch MVder Simic, M.; Bennell, K.L. Exercise for Osteoarthritis of the Knee. 2015|Cochrane Library. Available online: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD004376.pub3/full (accessed on 1 July 2025).
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef]
- Chavda, S.; Rabbani, S.A.; Wadhwa, T. Role and Effectiveness of Intra-articular Injection of Hyaluronic Acid in the Treatment of Knee Osteoarthritis: A Systematic Review. Cureus 2022, 14, e24503. [Google Scholar] [CrossRef]
- Giorgino, R.; Albano, D.; Fusco, S.; Peretti, G.M.; Mangiavini, L.; Messina, C. Knee Osteoarthritis: Epidemiology, Pathogenesis, and Mesenchymal Stem Cells: What Else Is New? An Update. Int. J. Mol. Sci. 2023, 24, 6405. [Google Scholar] [CrossRef] [PubMed]
- Bennell, K.L.; Paterson, K.L.; Metcalf, B.R.; Duong, V.; Eyles, J.; Kasza, J.; Wang, Y.; Cicuttini, F.; Buchbinder, R.; Forbes, A.; et al. Effect of Intra-articular Platelet-Rich Plasma vs Placebo Injection on Pain and Medial Tibial Cartilage Volume in Patients with Knee Osteoarthritis: The RESTORE Randomized Clinical Trial. J. Am. Med. Assoc. 2021, 326, 2021–2030. [Google Scholar] [CrossRef] [PubMed]
- Ishijima, M.; Nakamura, T.; Shimizu, K.; Hayashi, K.; Kikuchi, H.; Soen, S.; Omori, G.; Yamashita, T.; Uchio, Y.; Chiba, J.; et al. Intra-articular hyaluronic acid injection versus oral non-steroidal anti-inflammatory drug for the treatment of knee osteoarthritis: A multi-center, randomized, open-label, non-inferiority trial. Arthritis Res. Ther. 2014, 16, R18. [Google Scholar] [CrossRef] [PubMed]
- Ferkel, E.; Manjoo, A.; Martins, D.; Bhandari, M.; Sethi, P.; Nicholls, M. Intra-articular Hyaluronic Acid Treatments for Knee Osteoarthritis: A Systematic Review of Product Properties. Cartilage 2023, 14, 424–432. [Google Scholar] [CrossRef]
- Tang, J.Z.; Nie, M.J.; Zhao, J.Z.; Zhang, G.C.; Zhang, Q.; Wang, B. Platelet-rich plasma versus hyaluronic acid in the treatment of knee osteoarthritis: A meta-analysis. J. Orthop. Surg. Res. 2020, 15, 403. [Google Scholar] [CrossRef]
- Phillips, M.; Bhandari, M.; Grant, J.; Bedi, A.; Trojian, T.; Johnson, A.; Schemitsch, E. A Systematic Review of Current Clinical Practice Guidelines on Intra-articular Hyaluronic Acid, Corticosteroid, and Platelet-Rich Plasma Injection for Knee Osteoarthritis: An International Perspective. Orthop. J. Sports Med. 2021, 9, 23259671211030272. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2020, 12, 55–61. [Google Scholar] [CrossRef]
- Bernetti, A.; Agostini, F.; Alviti, F.; Giordan, N.; Martella, F.; Santilli, V.; Paoloni, M.; Mangonet, M. New Viscoelastic Hydrogel Hymovis MO.RE. Single Intra-articular Injection for the Treatment of Knee Osteoarthritis in Sportsmen: Safety and Efficacy Study Results. Front. Pharmacol. 2021, 12, 1097. [Google Scholar] [CrossRef]
