Efficacy of Dupilumab in Patients with Chronic Rhinosinusitis with Nasal Polyps and Eosinophilic Otitis Media: A Six-Month Observational Study
Abstract
1. Introduction
2. Materials and Methods
- SNOT-22: We used the validated Italian version of SNOT-22. Possible total score range: 0–110. A SNOT-22 score < 20 was suggestive of mild symptoms. During follow-up time, the minimal clinically important difference (MCID) in SNOT-22 scores was assumed for an 8.9-point increase as reported in previous studies.
- Nasal endoscopy (Nasal Polyp Score): Each side of the nasal cavity was separately evaluated and scored in a range from 0 to 4 (0 = no polyps; 1 = small polyps in the middle meatus not reaching below the inferior border of the middle turbinate; 2 = polyps reaching below the lower border of the middle turbinate; 3 = large polyps reaching the lower border of the inferior turbinate or polyps medial to the middle turbinate; and 4 = large polyps causing complete obstruction of the inferior nasal cavity). The sum of the scores for both nasal cavities was recorded as the NPS value.
- VAS for symptoms: Intensity of symptoms was measured on a horizontal 10 cm line. A mean score for each symptom analyzed was obtained using the average scores assigned to all patients for the same symptom.
- Diagnostic Criteria of Eosinophilic Otitis Media (EOM): Major criteria: Otitis media with effusion or chronic otitis media with eosinophilic dominant effusion. Minor criteria: Highly viscous middle ear effusion, resistance to conventional treatment for OM, association with asthma, and association with CRSwNP. Definitive case: Positive for major criteria + two or more minor criteria. Exclusion criteria: Eosinophilic Granulomatosis with Polyangiitis (EGPA) [2].
- Tympanogram: “A”, “B” or “C”.
- Chronic otitis media outcome test (COMOT-15): We used the validated Italian version of COMOT-15. The COMOT-15 consists of three subscales, categorized as ear symptoms (questions 1–6), hearing function (questions 7–9), and mental health (questions 10–13), which form the overall score. In addition to questions 1 to 13, the COMOT-15 contains two other questions: a general evaluation of the impact of chronic otitis media on QoL (question 14) and a question on the frequency of ENT visits as a result of chronic otitis media in the previous 6 months (question 15). The possible total score range is 0–75 [COMOT] [13].
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bachert, C.; Zhang, N.; Hellings, P.W.; Bousquet, J. Endotype-driven care pathways in patients with chronic rhinosinusitis. J. Allergy Clin. Immunol. 2022, 149, 1496–1506. [Google Scholar] [CrossRef]
