Abstract
Background and Objectives: The use of Artificial Intelligence (AI) in the medical field is rapidly expanding. This review aims to explore and summarize all published research on the development and validation of deep learning (DL) models in gynecologic laparoscopic surgeries. Materials and Methods: MEDLINE, IEEE Xplore, and Google scholar were searched for eligible studies published between January 2000 and May 2025. Selected studies developed a DL model using datasets derived from gynecologic laparoscopic procedures. The exclusion criteria included non-gynecologic datasets, non-laparoscopic datasets, non-Convolutional Neural Network (CNN) models, and non-English publications. Results: A total of 16 out of 621 studies met our inclusion criteria. The findings were categorized into four main application areas: (i) anatomy classification (n = 6), (ii) anatomy segmentation (n = 5), (iii) surgical instrument classification and segmentation (n = 5), and (iv) surgical action recognition (n = 5). Conclusions: This review emphasizes the growing role of AI in gynecologic laparoscopy, improving anatomy recognition, instrument tracking, and surgical action analysis. As datasets grow and computational capabilities advance, these technologies are poised to improve intraoperative guidance and standardize surgical training.
1. Introduction
Minimally Invasive Surgery (MIS), first developed in the 1980s and widely adopted in the 1990s, is now the standard approach for numerous procedures [1]. Despite the increase in reported procedures, the number of open surgeries performed continues to decline [2]. In gynecology, MIS is now the preferred approach for most benign and specific malignant conditions [3]. It offers numerous advantages, including decreased blood loss, shorter clinical stay, fewer wound infections, and reduced postoperative pain [4]. However, the technical demands of laparoscopic procedures, including limited degrees of freedom in instrument motion and two-dimensional visualization, pose significant challenges, particularly for inexperienced surgeons [5]. Enhancing intraoperative support through advanced technologies may help achieve more consistent surgical performance.
Deep learning (DL) is a subset of machine learning that uses algorithms inspired by the human brain’s structure and function to learn from unsupervised data [6]. Convolutional Neural Networks (CNNs) are a prominent DL method used in computer vision tasks like image classification, object detection, and segmentation. They consist of three main layers: convolutional layers, which extract features using kernels; pooling layers, which reduce the size of feature maps while preserving important features; and fully connected layers, which convert 2D feature maps into a 1D vector and perform classification. CNNs learn through a forward stage, where an input image passes through the layers, and a backward stage, which adjusts weights to minimize error [7].
DL has gained significant traction in the medical field in recent years. DL has been widely used in medical imaging for tasks such as image segmentation, classification, and reconstruction across various modalities like Magnetic Resonance Imaging (MRI), Computed Tomography (CT), Positron Emission Tomography (PET), ultrasound (US), and Optical Coherence Tomography (OCT) [8]. Beyond radiology, CNNs have demonstrated promising applications in multiple other medical domains, including polyp detection during colonoscopy [9], mitosis identification in breast cancer histology images [10], skin lesions classification in dermatology [11], brain tumor classification [12], and electrocardiogram (ECG) analysis [13], among numerous other uses. Laparoscopy offers large, high-resolution medical image datasets, providing a valuable resource for training CNN models.
CNNs have also been applied in endoscopic surgery, with laparoscopic cholecystectomy being the most frequently studied procedure, accounting for 72.73% of cases. Their applications include surgical tool detection and segmentation, anatomical structure recognition, tissue classification, and surgical phase identification. These technologies hold promise for enhancing intraoperative decision-making and improving surgical precision, though further clinical validation is necessary [14].
Despite growing interest, the use of DL in gynecologic laparoscopy remains relatively underexplored. The large volume of high-resolution visual data generated during laparoscopic procedures presents a valuable opportunity for the effective training of CNNs. This review aims to evaluate the current landscape of CNN applications in gynecologic laparoscopic surgery, focusing on tissue classification, segmentation of anatomical structures, and the automated recognition of surgical instruments and procedural phases.
2. Materials and Methods
Search strategy
A comprehensive literature search was conducted across MEDLINE, IEEE Xplore, and Google Scholar to identify studies published between January 2000 and May 2025 that examined DL applications in gynecologic laparoscopic surgery. To ensure completeness, the reference lists of all included studies were also manually reviewed to identify additional relevant publications.