- Skwara, A.; Peterlein, C.D.; Tibesku, C.O.; Rosenbaum, D.; Fuchs-Winkelmann, S. Changes of gait patterns and muscle activity after intraarticular treatment of patients with osteoarthritis of the knee. A prospective, randomised, doubleblind study. Knee 2009, 16, 466–472. [Google Scholar] [CrossRef]
- Lester, D.; Zhang, K. Gait Analysis of Knee Arthritis Treated with Hyaluronic Acid. J. Arthroplast. 2010, 25, 1290–1294. [Google Scholar] [CrossRef]
- Briem, K.; Axe, M.J.; Snyder-Mackler, L. Medial knee joint loading increases in those who respond to hyaluronan injection for medial knee osteoarthritis. J. Orthop. Res. 2009, 27, 1420–1425. [Google Scholar] [CrossRef]
- DeCaria, J.; Montero-Odasso, M.; Wolfe, D.; Chesworth, B.; Petrella, R. The effect of intra-articular hyaluronic acid treatment on gait velocity in older knee osteoarthritis patients: A randomized, controlled study. Arch. Gerontol. Geriatr. 2012, 55, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.C.W.; Hong, W.H.; Chen, H.C.; Tang, S.F.T. Intra-articular intervention by hyaluronic acid for knee osteoarthritis can modify locomotor pattern of muscle activity. Clin. Neurol. Neurosurg. 2015, 129 (Suppl. S1), S16–S20. [Google Scholar] [CrossRef] [PubMed]
- Skwara, A.; Ponelis, R.; Tibesku, C.O.; Rosenbaum, D.; Fuchs-Winkelmann, S. Gait patterns after intraarticular treatment of patients with osteoarthritis of the knee—Hyaluronan versus triamcinolone: A prospective, randomized, doubleblind, monocentric study. Eur. J. Med. Res. 2009, 14, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.F.; Chen, C.P.; Chen, M.J.; Pei, Y.C.; Lau, Y.C.; Leong, C.P. Changes in sagittal ground reaction forces after intra-articular hyaluronate injections for knee osteoarthritis. Arch. Phys. Med. Rehabil. 2004, 85, 951–955. [Google Scholar] [CrossRef]
- Vincent, H.; Montero, C.; Conrad, B.; Horodyski, M.; Connelly, J.; Martenson, M.; Seay, A.N.; Vincent, K.R. ‘Functional Pain,’ Functional Outcomes, and Quality of Life After Hyaluronic Acid Intra-articular Injection for Knee Osteoarthritis. PMR 2013, 5, 310–318. [Google Scholar] [CrossRef]
- Yavuzer, G.; Sonel, B.; Süldür, N.; Ergin, S. Effects of intra-articular hylan G-F 20 injections on clinical and biomechanical characteristics of the knee in osteoarthritis. Int. J. Rehabil. Res. 2005, 28, 371–374. [Google Scholar] [CrossRef]
- Tang, A.C.W.; Tang, S.F.T.; Hong, W.H.; Chen, H.C. Kinetics features changes before and after intra-articular hyaluronic acid injections in patients with knee osteoarthritis. Clin. Neurol. Neurosurg. 2015, 129 (Suppl. S1), S21–S26. [Google Scholar] [CrossRef]
- Wallny, T.; Brackmann, H.H.; Semper, H.; Schumpe, G.; Effenberger, W.; Hess, L.; Seuser, A. Intra-articular hyaluronic acid in the treatment of haemophilic arthropathy of the knee. Clinical, radiological and sonographical assessment. Haemophilia 2000, 6, 566–570. [Google Scholar] [CrossRef]
