Peripheral Parenteral Nutrition and Personalized Nutritional Approach After Colorectal Resection Surgery: A Comprehensive Review of Current Evidence
Abstract
1. Introduction: Postoperative Stress and Metabolic Disturbances
2. Perioperative Malnutrition
2.1. Malnutrition Screening Assessment
2.2. Prehabilitation and Exercise
2.3. Enhanced Recovery After Surgery (ERAS) Protocol
3. The Role of Peripheral Parenteral Nutrition
3.1. PPN in Colorectal Surgery
3.2. Practical Aspects Regarding PPN Use
3.3. Adverse Effects of PPN
4. Nutritional Modulation in Colorectal Surgery
5. Health Economics of PPN
5.1. PPN and Healthcare Cost Reduction
5.2. Bespoke Use of PPN
6. Gut Microbiota and Perioperative Nutrition
6.1. Malnutrition and Dysbiosis
6.2. Nutritional Enhancement and Microbiome
7. Immunonutrition and PPN
7.1. Immunonutrition in Gastrointestinal Surgery
- Glutamine, which serves as a primary fuel for enterocytes and immune cells, enhances heat shock protein expression and reduces oxidative stress [56].
- Arginine, which promotes nitric oxide synthesis, enhances T-cell function and improves wound healing [57].
- Omega-3 fatty acids, which displace arachidonic acid in cell membranes and reduce pro-inflammatory eicosanoids and cytokine release [58].
- Nucleotides and selenium, which support cellular replication and antioxidant defence, respectively [59].
7.2. Pharmaconutrition and PPN
8. Future Research
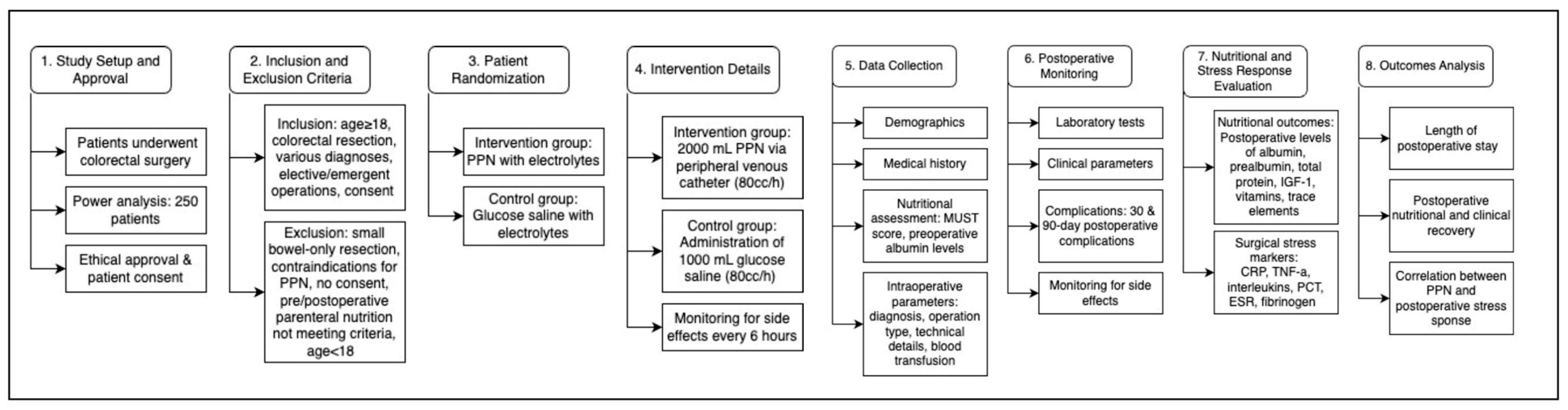
8.1. Study Population and Intervention
8.2. Study Outcomes
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bain, C.R.; Myles, P.S.; Martin, C.; Wallace, S.; Shulman, M.A.; Corcoran, T.; Bellomo, R.; Peyton, P.; Story, D.A.; Leslie, K.; et al. Postoperative systemic inflammation after major abdominal surgery: Patient-centred outcomes. Anaesthesia 2023, 78, 1365–1375. [Google Scholar] [CrossRef]
- Prete, A.; Yan, Q.; Al-Tarrah, K.; Akturk, H.K.; Prokop, L.J.; Alahdab, F.; Foster, M.A.; Lord, J.M.; Karavitaki, N.; Wass, J.A. The cortisol stress response induced by surgery: A systematic review and meta-analysis. Clin. Endocrinol. 2018, 89, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Tewari, N.; Awad, S.; Duška, F.; Williams, J.P.; Bennett, A.; Macdonald, I.A.; Lobo, D.N. Postoperative inflammation and insulin resistance in relation to body composition, adiposity and carbohydrate treatment: A randomised controlled study. Clin. Nutr. 2019, 38, 204–212. [Google Scholar] [CrossRef]
- Yoshida, T.; Delafontaine, P. Mechanisms of igf-1-mediated regulation of skeletal muscle hypertrophy and atrophy. Cells 2020, 9, 1970. [Google Scholar] [CrossRef]
- Stitt, T.N.; Drujan, D.; Clarke, B.A.; Panaro, F.; Timofeyva, Y.; Kline, W.O.; Gonzalez, M.; Yancopoulos, G.D.; Glass, D.J. The igf-1/pi3k/akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting foxo transcription factors. Mol. Cell 2004, 14, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Cañueto, D.; Salek, R.M.; Bulló, M.; Correig, X.; Cañellas, N. Application of machine learning solutions to optimize parameter prediction to enhance automatic nmr metabolite profiling. Metabolites 2022, 12, 283. [Google Scholar] [CrossRef]