Search queries were tailored for each database as follows:
- (1)
- MEDLINE: “Laparoscopy”[Mesh] AND (“Artificial Intelligence”[Mesh] OR “Deep Learning”[Mesh]) AND (“Gynecology”[Mesh] OR “Hysterectomy”[Mesh] OR “Uterine Myomectomy”[Mesh] OR “Endometriosis”[Mesh]).
- (2)
- IEEE Xplore: Laparoscopy AND Deep learning.
- (3)
- Google scholar: intitle: Laparoscopy OR intitle: Laparoscopic AND “Deep Learning” AND (“Gynecology” OR “Hysterectomy” OR “Uterine Myomectomy” OR “Endometriosis”).
Eligibility criteria
Studies were included if they developed and validated a CNN using datasets from gynecologic laparoscopic surgeries and addressed one or more of the following applications: (i) classification or segmentation of anatomical structures, (ii) detection, classification, or segmentation of surgical instruments, and (iii) recognition of surgical actions or procedural steps. The exclusion criteria were as follows: (i) studies using datasets not exclusively derived from gynecologic laparoscopic surgery, (ii) studies using datasets originating from cadaveric or laparoscopic training boxes, (iii) studies employing DL models other than CNNs, (iv) non-English publications, and (v) non-original research such as abstracts, reviews, editorials, and letters to the editor.
Study eligibility was independently assessed by two reviewers through title and abstract screening. Any disagreements were resolved by a third reviewer. Data extraction was also performed independently and cross-validated for accuracy. The extracted data included the study design, the type of surgical procedures analyzed, the basic CNN architecture employed for model development, the clinical application or task addressed by the model, and the performance metrics reported. A flowchart illustrating the study inclusion process is presented in Figure 1.
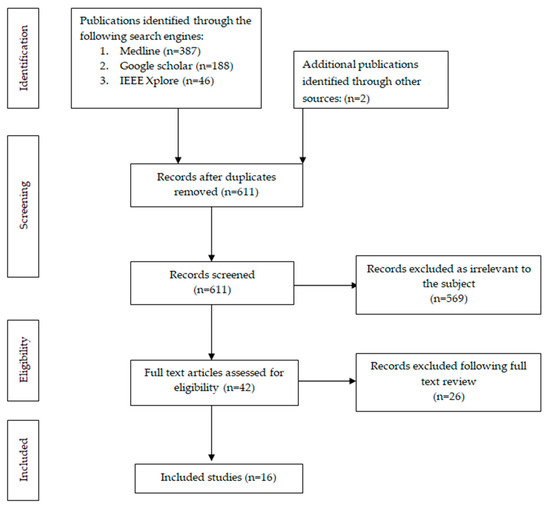
Figure 1.
Flowchart of search and inclusion process.
Most of the included studies developed CNNs trained on annotated laparoscopic images from gynecologic surgeries, often applying preprocessing techniques such as data augmentation when needed. Models were either trained from scratch with randomly initialized weights or developed through transfer learning by fine-tuning architectures like GoogLeNet Inception v1, developed by Google LLC (Mountain View, CA, USA), that are pretrained on large datasets. CNNs are trained on a training dataset, where they learn to recognize patterns by adjusting their internal parameters. After training, CNNs are evaluated on a separate test set containing new data to measure how well they generalize beyond the training examples. This performance is evaluated by metrics including accuracy, precision, recall, f1 score, Dice score, and intersection over union (IoU) [15].
3. Results
The results of the final studies that met our inclusion criteria (n = 16) are presented in Table 1. The main findings were as follows: (i) six studies (37.5%) addressed anatomy classification, (ii) five studies (31.2%) focused on anatomical structure segmentation, (iii) five studies (31.2%) covered surgical instrument classification and segmentation, and (iv) five studies (31.2%) investigated surgical action recognition. The evaluation metrics used in this paper are presented in Appendix A Table A1.

Table 1.
Included studies.