- Metsavaht, L.; Leporace, G.; Crespo, B.; Gonzalez, F.; Pereira, M.M.; Guadagnin, E.C.; Chahla, J.; Franciozi, C.E.; Luzo, M.V.M. Gait kinematics of osteoarthritic knees after intra-articular viscosupplementation: A double-blinded randomized controlled trial. Knee 2024, 47, 102–111. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Br. Med. J. 2021, 372, n71. [Google Scholar] [CrossRef]
- Cesari, M.; Pahor, M.; Lauretani, F.; Zamboni, V.; Bandinelli, S.; Bernabei, R.; Guralnik, J.M.; Ferrucci, L. Skeletal muscle and mortality results from the InCHIANTI Study. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Wallis, J.A.; Webster, K.E.; Levinger, P.; Singh, P.J.; Fong, C.; Taylor, N.F. A walking program for people with severe knee osteoarthritis did not reduce pain but may have benefits for cardiovascular health: A phase II randomised controlled trial. Osteoarthr. Cartil. 2017, 25, 1969–1979. [Google Scholar] [CrossRef] [PubMed]
- Uematsu, H.; Osako, S.; Hakata, S.; Kabata, D.; Shintani, A.; Kawazoe, D.; Mizuno, K.; Fujino, Y.; Matsuda, Y. A Double-Blind, Placebo-Controlled Study of Ultrasound-Guided Pulsed Radiofrequency Treatment of the Saphenous Nerve for Refractory Osteoarthritis-Associated Knee Pain. Pain Physician 2021, 24, E761–E769. [Google Scholar] [PubMed]
- Jarchi, D.; Pope, J.; Lee, T.K.M.; Tamjidi, L.; Mirzaei, A.; Sanei, S. A Review on Accelerometry-Based Gait Analysis and Emerging Clinical Applications. IEEE Rev. Biomed. Eng. 2018, 11, 177–194. [Google Scholar] [CrossRef]
- Klöpfer-Krämer, I.; Brand, A.; Wackerle, H.; Müßig, J.; Kröger, I.; Augat, P. Gait analysis—Available platforms for outcome assessment. Injury 2020, 51 (Suppl. S2), S90–S96. [Google Scholar] [CrossRef]
- DeJong, P.; Hatamiya, N.S.; Barkley, L.C. Running Gait Analysis and Biomechanics. Curr. Sports Med. Rep. 2022, 21, 107–108. [Google Scholar] [CrossRef]
- Hecht, G.G.; Van Rysselberghe, N.L.; Young, J.L.; Gardner, M.J. Gait Analysis in Orthopaedic Surgery: History, Limitations, and Future Directions. J. Am. Acad. Orthop. Surg. 2022, 30, e1366–e1373. [Google Scholar] [CrossRef]
- Subramaniam, S.; Faisal, A.I.; Deen, M.J. Wearable Sensor Systems for Fall Risk Assessment: A Review. Front. Digit. Health 2022, 4, 921506. [Google Scholar] [CrossRef]
- Pallavi, P.; Ranjan, S.; Patel, N.; Kanetkar, M.; Lahiri, U. Smart Wearable Device for Quantification of Risk of Fall: Exploring Role of Gait Phases and Knee Bending Angle for Parkinson’s Patients. In Proceedings of the 2022 44th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Glasgow, UK, 11–15 July 2022. [Google Scholar]
- Liang, S.; Jia, H.; Li, Z.; Li, H.; Gao, X.; Ma, Z.; Ma, Y.; Zhao, G. Fall risk factors analysis based on sample entropy of plantar kinematic signal during stance phase. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2016, 2016, 4832–4836. [Google Scholar]