- Gillis, C.; Carli, F. Promoting perioperative metabolic and nutritional care. Anesthesiology 2015, 123, 1455–1472. [Google Scholar] [CrossRef]
- Cardinale, F.; Chinellato, I.; Caimmi, S.; Peroni, D.G.; Franceschini, F.; Del Giudice, M.M.; Bernardini, R. Perioperative period: Immunological modifications. Int. J. Immunopathol. Pharmacol. 2011, 24, S3–S12. [Google Scholar] [CrossRef]
- Yan, Y.; Jin, P.; Lu, J.; Cheng, D.; Xu, J.; Yuan, J.; Yu, Z.; Hu, Y. Postoperative cytokine levels and their predictive value in critical patients after major abdominal surgery: A retrospective cohort study. Ann. Palliat. Med. 2022, 11, 15. [Google Scholar] [CrossRef]
- Meissner, C.; Tiegges, S.; Broehl, M.; Otto, R.; Ridwelski, K. International study on the prevalence of malnutrition in centralized care for colorectal cancer patients. Innov. Surg. Sci. 2023, 8, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Guida, A.M.; Franceschilli, M.; Leonetti, G.; Bellato, V.; Pirozzi, B.M.; Fiorani, C.; Siragusa, L.; Sica, G.S. Pathophysiology of metabolic changes and malnutrition in colorectal cancer’s patients. AME Med. J. 2025, 10, 13. [Google Scholar] [CrossRef]
- Rémond, D.; Shahar, D.R.; Gille, D.; Pinto, P.; Kachal, J.; Peyron, M.A.; Santos, C.N.D.; Walther, B.; Bordoni, A.; Dupont, D.; et al. Understanding the gastrointestinal tract of the elderly to develop dietary solutions that prevent malnutrition. Oncotarget 2015, 6, 13858–13898. [Google Scholar] [CrossRef]
- Shin, C.H.; Long, D.R.; McLean, D.; Grabitz, S.D.; Ladha, K.; Timm, F.P.; Thevathasan, T.; Pieretti, A.; Ferrone, C.; Hoeft, A.; et al. Effects of intraoperative fluid management on postoperative outcomes: A hospital registry study. Ann. Surg. 2018, 267, 1084–1092. [Google Scholar] [CrossRef]
- Xu, H.; Kong, F. Malnutrition-related factors increased the risk of anastomotic leak for rectal cancer patients undergoing surgery. Biomed. Res. Int. 2020, 2020, 5059670. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Baracos, V.; Bertz, H.; Bozzetti, F.; Calder, P.C.; Deutz, N.E.P.; Erickson, N.; Laviano, A.; Lisanti, M.P.; Lobo, D.N.; et al. Espen expert group recommendations for action against cancer-related malnutrition. Clin. Nutr. 2017, 36, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Gazouli, A.; Georgiou, K.; Frountzas, M.; Tsourouflis, G.; Boyanov, N.; Nikiteas, N.; Gazouli, M.; Theodoropoulos, G.E. Perioperative nutritional assessment and management of patients undergoing gastrointestinal surgery. Ann. Gastroenterol. 2024, 37, 142–154. [Google Scholar] [CrossRef]
- Knight, S.R.; Qureshi, A.U.; Drake, T.M.; Lapitan, M.C.M.; Maimbo, M.; Yenli, E.; Tabiri, S.; Ghosh, D.; Kingsley, P.A.; Sundar, S. The impact of preoperative oral nutrition supplementation on outcomes in patients undergoing gastrointestinal surgery for cancer in low-and middle-income countries: A systematic review and meta-analysis. Sci. Rep. 2022, 12, 12456. [Google Scholar] [CrossRef]
- Bausys, A.; Kryzauskas, M.; Abeciunas, V.; Degutyte, A.E.; Bausys, R.; Strupas, K.; Poskus, T. Prehabilitation in modern colorectal cancer surgery: A comprehensive review. Cancers 2022, 14, 5017. [Google Scholar] [CrossRef]