- Iino, Y. Eosinophilic otitis media: A new middle ear disease entity. Curr. Allergy Asthma Rep. 2008, 8, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Iino, Y.; Tomioka-Matsutani, S.; Matsubara, A.; Nakagawa, T.; Nonaka, M. Diagnostic criteria of eosinophilic otitis media, a newly recognized middle ear disease. Auris Nasus Larynx 2011, 38, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Bachert, C.; Han, J.K.; Desrosiers, M.; Hellings, P.W.; Amin, N.; Lee, S.E.; Mullol, J.; Greos, L.S.; Bosso, J.V.; Laidlaw, T.M.; et al. Efficacy and safety of dupilumab in CRSwNP: Results from the SINUS-24 and SINUS-52 trials. N. Engl. J. Med. 2019, 381, 55–69. [Google Scholar]
- Breslin, N.K.; Heindel, N.H.; Haberman, R.S. Role of Interleukin 5 Inhibition in the Treatment of Eosinophilic Otitis Media. OTO Open 2021, 5, 2473974X21991449. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morse, J.C.; Miller, C.; Senior, B. Management of Chronic Rhinosinusitis with Nasal Polyposis in the Era of Biologics. J. Asthma Allergy 2021, 14, 873–882. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wenzel, S.; Castro, M.; Corren, J.; Maspero, J.; Wang, L.; Zhang, B.; Pirozzi, G.; Sutherland, E.R.; Evans, R.R.; Joish, V.N.; et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma. N. Engl. J. Med. 2013, 368, 2455–2466. [Google Scholar] [CrossRef] [PubMed]
- Baumann, I.; Kurpiers, B.; Plinkert, P.K.; Praetorius, M. Development and validation of the Chronic Otitis Media Outcome Test 15 (COMOT-15). Otol. Neurotol. 2009, 30, 1190–1196. [Google Scholar]
- Prell, J.; Schick, B.; Reiners, C.; Schwab, B.; Plinkert, P.K.; Baumann, I. Health-related quality of life measured by the COMOT-15 in patients with chronic otitis media—Validation of the German version. Health Qual. Life Outcomes 2007, 5, 2. [Google Scholar]
- Seys, S.F.; Schneider, S.; de Kinderen, J.; Reitsma, S.; Cavaliere, C.; Tomazic, P.-V.; Morgenstern, C.; Mortuaire, G.; Wagenmann, M.; Bettio, G.; et al. Real-world effectiveness of dupilumab in a European cohort of chronic rhinosinusitis with nasal polyps (CHRINOSOR). J. Allergy Clin. Immunol. 2025, 155, 451–460. [Google Scholar] [CrossRef]
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 58 (Suppl. S29), 1–464. [Google Scholar] [CrossRef]
- Iino, Y.; Tomioka-Matsutani, S. Eosinophilic otitis media: An update. Auris Nasus Larynx 2020, 47, 505–513. [Google Scholar]