- Kuan, Y.C.; Huang, L.K.; Wang, Y.H.; Hu, C.J.; Tseng, I.J.; Chen, H.C.; Lin, L.F. Balance and gait performance in older adults with early-stage cognitive impairment. Eur. J. Phys. Rehabil. Med. 2021, 57, 560–567. [Google Scholar] [CrossRef]
- Miyazaki, T.; Wada, M.; Kawahara, H.; Sato, M.; Baba, H.; Shimada, S. Dynamic load at baseline can predict radiographic disease progression in medial compartment knee osteoarthritis. Ann. Rheum. Dis. 2002, 61, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Bartholdy, C.; Juhl, C.; Christensen, R.; Lund, H.; Zhang, W.; Henriksen, M. The role of muscle strengthening in exercise therapy for knee osteoarthritis: A systematic review and meta-regression analysis of randomized trials. Semin. Arthritis Rheum. 2017, 47, 9–21. [Google Scholar] [CrossRef] [PubMed]
- DeVita, P.; Aaboe, J.; Bartholdy, C.; Leonardis, J.M.; Bliddal, H.; Henriksen, M. Quadriceps-strengthening exercise and quadriceps and knee biomechanics during walking in knee osteoarthritis: A two-centre randomized controlled trial. Clin. Biomech. 2018, 59, 199–206. [Google Scholar] [CrossRef]
- Henriksen, M.; Simonsen, E.B.; Alkjær, T.; Lund, H.; Graven-Nielsen, T.; Danneskiold-Samsøe, B.; Bliddal, H. Increased joint loads during walking—A consequence of pain relief in knee osteoarthritis. Knee 2006, 13, 445–450. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, G.; Zhang, L.; Gong, R.; Fu, P.; Han, B.; Li, H.; Saarakkala, S. Biomechanical Effect of Valgus Knee Braces on the Treatment of Medial Gonarthrosis: A Systematic Review. Appl. Bionics Biomech. 2022, 2022, 4194472. [Google Scholar] [CrossRef]
- Shelburne, K.B.; Torry, M.R.; Steadman, J.R.; Pandy, M.G. Effects of foot orthoses and valgus bracing on the knee adduction moment and medial joint load during gait. Clin. Biomech. 2008, 23, 814–821. [Google Scholar] [CrossRef]
- Monticone, M.; Frizziero, A.; Rovere, G.; Vittadini, F.; Uliano, D.; La Bruna, S.; Gatto, R.; Nava, C.; Leggero, V.; Masiero, S. Hyaluronic acid intra-articular injection and exercise therapy: Effects on pain and disability in subjects affected by lower limb joints osteoarthritis. A systematic review by the Italian Society of Physical and Rehabilitation Medicine (SIMFER). Eur. J. Phys. Rehabil. Med. 2016, 52, 389–399. [Google Scholar]
- Saccomanno, M.F.; Donati, F.; Careri, S.; Bartoli, M.; Severini, G.; Milano, G. Efficacy of intra-articular hyaluronic acid injections and exercise-based rehabilitation programme, administered as isolated or integrated therapeutic regimens for the treatment of knee osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 1686–1694. [Google Scholar] [CrossRef]
- Smith, C.; Patel, R.; Vannabouathong, C.; Sales, B.; Rabinovich, A.; McCormack, R.; Belzile, E.L.; Bhandari, M. Combined intra-articular injection of corticosteroid and hyaluronic acid reduces pain compared to hyaluronic acid alone in the treatment of knee osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 1974–1983. [Google Scholar] [CrossRef]