- Gillis, C.; Buhler, K.; Bresee, L.; Carli, F.; Gramlich, L.; Culos-Reed, N.; Sajobi, T.T.; Fenton, T.R. Effects of nutritional prehabilitation, with and without exercise, on outcomes of patients who undergo colorectal surgery: A systematic review and meta-analysis. Gastroenterology 2018, 155, 391–410.e4. [Google Scholar] [CrossRef] [PubMed]
- Molenaar, C.J.L.; Minnella, E.M.; Coca-Martinez, M.; Cate, D.W.G.T.; Regis, M.; Awasthi, R.; Martínez-Palli, G.; López-Baamonde, M.; Sebio-Garcia, R.; Feo, C.V. Effect of multimodal prehabilitation on reducing postoperative complications and enhancing functional capacity following colorectal cancer surgery: The prehab randomized clinical trial. JAMA Surg. 2023, 158, 572–581. [Google Scholar] [CrossRef]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hübner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.G. Espen practical guideline: Clinical nutrition in surgery. Clin. Nutr. 2021, 40, 4745–4761. [Google Scholar] [CrossRef]
- Jochum, S.B.; Ritz, E.M.; Bhama, A.R.; Hayden, D.M.; Saclarides, T.J.; Favuzza, J. Early feeding in colorectal surgery patients: Safe and cost effective. Int. J. Color. Dis. 2020, 35, 465–469. [Google Scholar] [CrossRef]
- Herbert, G.; Perry, R.; Andersen, H.K.; Atkinson, C.; Penfold, C.; Lewis, S.J.; Ness, A.R.; Thomas, S. Early enteral nutrition within 24 hours of lower gastrointestinal surgery versus later commencement for length of hospital stay and postoperative complications. Cochrane Database Syst. Rev. 2019, 7, CD004080. [Google Scholar] [CrossRef]
- Byrnes, A.; Worrall, J.; Young, A.; Mudge, A.; Banks, M.; Bauer, J. Early post-operative diet upgrade in older patients may improve energy and protein intake but patients still eat poorly: An observational pilot study. J. Hum. Nutr. Diet. 2018, 31, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Beard, T.L.; Leslie, J.B.; Nemeth, J. The opioid component of delayed gastrointestinal recovery after bowel resection. J. Gastrointest. Surg. 2011, 15, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Studer, P.; Räber, G.; Ott, D.; Candinas, D.; Schnüriger, B. Risk factors for fatal outcome in surgical patients with postoperative aspiration pneumonia. Int. J. Surg. 2016, 27, 21–25. [Google Scholar] [CrossRef]
- Senkal, M.; Bonavina, L.; Reith, B.; Caruso, R.; Matern, U.; Duran, M. Perioperative peripheral parenteral nutrition to support major gastrointestinal surgery: Expert opinion on treating the right patients at the right time. Clin. Nutr. ESPEN 2021, 43, 16–24. [Google Scholar] [CrossRef]
- Iresjö, B.M.; Engström, C.; Smedh, U.; Lundholm, K. Overnight steady-state infusions of parenteral nutrition on myosin heavy chain transcripts in rectus abdominis muscle related to amino acid transporters, insulin-like growth factor 1, and blood amino acids in patients aimed at major surgery. JPEN J. Parenter. Enter. Nutr. 2019, 43, 497–507. [Google Scholar] [CrossRef]
- Kornbau, C.; Lee, K.C.; Hughes, G.D.; Firstenberg, M.S. Central line complications. Int. J. Crit. Illn. Inj. Sci. 2015, 5, 170–178. [Google Scholar] [CrossRef]