3.1. Anatomy Classification
Three studies applied CNNs to classify images based on the anatomical structures depicted. Leibetseder et al. implemented GoogleNet for image analysis of their dataset, LapGyn4, which consisted of 111 laparoscopic gynecologic procedures. The model achieved the highest recall rate for the ovary at 94.5%, followed by the colon at 91.9% and the uterus at 91.7%. The oviduct and liver achieved lower recall rates of 84.1% and 82.2%, respectively [20]. Petscharning et al. conducted a similar study, implementing both GoogleNet and AlexNet in their dataset of 111 laparoscopic gynecologic procedures, yielding less impressive results. GoogleNet outperformed AlexNet, achieving recall rates of 88.8% for the ovaries, 86.2% for the liver, 79.5% for the colon, 74.3% for the uterus, and 62.3% for the oviduct [21]. In their recent study, Konduri et al. utilized a full-resolution CNN (FrCNN), which distinguishes itself from traditional CNNs by maintaining the full resolution of input images through the replacement of conventional pooling layers with full resolution layers. LapGyn4 was used as their dataset and achieved outstanding results in organ classification, with an average precision and recall rate of 98.6% and 99.1%, respectively [22].
Three studies [24,25,26] focused on classifying images based on the presence or absence of endometriotic lesions. These studies utilized images from an open-access database, the GLENDA dataset, which comprises a large collection of images from laparoscopic surgery with annotated endometriotic lesions [31]. Among these studies, two [24,25] implemented ResNet50 as the foundational CNN architecture for image analysis and classification, while the third [26] employed eENet. Of note, the study by Nifora et al. demonstrated the highest performance, achieving a precision and recall rate of 99% [24] [Table 2].

Table 2.
Anatomy classification.
3.2. Anatomy Segmentation
Three studies applied CNNs to anatomy segmentation [16,17,18]. Madad Zadeh et al. [16] employed Mask R-CNN for image analysis on their proprietary dataset named SurgAI, consisting of eight laparoscopic hysterectomies. The study focused on segmenting the uterus and ovaries; uterus segmentation demonstrated good performance with an IoU of 84.5%, while ovary segmentation performed poorly with an IoU of 29.6% due to limited annotations and significant morphologic variability among patients [16]. In a subsequent study, Madad Zadeh et al. [17] implemented U-Net in their expanded SurgAI3.8K dataset consisting of 79 laparoscopic procedures: 48 hysterectomies, 21 endometriosis excisions, and 10 fertility explorations. The performance of uterus segmentation remained consistent, with an IoU of 84.9% [17].
Serban et al. applied the U-Net architecture to address the challenging task of segmenting critical anatomical structures—uterine arteries, ureters, and nerves—in a proprietary dataset comprising 38 laparoscopic gynecologic surgeries. Accurate segmentation of these structures is clinically significant, as it can help reduce the risk of injury during surgery, where differentiation between these tissues is often difficult. The initial multiclass model demonstrated a limited ability to differentiate between these three structures. To overcome this limitation, the researchers constructed separate binary models for each structure and then combined them using four ensemble techniques. The weighted pixel-wise ensemble technique achieved superior performance in segmenting the ureter, yielding an IoU of 42.01% and a Dice score of 49.99%. However, the weighted region-based ensemble proved more effective in segmenting uterine arteries and nerves, with IoU scores of 27.12% and 55.48%, respectively, along with corresponding Dice scores of 33.72% and 60.25%, respectively [18]. Wang et al. also employed U-Net to segment the ureter in their dataset, which consisted of eleven laparoscopic procedures—two hysterectomies, six hysterectomies with lymphadenectomy, and three endometriosis excisions. After fine-tuning, the model achieved an impressive performance, reaching a Dice coefficient of 77% in an independent test [19].
Leibetseder et al. aimed to advance endometriosis image classification by employing Mask R-CNN and the GLENDA dataset to segment lesions according to their anatomical location, i.e., peritoneum, ovary, uterus, and deep infiltrating endometriosis. They further classified the lesions by visual appearance into categories like ‘mucus’, ‘vesicles’, ‘implants’, and ‘abnormal tissue’. The results for both regional and appearance-based segmentation were disappointing, except for the ‘implants’ category. To address this, they created a new dataset focused solely on ‘implants’ lesions, named ENID, and achieved a mAP50 of 56.1% in segmenting those lesions [27] [Table 3]

Table 3.