- Karasavvidis, T.; Totlis, T.; Gilat, R.; Cole, B.J. Platelet-Rich Plasma Combined with Hyaluronic Acid Improves Pain and Function Compared With Hyaluronic Acid Alone in Knee Osteoarthritis: A Systematic Review and Meta-analysis. Arthrosc. J. Arthrosc. Relat. Surg. 2021, 37, 1277–1287.e1. [Google Scholar] [CrossRef] [PubMed]
- Fransen, M.; Crosbie, J.; Edmonds, J. Physical therapy is effective for patients with osteoarthritis of the knee: A randomized controlled clinical trial. J. Rheumatol. 2001, 28, 156–164. [Google Scholar] [PubMed]
- Schmalz, T.; Knopf, E.; Drewitz, H.; Blumentritt, S. Analysis of biomechanical effectiveness of valgus-inducing knee brace for osteoarthritis of knee. J. Rehabil. Res. Dev. 2010, 47, 419–429. [Google Scholar] [CrossRef]
- Srinivasan, V.; Ethiraj, P.; Agarawal, S.; HSA; Parmanantham, M. Comparison of Various Modalities in the Treatment of Early Knee Osteoarthritis: An Unsolved Controversy. Cureus 2023, 15, e33630. [Google Scholar] [CrossRef]
- Demeco, A.; Marotta, N.; Moggio, L.; Pino, I.; Marinaro, C.; Barletta, M.; Petraroli, A.; Palumbo, A.; Ammendolia, A. Quantitative analysis of movements in facial nerve palsy with surface electromyography and kinematic analysis. J. Electromyogr. Kinesiol. 2021, 56, 102485. [Google Scholar] [CrossRef]
| PubMed ((gait analysis) OR (gait) OR (walk) OR (inertial sensor) OR (inertial measurement unit) OR (accelerometer) OR (surface electromyography) OR (kinematic analysis)) AND (knee) AND ((arthrosis) OR (arthritis) OR (osteoarthrosis) OR (gonarthritis) OR (gonarthrosis)) AND ((injection) OR (infiltration)) AND ((viscosupplementation) OR (viscoinduction) OR (hyaluronic acid)) |
| Web of Science (((gait analysis) OR (gait) OR (walk) OR (inertial sensor) OR (inertial measurement unit) OR (accelerometer) OR (surface electromyography) OR (kinematic analysis)) AND (knee) AND ((arthrosis) OR (arthritis) OR (osteoarthrosis) OR (gonarthritis) OR (gonarthrosis)) AND ((injection) OR (infiltration)) AND ((viscosupplementation) OR (viscoinduction) OR (hyaluronic acid))) |
| Scopus TITLE-ABS-KEY((“gait analysis”) OR (“gait”) OR (“walk”) OR (“inertial sensor”) OR (“inertial measurement unit”) OR (“accelerometer”) OR (“surface electromyography”) OR (“kinematic analysis”)) AND (“knee”) AND ((“arthrosis”) OR (“arthritis”) OR (“osteoarthrosis”) OR (“gonarthritis”) OR (“gonarthrosis”)) AND ((“injection”) OR (“infiltration”)) AND ((“viscosupplementation”) OR (“viscoinduction”) OR (“hyaluronic acid”)) |
| Variables | Study | Follow up Time | Intervention Mean | Comparison Mean | p Value |
|---|---|---|---|---|---|
| CADENCE (step/minute) | Bernetti et al., 2021 [30] | Baseline | 103.39 ± 9.07 | / | p = 0.005 |
| Day 30 | / | ||||
| Day 90 | 110.95 ± 11.29 | ||||
| Day 180 | / | ||||
| Day 360 | / | ||||
| Vincent et al., 2013 [38] | Baseline | 97.9 ± 14.8 | 108.1 ± 10.4 | p < 0.05 | |