- Cavaliere, M.; Capriglione, P.; Cavaliere, F.; de Corso, E.; Zanoletti, E.; Motta, G.; Iengo, M.; Cantone, E. Cross-cultural adaptation and Italian validation of chronic otitis media outcome test 15 (COMOT-15). Acta Otorhinolaryngol. Ital. 2021, 41, 277–281. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schilder, A.G.; Chonmaitree, T.; Cripps, A.W.; Rosenfeld, R.M.; Casselbrant, M.L.; Haggard, M.P.; Venekamp, R.P. Otitis media. Nat. Rev. Dis. Primers 2016, 2, 16063. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Galletti, C.; Ragusa, M.; Sireci, F.; Ciodaro, F.; Barbieri, M.A.; Giunta, G.; Grigaliute, E.; Immorodino, A.; Lorusso, F.; Dispenza, F.; et al. Dupilumab in chronic rhinosinusitis with nasal polyps: Real life data in a multicentric Sicilian experience. Am. J. Otolaryngol. 2023, 45, 104106. [Google Scholar] [CrossRef] [PubMed]
- Nadol, J.B., Jr.; Staecker, H.; Gluth, M.B. Patient-reported outcome measures in otology: An overview. Otol. Neurotol. 2020, 41, 131–140. [Google Scholar]
- Galletti, C.; Barbieri, M.A.; Ciodaro, F.; Freni, F.; Galletti, F.; Spina, E.; Galletti, B. Effectiveness and safety profile of Dupilumab in chronic rhinosinusitis with nasal polyps: Real- life data in tertiary care. Pharmaceuticals 2023, 16, 630. [Google Scholar] [CrossRef]
- Kanazawa, H.; Yoshida, N.; Iino, Y. New insights into eosinophilic otitis media. Curr. Allergy Asthma Rep. 2015, 15, 76. [Google Scholar] [CrossRef]
- Komori, M.; Teraura, H.; Iino, Y. Long-term outcomes of eosinophilic otitis media and the effectiveness of early biologic therapy. Auris Nasus Larynx 2023, 50, 51–58. [Google Scholar]
- Lee, S.E.; Hopkins, C.; Mullol, J.; Msihid, J.; Guillemin, I.; Amin, N.; Mannent, L.P.; Li, Y.; Siddiqui, S.; Chuang, C.C.; et al. Dupilumab improves health related quality of life: Results from the phase 3 SINUS studies. Allergy 2022, 77, 2211–2221. [Google Scholar] [CrossRef]
- Bachert, C.; Luong, A.U.; Gevaert, P.; Mullol, J.; Smith, S.G.; Silver, J.; Sousa, A.R.; Howarth, P.H.; Benson, V.S.; Mayer, B.; et al. The unified airway hypothesis: Evidence from specific intervention with anti–IL-5 biologic therapy. J. Allergy Clin. Immunol. Pract. 2023, 11, 2630–2641. [Google Scholar] [CrossRef]
- De Corso, E.; Montuori, C.; Settimi, S.; Mele, D.A.; Cantiani, A.; Corbò, M.; Cantone, E.; Paludetti, G.; Galli, J. Efficacy of Biologics on Refractory Eosinophilic Otitis Media Associated with Bronchial Asthma or Severe Uncontrolled CRSwNP. J. Clin. Med. 2022, 11, 926. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Population Sample at the Baseline (n = 22) | |