Anatomy segmentation.
3.3. Surgical Instruments
Two studies [16,23] focused on surgical instrument segmentation, a third [22] on classifying surgical instruments, a fourth [20] on classifying images by the number of instruments, and a fifth [15] on classifying images based on the presence or absence of instruments. Kletz et al. conducted notable work by applying Mask R-CNN to a proprietary dataset of 333 video frames from laparoscopic hysterectomy and myomectomy procedures. The CNN provided segmentation masks and bounding boxes for eleven surgical instruments: bipolar, grasper, hook, irrigator, knot-pusher, morcellator, needle, needle-holder, scissors, sealer and divider, and trocar. The model achieved an AP50:95 of 42.9%, with an AP50 of 61.3% and an AR of 47.7% [23]. Madad Zadeh et al. utilized Mask R-CNN in the aforementioned SurgAI dataset for surgical tool segmentation without specifying the individual tool, achieving a mean IoU of 54.5%, an AP50 of 88%, and an AR of 86% [16]. Konduri et al. used the LapGyn4 dataset to train the aforementioned FrCNN to classify surgical instruments in the following three categories: grasper, hook, and scissors. The results were highly promising, yielding a precision of 89.6% and a recall of 98.9% [22]. Leibetseder et al. applied GoogleNet to categorize images based on the presence of zero, one, two, or three surgical instruments, with the goal of identifying the corresponding phase of the procedure. The dataset included 411 gynecologic laparoscopic surgeries (LapGyn4) along with the publicly available Cholec80 dataset, which contains images from laparoscopic cholecystectomy procedures. The model demonstrated a strong performance, achieving an average recall of 84.2% and an average precision of 84.1%. Kletz et al., in another study, utilized the LapGyn4 and Cholec80 datasets, employing GoogleNet to classify images as either containing a surgical instrument or not. The CNN was evaluated using a gynecologic dataset, achieving a recall of 95% for instrument presence and 77% for non-instrument presence [15] [Table 4].

Table 4.
Surgical instruments.
3.4. Surgical Action Recognition
Leibetseder et al. and Petscharing et al. implemented GoogleNet and both GoogleNet and AlexNet, respectively, in their aforementioned proprietary datasets to classify images based on the surgical actions depicted [20,21]. The surgical actions were categorized into eight types: blunt dissection, coagulation, cutting cold, cutting, hysterectomy (sling), injection, suction and irrigation, and suture. Leibetseder’s CNN achieved a better performance than Petscharing’s, with an average precision rate of 92.4% and a recall rate of 92.5% [20]. In Petscharing’s study, GoogleNet outperformed AlexNet, achieving an average precision of 59% and a recall rate of 61.7% [21]. In a subsequent paper, Petcharing et al. implemented a series of techniques to enhance the input images, yielding less impressive results than Leibetseider et al. [28]. Nasirihaghighi et al. developed a Convolutional Neural Network–Recurrent Neural Network (CNN-RNN) model, where the feature maps from the CNN layers are passed to the RNN layers to capture the temporal dependencies in sequential data. For the CNN backbone, they used VGG16, ResNet50, EfficientNetB2, and DensNeet121, with the RNN component being evaluated using four different architectural types. Their proprietary dataset consisted of 18 laparoscopic gynecologic surgeries. The CNN-RNN model with the best performance utilized ResNet50 as the CNN backbone, achieving an average accuracy of 86.78% for surgical action classification [29]. In addition, Müenzer et al. employed a proprietary dataset called SurgicalActions160, consisting of 160 five-second video clips extracted from gynecologic laparoscopic procedures. They classified these clips using DL methods across 16 surgical actions, ranking them from highest to lowest probability within each category. The average precision for each classification was then calculated, yielding moderate performance results [30]. Leibetseder et al. also trained a CNN to classify images into the following four categories of surgical action on anatomy: suturing the uterus, ovary, vagina, and others. However, the results were less notable, with an average precision of 63.4% and a recall of 62% [20] [Table 5].

Table 5.
Surgical action recognition.