| 6 months | 106.7 ± 10.7 | 108.6 ± 10.8 | |||
| Tang et al., 2004 [37] | Baseline | 96.1 ± 11.0 | 106.3 ± 12.3 | p < 0.05 | |
| 1 week | 108.8 ± 10.1 | ||||
| 3 months | 110.7 ± 12.01 | ||||
| 6 months | 12.7 ± 14.3 | ||||
| WALKING SPEED m/s | Bernetti et al., 2021 [30] | Baseline | 1.10 ± 0.14 | / | p = 0.001 |
| Day 30 | / | ||||
| Day 90 | 1.28 ± 0.21 | ||||
| Day 180 | / | ||||
| Day 360 | / | ||||
| Vincent et al., 2013 (cm/s) [38] | Baseline | 98.0 ± 31.4 | 118.8 ± 22.9 | p = 0.002 | |
| 6 months | 110.2 ± 30.4 | 118.6 ± 23.1 | |||
| Tang AC et al., 2015 (%BH/sec) [40] | Baseline | 49.4% ± 11.7 | ~62(box plot) | p < 0.001 | |
| 1 week | ~58(box plot) | ||||
| 3 months | ~60(box plot) | ||||
| 6 months | ~60(box plot) | ||||
| DeCaria et al., 2012 [34] | Week 4 3 months 6 months | 12.32 (11.50) 11.10 (11.43) 9.53 (14.33) | 8.23 (10.46) 9.86 (10.22) 12.83 (11.89) | p = 0.01 | |
| Tang et al., 2004 (%BH/sec) [37] | Baseline | 50.2 ± 11.9 | 59.5 ± 10.7 | p < 0.05 | |
| 1 week | 62.2 ± 10.8 | ||||
| 3 months | 63.1 ± 16.8 | ||||
| 6 months | 61.8 ± 16.2 | ||||
| STEP LENGTH cm | Vincent et al., 2013 [38] | Baseline | 58.6 ± 13.4 | 67.4 ± 15.5 | p < 0.05 |
| 6 month | 60.5 ± 13.1 | 66.9 ± 15.8 | |||
| Tang AC et al., 2015 [40] | Baseline | 30.8 ± 5.2 | ~38(box plot) | p = 0.01 | |
| 1 week | ~35(box plot) | ||||
| 3 months | ~34(box plot) | ||||
| 6 months | ~33(box plot) | ||||
| Tang et al., 2004 [37] | Baseline | 30.4± 5.4 | 35.6 ± 4.3 | p < 0.05 | |
| 1 week | 34.6 ± 4.7 | ||||
| 3 months | 33.7 ± 7.0 | ||||
| 6 months | 33.5 ± 6.8 | ||||
| STRIDE LENGTH cm | Vincent et al., 2013 [38] | Baseline | 118.0 ± 27.0 | 131.4 ± 18.0 126.6 ± 29.5 | p < 0.05 |
| 6 month | 122.34 ± 26.2 | ||||
| SINGLE LEG STANCE sec | Vincent et al., 2013 [38] | Baseline | 32.8 ± 4.0 | 35.4 ± 2.1 | p < 0.05 |
| 6 month | 33.8 ± 4.2 | 35.3 ± 1.9 | |||
| Metsavaht et al., 2024 [42] | Baseline | 38.8% | 38.0% | p < 0.05 | |
| 1 week | 38.4% | 39.6% | |||
| 6 weeks | 38.8 % | 38.5 % | |||
| 8 weeks | 38.3% | 38.9% | |||
| BIPEDAL SUPPORT TIME (ms) | Lester et al., 2010 [32] | Baseline 3 weeks | 159.74 | 168.65 | p = 0.04 |
| Variables | Study | Follow Up Time | Intervention Mean | Comparison Mean | p Value |
|---|---|---|---|---|---|
| SAGITTAL GROUND REACTION 1st peak force (%BW) | Tang et al., 2004 [37] | Baseline | 100.7 ± 5.1 | 102.3 ± 6.5 | p < 0.05 |
| 1 week | 103.5 ± 10.0 | ||||
| 3 months | 106.0 ± 8.7 | ||||
| 6 months | 105.5 ± 6.9 | ||||
| Skwara et al., 2009 [31] Vertical force maximum 1 and 2 (BW) | Baseline 1 week 12 weeks | 1.1 / 1.1 | 1.0 / 1.0 | p = 0.018 | |
| KNEE FLEXION (Nm/Kg) | Tang AC et al., 2015 [40] | Baseline 1 week 3 months 6 months | larger knee flexion moments at terminal stance (graphical data) | / | p < 0.01 |
| Metsavaht et al., 2024 (angle°) [42] | baseline | 12.9 | 14.8 | p < 0.001 | |
| 1 week | 11.0 | 14.8 | |||
| 6 week | 12.7 | 15.5 | |||