|---|---|
| Mean Age, y | 55 ± 11.9 |
| Male | 11 (50%) |
| Smoking | 22 (31.8%) |
| CCS | 22 (100%) |
| OCS | 22 (100%) |
| Previous ESS | 22 (100%) |
| Asthma | 16 (72.7%) |
| Allergy | 20 (90.9%) |
| AERD | 9 (40.9%) |
| ASA TRIAD | 9 (40.9%) |
| Mean IgE | 108.8 ± 88.7 |
| Outcome Measure | Baseline Median (IQR) | 6-Months Median (IQR) | p-Value (95% CI) | Test Used |
|---|---|---|---|---|
| Mean EOS (0.1 × 103/μL) | 0.80 ± 0.49 | 0.84 ± 0.58 | 0.834 (−0.38–0.31) | Wilcoxon signed-rank |
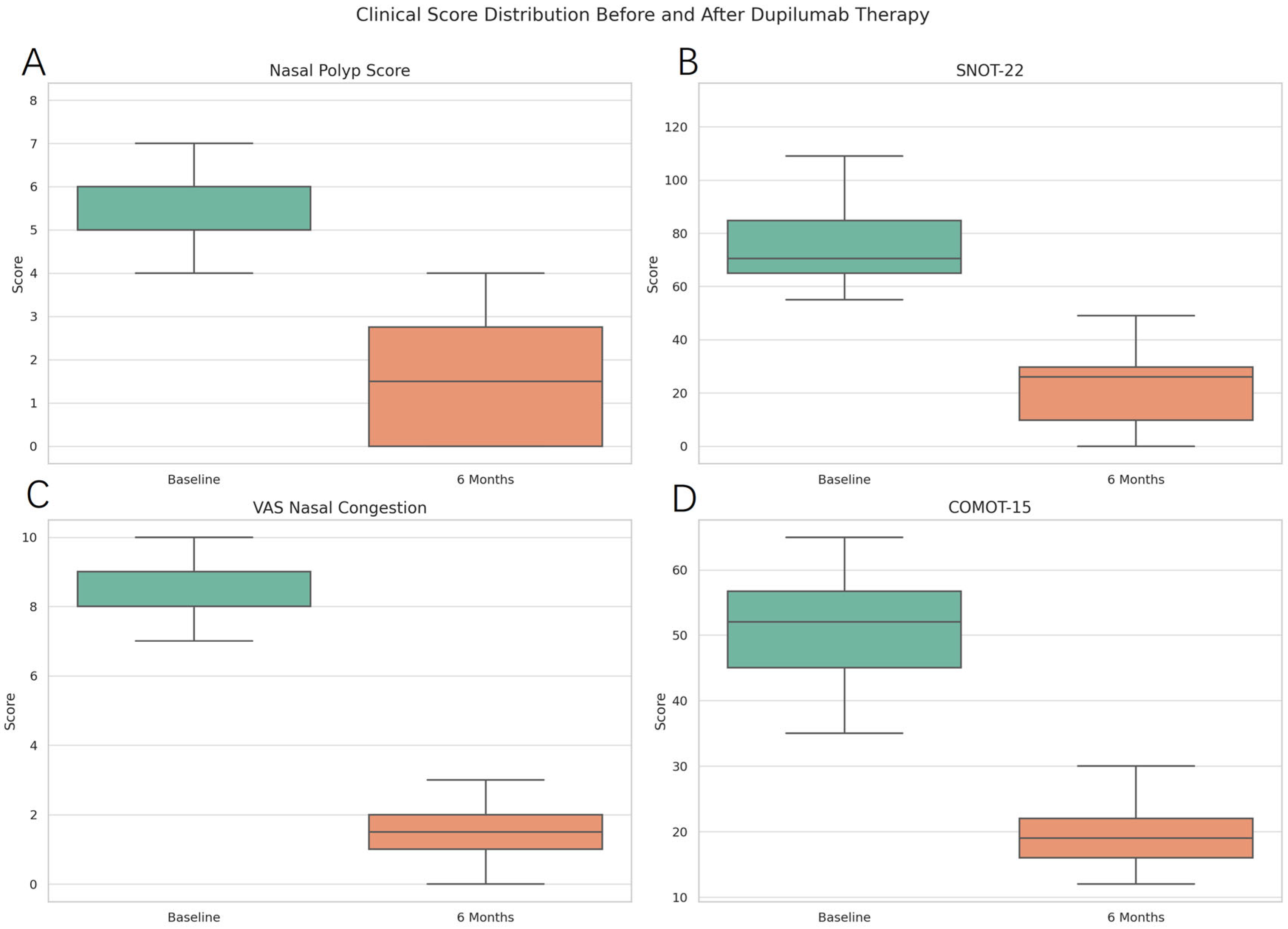
| Mean NPS | 5.7 ± 1.2 | 1.5 ± 1.3 | <0.0001 (3.61–4.76) | Wilcoxon signed-rank |
| Mean SNOT 22 | 77.6 ± 19.0 | 21.5 ± 13.4 | <0.0001 (45.13–66.96) | Wilcoxon signed-rank |
| Mean VAS | 8.4 ± 1.1 | 1.7 ± 1.2 | <0.0001 (5.92–7.54) | Wilcoxon signed-rank |
| Mean COMOT 15 | 51.3 ± 8.4 | 19.2 ± 5.0 | <0.0001 (28.90–35.28) | Wilcoxon signed-rank |
| Mean EOS | 0.80 ± 0.49 | 0.84 ± 0.58 | 0.834 (−0.38–0.31) | Wilcoxon signed-rank |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galletti, C.; Giammona Indaco, F.; Portelli, D.; Laterra, G.; Zambito, P.; Ferrisi, M.G.; Freni, L.; Ciodaro, F.; Freni, F.; Maira, S.; et al. Efficacy of Dupilumab in Patients with Chronic Rhinosinusitis with Nasal Polyps and Eosinophilic Otitis Media: A Six-Month Observational Study. Medicina 2025, 61, 1471. https://doi.org/10.3390/medicina61081471
Galletti C, Giammona Indaco F, Portelli D, Laterra G, Zambito P, Ferrisi MG, Freni L, Ciodaro F, Freni F, Maira S, et al. Efficacy of Dupilumab in Patients with Chronic Rhinosinusitis with Nasal Polyps and Eosinophilic Otitis Media: A Six-Month Observational Study. Medicina. 2025; 61(8):1471. https://doi.org/10.3390/medicina61081471
Chicago/Turabian StyleGalletti, Cosimo, Federica Giammona Indaco, Daniele Portelli, Giulia Laterra, Patrizia Zambito, Maria Grazia Ferrisi, Leonard Freni, Francesco Ciodaro, Francesco Freni, Salvatore Maira, and et al. 2025. "Efficacy of Dupilumab in Patients with Chronic Rhinosinusitis with Nasal Polyps and Eosinophilic Otitis Media: A Six-Month Observational Study" Medicina 61, no. 8: 1471. https://doi.org/10.3390/medicina61081471
APA StyleGalletti, C., Giammona Indaco, F., Portelli, D., Laterra, G., Zambito, P., Ferrisi, M. G., Freni, L., Ciodaro, F., Freni, F., Maira, S., & Galletti, B. (2025). Efficacy of Dupilumab in Patients with Chronic Rhinosinusitis with Nasal Polyps and Eosinophilic Otitis Media: A Six-Month Observational Study. Medicina, 61(8), 1471. https://doi.org/10.3390/medicina61081471