4. Discussion
This review presents the findings of published studies investigating the application of DL in gynecologic laparoscopic surgeries. The included studies address a range of tasks, including classification and segmentation of both prominent and obscure anatomical structures, surgical instrument detection, and surgical action recognition. Overall, these studies demonstrate progressive advances in applying DL to surgical contexts, evolving from foundational tasks to more advanced applications like specific endometriotic lesion identification and distinct surgical step recognition.
4.1. Interpretation of Results
The findings related to anatomical classification and segmentation demonstrate that DL models are effective in identifying key anatomical structures in laparoscopic images derived from gynecologic procedures. Accurate anatomical segmentation through DL models holds significant clinical value by improving intraoperative orientation and enabling safer dissection of pelvic structures. High precision for major organs such as the uterus, ovary, and colon highlights the potential of these models to reduce operative time and prevent complications related to misidentification. This is particularly impactful in complex cases, where identifying key structures can be challenging. However, the performance of DL models remains limited for smaller yet vital structures, such as the ureter and uterine artery, where segmentation errors may directly compromise surgical safety. This is mainly caused by morphological variability and insufficiently annotated datasets. Addressing these gaps is essential to ensure that DL models can support efficiency and safety in surgical practice.
DL models are increasingly capable of correctly identifying surgical instruments, enabling a range of clinically valuable applications. By analyzing tool usage patterns, these models can support the indirect recognition of procedural phases, reducing reliance on manual annotations. Instrument recognition could also aid hospital workflow by linking detected tools to specific procedural codes, supporting accurate billing for consumables. Additionally, real-time detection of inappropriate instrument use near vital structures could serve as a decision support tool, issuing warnings that enhance intraoperative safety. These applications illustrate how DL can move beyond passive recognition toward intelligent surgical assistance systems that promote efficiency and safety in operating room environments.
The recognition of surgical actions and procedural phases by DL models represents a complex yet feasible application. By decomposing complex procedures into clearly defined steps, these models provide a structured framework that helps trainees understand the sequence and purpose of each phase. This enables focused practice of individual surgical phases, allowing learners to develop specific technical skills and strengthen decision-making processes tailored to each step of the procedure. Ultimately, this structured approach leads to better surgical proficiency, increased confidence, and improved competence for real-life operations.
4.2. Future Directions
The promising results from DL applications in laparoscopic surgery suggest a bright future, with much potential for further development. A primary future application is the development of real-time DL systems. Most existing models function retrospectively, analyzing images from recorded laparoscopic surgical videos rather than delivering live intraoperative feedback. For clinical utility, DL models must perform within real-time constraints while sustaining high accuracy. Object detection models such as You Only Look Once (YOLO), which process images in a single pass with rapid inference speeds, represent promising candidates for real-time surgical applications [32]. Future DL models could provide real-time decision support by recognizing surgical phases, identifying anatomical structures, and flagging potential risks, such as vascular or ureteral proximity during dissection. This could reduce errors and guide less experienced surgeons during complex procedures. Another potential application of DL lies in the objective evaluation of surgical performance. By analyzing tool usage patterns, movement precision, timing, and intraoperative error rates, DL models can generate quantitative assessments of technical skills. In residency training programs, such tools could support individualized feedback, identify specific areas for improvement, and ensure that competency benchmarks are consistently met. Another potential application is the use of DL models in preoperative planning, where analysis of imaging and clinical parameters could help predict surgical complexity and anticipate potential complications. Such predictive capabilities could enhance surgical planning and contribute to more efficient operating room time management. Lastly, by recognizing surgical instruments, procedural phases, and surgeon actions, DL systems have the potential to generate detailed and accurate operative reports in real time. This capability can significantly reduce the bureaucratic workload of surgical teams while maintaining the completeness and quality of surgical documentation.