| 12 week | 13.1 | 14.2 | |||
| Bernetti et al., 2021 [30] | Baseline | 0.300 ± 0.242 | 0.440 ± 0.340 | p < 0.05 | |
| Day 30 | 0.080 ± 0.380 | 0.540 ± 2.377 | |||
| Day 90 | 0.160 ± 0.360 | −0.06 ± 0.426 | |||
| Day 180 | 0.200 ± 0.458 | −0.110 ± 0.460 | |||
| Day 360 | 0.190 ± 0.970 | −0.10 ± 0.399 | |||
| KNEE ADDUCTION MOMENT and KNEE VARO-VALGUS MOMENT Nm/Kg | Briem et al., 2009 [33] | Baseline 3 weeks 5 months | Increased peek knee adduction moment (graphical data) | / | p = 0.001 |
| Bernetti et al., 2021 [30] | Baseline | 0.350 ± 0.246 | 0.430 ± 0.290 | p < 0.05 | |
| Day 30 | 0.030 ± 0.250 | 0.060 ± 0.341 | |||
| Day 90 | 0.080 ± 0.267 | −0.07 ± 0.285 | |||
| Day 180 | 0.160 ± 0.269 | 0.050 ± 0.242 | |||
| Day 360 | 0.080 ± 0.298 | 0.100 ± 0.360 | |||
| Tang AC et al., 2015 [40] | Baseline | increased | / | p < 0.001 | |
| 1 week | (more varus) | ||||
| 3 months | (graphical data) | ||||
| 6 months | |||||
| Yavuzer et al., 2005 [39] | Baseline | 0.45 ± 0.1 | / | p = 0.047 | |
| 1 week | 0.41 ± 0.1 | ||||
| KNEE ABDUCTION MOMENT Nm/Kg | Skwara et al., 2009 [31] | Baseline | / | / | p = 0.007 |
| 1 week | 0.2 | 0.3 | |||
| 12 weeks | 0.3 | 0.3 | |||
| HIP FLEXION ANGLE | Briem et al., 2009 [33] | Baseline | Increased hip | / | p = 0.018 |
| 3 weeks | flexion angle | ||||
| 5 months | (graphical data) | ||||
| Skwara et al., 2009 [36] | Baseline | 38.74 | 36.16 | p = 0.0177 | |
| 12 weeks | 42.48 | 38.33 | |||
| PEAK HIP AB-ADDUCTION MOMENT | Bernetti et al., 2021 [30] | Baseline | 0.490 ± 0.296 | 0.560 ± 0.304 | p < 0.05 |
| Day 30 | 0.120 ± 0.392 | 0.170 ± 0.471 | |||
| Day 90 | 0.190 ± 0.383 | 0.000 ± 0.341 | |||
| Day 180 | 0.220 ± 0.353 | 0.140 ± 0.521 | |||
| Day 360 | 0.050 ± 0.398 | 0.070 ± 0.444 | |||
| Tang AC et al., 2015 [40] | Baseline 1 week 3 months 6 months | Increased adduction at early stance (graphical data) | / | p < 0.05 |
| Variables | Study | Follow Up Time | Intervention Mean | Comparison Mean | p Value |
|---|---|---|---|---|---|
| WOMAC pain | Yavuzer et al., 2005 [39] | Baseline | 9.2 ± 2.7 | / | p = 0.005 |
| 1 week | 4.8 ± 3.1 | ||||
| Bernetti et al., 2021 [30] | Baseline | 11.77 ± 10.53 | / | p < 0.001 | |
| Day 30 | 11.77 ± 10.53 | ||||
| Day 90 | 7.410 ± 9.320 | ||||
| Day 180 | 8.040 ± 9.620 | ||||
| Day 360 | 8.040 ± 9.620 | ||||
| Vincent et al., 2013 [38] | Baseline | 7.5 ± 3.7 | 5.7 ± 3.3 | p = 0.009 | |
| 6 month | 5.5 ± 4.7 | 7.2 ± 5.2 | |||
| DeCaria et al., 2012 [34] | Baseline | 11.25 ± 4.17 | p = 0.008 | ||
| 1 week | 8.50 ± 2.98 | ||||
| VAS mm | Skwara et al., 2009 [31] | Baseline | 53.1 | 57.9 | p < 0.001 |
| 1 week | 23.2 | 20.5 | |||
| 12 weeks | 33.6 | 32.00 | |||
| Bernetti et al., 2021 [30] | Baseline | 68.65 ± 11.53 | / | p < 0.001 | |
| Day 30 | 20.94 ± 19.10 | ||||
| Day 90 | 16.48 ± 18.65 | ||||
| Day 180 | 12.15 ± 17.29 | ||||
| Day 360 | 14.39 ± 15.41 | ||||