4.3. Limitations
There are several limitations to consider. Firstly, DL models failed to achieve acceptable performance in tasks such as the segmentation of less prominent but clinically crucial structures, such as the ureter, uterine artery, and nerves. This challenge likely arises from the morphological and topographic variability in these structures, as well as from insufficiently annotated or limited datasets. Therefore, the development of large-scale, publicly available, and medically annotated datasets derived from gynecologic surgeries is essential. Existing datasets are often limited to single institutions and lack procedural diversity, which restricts the generalizability and reproducibility of research findings. Expanding the diversity and volume of such data with multi-institutional collaborations will significantly enhance the performance of DL models. Another important limitation is the significant variation in evaluation metrics across studies and applications, making direct comparisons and meta-analyses of data non-feasible. Additionally, most studies are published in computational journals, which often focus on technical aspects rather than medical context, meaning that crucial clinical features and medical insights may be underreported or not sufficiently emphasized. Lastly, although evaluation metrics assess model performance, there is a critical need to conduct large clinical trials to evaluate the impact of DL models on key surgical outcomes, such as operative time, complication rates, and patient recovery, ensuring that these technologies provide measurable benefits to patient care.
5. Conclusions
This review highlights the promising results of AI models within the medical field of gynecologic laparoscopy, with applications ranging from foundational to more complex tasks. In the near future, the availability of large, high-quality datasets will support the development of advanced DL models, enabling their application to surgical practice. The successful integration of these models has the potential to significantly impact clinical practices and training protocols, shaping the future of gynecologic surgery. However, the true extent of this impact will need to be evaluated through clinical trials.
Author Contributions
Conceptualization, V.B., F.G., M.P. and A.D.; methodology, V.B., F.G. and A.D.; investigation, V.B., C.S., D.R.K., G.G. and A.V.; writing—original draft preparation, V.B. and F.G.; writing—review and editing, F.G. and A.D.; supervision, F.G., M.P. and A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors have reviewed and edited the output and take full responsibility for the content of this publication. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Evaluation metrics.
Table A1.
Evaluation metrics.
| Evaluation Metrics |
|---|
| Accuracy = TP + TN/TP + TN + FP + FN |
| Precision = TP/TP + FP |
| Recall = TP/TP + FN |
| spec = TN/FP + TN |
| f1 = 2TP/2TP + FP + FN |
| mcc = TP × TN − FP × FN/ |
| Jaccard = IoU = |gt ∩ P|/|gt ∪ P| |
| Dice score = 2|gt ∩ P|/|gt| + |P| |
| AP50: average precision for IoU threshold of 50% |
| AP50:95: precision averaged over IoU thresholds of 50% to 90% obtained in increments of 5% |
| AR: average recall |
TP: true positive; TN: true negative; FP: false positive; FN: false negative; IoU: intersection over union; gt: ground truth; P: prediction.
References
- St John, A.; Caturegli, I.; Kubicki, N.S.; Kavic, S.M. The Rise of Minimally Invasive Surgery: 16 Year Analysis of the Progressive Replacement of Open Surgery with Laparoscopy. JSLS 2020, 24, e2020.00076. [Google Scholar] [CrossRef]
- Bingmer, K.; Ofshteyn, A.; Stein, S.L.; Marks, J.M.; Steinhagen, E. Decline of Open Surgical Experience for General Surgery Residents. Surg. Endosc. 2020, 34, 967–972. [Google Scholar] [CrossRef]
- Bankar, G.R.; Keoliya, A. Robot-Assisted Surgery in Gynecology. Cureus 2019, 14, e29190. [Google Scholar] [CrossRef]
- Shi, J.; Cui, R.; Wang, Z.; Yan, Q.; Ping, L.; Zhou, H.; Gao, J.; Fang, C.; Han, X.; Hua, S. Deep Learning HRNet-FCN for Blood Vessel Identification in Laparoscopic Pancreatic Surgery. npj Digit. Med. 2025, 8, 235. [Google Scholar] [CrossRef] [PubMed]
- Ballantyne, G.H. The Pitfalls of Laparoscopic Surgery: Challenges for Robotics and Telerobotic Surgery. Surg. Laparosc. Endosc. Percutan. Tech. 2002, 12, 1–5. [Google Scholar] [CrossRef]
- Bashar, A. Survey on Evolving Deep Learning Neural Network Architectures. J. Artif. Intell. 2019, 1, 73–82. [Google Scholar]
- Guo, Y.; Liu, Y.; Oerlemans, A.; Lao, S.; Wu, S.; Lew, M.S. Deep Learning for Visual Understanding. Neurocomputing 2016, 187, 27–48. [Google Scholar] [CrossRef]
- Zhang, H.; Qie, Y. Applying Deep Learning to Medical Imaging: A Review. Appl. Sci. 2023, 13, 10521. [Google Scholar] [CrossRef]
- Park, S.Y.; Sargent, D. Colonoscopic Polyp Detection Using Convolutional Neural Networks. In Proceedings of the Medical Imaging 2016: Computer-Aided Diagnosis, San Diego, CA, USA, 27 February–3 March 2016; SPIE: Bellingham, WA, USA, 2016; Volume 9785, pp. 577–582. [Google Scholar]
- Albarqouni, S.; Baur, C.; Achilles, F.; Belagiannis, V.; Demirci, S.; Navab, N. AggNet: Deep Learning From Crowds for Mitosis Detection in Breast Cancer Histology Images. IEEE Trans. Med. Imaging 2016, 35, 1313–1321. [Google Scholar] [CrossRef]
- Esteva, A.; Kuprel, B.; Novoa, R.A.; Ko, J.; Swetter, S.M.; Blau, H.M.; Thrun, S. Dermatologist-Level Classification of Skin Cancer with Deep Neural Networks. Nature 2017, 542, 115–118. [Google Scholar] [CrossRef]
- Deepak, S.; Ameer, P.M. Brain Tumor Classification Using Deep CNN Features via Transfer Learning. Comput. Biol. Med. 2019, 111, 103345. [Google Scholar] [CrossRef]
- Burger, A.; Qian, C.; Schiele, G.; Helms, D. An Embedded CNN Implementation for On-Device ECG Analysis. In Proceedings of the 2020 IEEE International Conference on Pervasive Computing and Communications Workshops (PerCom Workshops), Austin, TX, USA, 23–27 March 2020; pp. 1–6. [Google Scholar]
- Fernandes, N.; Oliveira, E.; Rodrigues, N.F. Future Perspectives of Deep Learning in Laparoscopic Tool Detection, Classification, and Segmentation: A Systematic Review. In Proceedings of the 2023 IEEE 11th International Conference on Serious Games and Applications for Health (SeGAH), Athens, Greece, 28–30 December 2023; pp. 1–8. [Google Scholar]
- Kletz, S.; Schoeffmann, K.; Husslein, H. Learning the Representation of Instrument Images in Laparoscopy Videos. Healthc. Technol. Lett. 2019, 6, 197–203. [Google Scholar] [CrossRef]
- Madad Zadeh, S.; Francois, T.; Calvet, L.; Chauvet, P.; Canis, M.; Bartoli, A.; Bourdel, N. SurgAI: Deep Learning for Computerized Laparoscopic Image Understanding in Gynaecology. Surg. Endosc. 2020, 34, 5377–5383. [Google Scholar] [CrossRef]
- Madad Zadeh, S.; François, T.; Comptour, A.; Canis, M.; Bourdel, N.; Bartoli, A. SurgAI3.8K: A Labeled Dataset of Gynecologic Organs in Laparoscopy with Application to Automatic Augmented Reality Surgical Guidance. J. Minim. Invasive Gynecol. 2023, 30, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Serban, N.; Kupas, D.; Hajdu, A.; Török, P.; Harangi, B. Distinguishing the Uterine Artery, the Ureter, and Nerves in Laparoscopic Surgical Images Using Ensembles of Binary Semantic Segmentation Networks. Sensors 2024, 24, 2926. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, C.; Zhang, Z.; Deng, Y.; Xiao, M.; Zhang, Z.; Dekker, A.; Wang, S.; Liu, Y.; Qian, L.; et al. Real-time Auto-segmentation of the Ureter in Video Sequences of Gynaecological Laparoscopic Surgery. Int. J. Med. Robot. 2024, 20, e2604. [Google Scholar] [CrossRef] [PubMed]