| Tang AC et al., 2015 [40] | Baseline | 54.6 ± 12.4 | / | p < 0.001 | |
| 1 week | 38.5 ± 11.2 | ||||
| 3 months | 36.8 ± 10.3 | ||||
| 6 month | 42.4 ± 10.0 | ||||
| Wallny et al., 2000 (cm) [41] | Baseline | 5.4 | / | / | |
| 3 months | 3.8 | ||||
| KOOS | Bernetti et al., 2021 [30] | Baseline | 41.61 ± 20.95 | / | p < 0.001 |
| Day 30 | 74.66 ± 21.63 | ||||
| Day 90 | 74.66 ± 21.63 | ||||
| Day 180 | 76.15 ± 18.46 | ||||
| Day 360 | 78.04 ± 19.53 | ||||
| Briem et al., 2009 [33] | Baseline | 61 | 61 | p = 0.001 | |
| 3 weeks | 79 | 61 | |||
| 5 months | 75 | 66 | |||
| KSS | Skwara et al., 2009 [31] | Baseline | 128.2 | 132.1 | p < 0.001 |
| 1 week | 147.0 | 155.7 | |||
| 12 weeks | 140.6 | 154.7 | |||
| LI | Skwara et al., 2009 [31] | Baseline | 11.5 | 12.3 | p < 0.001 |
| 1 week | 8.3 | 7.6 | |||
| 12 weeks | 8.8 | 9.1 | |||
| Tang AC et al., 2015 [40] | Baseline | 5.6 ± 1.9 | / | p = 0.01 | |
| 1 week | 2.2 ± 1.0 | ||||
| 3 months | 2.4 ± 1.7 | ||||
| 6 months | 3.0 ± 1.5 | ||||
| KOS | Briem et al., 2009 [33] | Baseline | 62 | 71 | p = 0.005 |
| 3 weeks | 78 | 73 | |||
| 5 months | 76 | 72 | |||
| SF36 | Skwara et al., 2009 [31,36] | Baseline 1 week 12 weeks | Improved (graphical data) | / | p = 0.002 |
| Vincent et al., 2013 [38] | |||||
| Role Physical | Baseline | 33.6 ± 10.3 | 49.1 ± 9.2 | p = 0.025 | |
| 6 months | 35.4 ± 10.6 | 37.7 ± 10.6 | |||
| Vitality | Baseline | 44.6 ± 8.2 | 50.9 ± 7.4 | p = 0.013 | |
| 6 months | 50.4 ± 5.9 | 48.8 ± 10.8 | |||
| Role Emotional | Baseline | 38.9 ± 13.8 | 50.0 ± 5.6 | p = 0.024 | |
| 6 months | 45.8 ± 12.7 | 51.3 ± 11.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costantino, C.; Ronzoni, S.; Ingletto, A.; Sabato, R.; Salerno, A.; Palermi, S.; Foresti, R.; Martini, C.; Demeco, A. The Effects of Hyaluronic Acid on Gait Parameters in Patients with Knee Osteoarthritis: A Systematic Literature Review. Medicina 2025, 61, 1488. https://doi.org/10.3390/medicina61081488
Costantino C, Ronzoni S, Ingletto A, Sabato R, Salerno A, Palermi S, Foresti R, Martini C, Demeco A. The Effects of Hyaluronic Acid on Gait Parameters in Patients with Knee Osteoarthritis: A Systematic Literature Review. Medicina. 2025; 61(8):1488. https://doi.org/10.3390/medicina61081488
Chicago/Turabian StyleCostantino, Cosimo, Sara Ronzoni, Annalisa Ingletto, Roberto Sabato, Antonello Salerno, Stefano Palermi, Ruben Foresti, Chiara Martini, and Andrea Demeco. 2025. "The Effects of Hyaluronic Acid on Gait Parameters in Patients with Knee Osteoarthritis: A Systematic Literature Review" Medicina 61, no. 8: 1488. https://doi.org/10.3390/medicina61081488
APA StyleCostantino, C., Ronzoni, S., Ingletto, A., Sabato, R., Salerno, A., Palermi, S., Foresti, R., Martini, C., & Demeco, A. (2025). The Effects of Hyaluronic Acid on Gait Parameters in Patients with Knee Osteoarthritis: A Systematic Literature Review. Medicina, 61(8), 1488. https://doi.org/10.3390/medicina61081488