- Leibetseder, A.; Petscharnig, S.; Primus, M.J.; Kletz, S.; Münzer, B.; Schoeffmann, K.; Keckstein, J. Lapgyn4: A Dataset for 4 Automatic Content Analysis Problems in the Domain of Laparoscopic Gynecology. In Proceedings of the 9th ACM Multimedia Systems Conference, Amsterdam, The Netherlands, 12–15 June 2018; ACM: New York, NY, USA, 2018; pp. 357–362. [Google Scholar]
- Petscharnig, S.; Schöffmann, K. Learning Laparoscopic Video Shot Classification for Gynecological Surgery. Multimed. Tools Appl. 2018, 77, 8061–8079. [Google Scholar] [CrossRef]
- Konduri, P.S.; Rao, G.S.N. Full Resolution Convolutional Neural Network Based Organ and Surgical Instrument Classification on Laparoscopic Image Data. Biomed. Signal Process. Control 2024, 87, 105533. [Google Scholar] [CrossRef]
- Kletz, S.; Schoeffmann, K.; Benois-Pineau, J.; Husslein, H. Identifying Surgical Instruments in Laparoscopy Using Deep Learning Instance Segmentation. In Proceedings of the 2019 International Conference on Content-Based Multimedia Indexing (CBMI), Dublin, Ireland, 4–6 September 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 1–6. [Google Scholar]
- Nifora, C.; Chasapi, L.; Chasapi, M.-K.; Koutsojannis, C. Deep Learning Improves Accuracy of Laparoscopic Imaging Classification for Endometriosis Diagnosis. J. Clin. Med. Surg. 2024, 4, 1137. [Google Scholar]
- Visalaxi, S.; Muthu, T.S. Automated Prediction of Endometriosis Using Deep Learning. Int. J. Nonlinear Anal. Appl. 2021, 12, 2403–2416. [Google Scholar]
- Acharya, D.; Guda, R.K.S.; Raovenkatajammalamadaka, K. Enhanced Efficientnet Network for Classifying Laparoscopy Videos Using Transfer Learning Technique. In Proceedings of the 2022 International Joint Conference on Neural Networks (IJCNN), Padua, Italy, 18–23 July 2022; IEEE: Piscataway, NJ, USA, 2022; pp. 1–9. [Google Scholar]
- Leibetseder, A.; Schoeffmann, K.; Keckstein, J.; Keckstein, S. Endometriosis Detection and Localization in Laparoscopic Gynecology. Multimed. Tools Appl. 2022, 81, 6191–6215. [Google Scholar] [CrossRef]
- Petscharnig, S.; Schöffmann, K.; Benois-Pineau, J.; Chaabouni, S.; Keckstein, J. Early and Late Fusion of Temporal Information for Classification of Surgical Actions in Laparoscopic Gynecology. In Proceedings of the 2018 IEEE 31st International Symposium on Computer-Based Medical Systems (CBMS), Karlstad, Sweden, 18–21 June 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 369–374. [Google Scholar]
- Nasirihaghighi, S.; Ghamsarian, N.; Stefanics, D.; Schoeffmann, K.; Husslein, H. Action Recognition in Video Recordings from Gynecologic Laparoscopy. In Proceedings of the 2023 IEEE 36th International Symposium on Computer-Based Medical Systems (CBMS), L’Aquila, Italy, 22–24 June 2023; IEEE: Piscataway, NJ, USA, 2023; pp. 29–34. [Google Scholar]
- Müenzer, B.; Primus, M.J.; Kletz, S.; Petscharnig, S.; Schoeffmann, K. Static vs. Dynamic Content Descriptors for Video Retrieval in Laparoscopy. In Proceedings of the 2017 IEEE International Symposium on Multimedia (ISM), Taichung, Taiwan, 11–13 December 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 216–223. [Google Scholar]
- Leibetseder, A.; Kletz, S.; Schoeffmann, K.; Keckstein, S.; Keckstein, J. GLENDA: Gynecologic Laparoscopy Endometriosis Dataset. In International Conference on Multimedia Modeling; Springer International Publishing: Cham, Switzerland, 2019; pp. 439–450. [Google Scholar]
- Jiang, P.; Ergu, D.; Liu, F.; Cai, Y.; Ma, B. A Review of Yolo Algorithm Developments. Procedia Comput. Sci. 2022, 199, 1066–1073. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).