- Inayat-Hussain, A.; Falck, H.; Oorschot, S.; Picardo, S.; So, K. Peripheral parenteral nutrition: An evaluation of its use, safety and cost implications in a tertiary hospital setting. Clin. Nutr. ESPEN 2023, 56, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Scolapio, J.S.; Picco, M.F.; Tarrosa, V.B. Enteral versus parenteral nutrition: The patient’s preference. JPEN J. Parenter. Enter. Nutr. 2002, 26, 248–250. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Laing, E.; Beaumont, A.; Wong, J.; Warrier, S.; Heriot, A. Peripheral parenteral nutrition in surgery—A systematic review and meta-analysis. Clin. Nutr. ESPEN 2023, 54, 337–348. [Google Scholar] [CrossRef]
- Gys, T.; Peeters, R.; Hubens, A. The value of short-term peripheral parenteral nutrition after colorectal surgery: A comparative study with conventional postoperative intravenous fluid. Acta Chir. Belg. 1990, 90, 234–239. [Google Scholar]
- Liu, M.-Y.; Tang, H.-C.; Yang, H.-L.; Huang, H.-H.; Chang, S.-J. Hypo-calories with micronutrients and fat emulsion of pre-operative peripheral parenteral nutrition in malnutrition risk rectal cancer patients: A retrospective cross-sectional study. Food Nutr. Sci. 2013, 4, 821–826. [Google Scholar] [CrossRef]
- Sánchez-Guillén, L.; Soriano-Irigaray, L.; López-Rodríguez-Arias, F.; Barber, X.; Murcia, A.; Alcaide, M.J.; Aranaz-Ostáriz, V.; Soler-Silva, Á.; Navarro-Ruiz, A.; Arroyo, A. Effect of early peripheral parenteral nutrition support in an enhanced recovery program for colorectal cancer surgery: A randomized open trial. J. Clin. Med. 2021, 10, 3647. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Chen, L.-J.; Tsao, L.-Y.; Chen, H.-N.; Lee, C.-H.; Hsiao, C.-C. Parenteral nutrition with fish oil-based lipid emulsion reduces the risk of cholestasis in preterm infants. J. Int. Med. Res. 2021, 49, 3000605211011805. [Google Scholar] [CrossRef]
- Nightingale, J. Nutrition support teams: How they work, are set up and maintained. Frontline Gastroenterol. 2010, 1, 171–177. [Google Scholar] [CrossRef]
- Worthington, P.; Gura, K.M.; Kraft, M.D.; Nishikawa, R.; Guenter, P.; Sacks, G.S. Update on the use of filters for parenteral nutrition: An aspen position paper. Nutr. Clin. Pract. 2021, 36, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Solomon, D.M.; Emery, E.Z.; Kavelak, H.L.; Pontiggia, L.; Hollands, J.M.; Bingham, A.L. Impact of implementation of the american society for parenteral and enteral nutrition model for parenteral nutrition order writing and review on competency, attitudes, and perceptions. Nutr. Clin. Pract. 2019, 34, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Sun, Y.; Hou, F.; Yun, L. Severe side effects caused by parenteral nutrition therapy with fat emulsion (10%)/amino acids (15)/glucose (20%) injection: 2 case reports. Transl. Cancer Res. 2022, 11, 1813–1818. [Google Scholar] [CrossRef] [PubMed]
- Sadu Singh, B.K.; Narayanan, S.S.; Khor, B.H.; Sahathevan, S.; Gafor, A.H.A.; Fiaccadori, E.; Sundram, K.; Karupaiah, T. Composition and functionality of lipid emulsions in parenteral nutrition: Examining evidence in clinical applications. Front. Pharmacol. 2020, 11, 506. [Google Scholar] [CrossRef]
- Calder, P.C.; Waitzberg, D.L.; Klek, S.; Martindale, R.G. Lipids in parenteral nutrition: Biological aspects. J. Parenter. Enter. Nutr. 2020, 44, S21–S27. [Google Scholar] [CrossRef] [PubMed]
- Boersema, G.S.A.; Wu, Z.; Menon, A.G.; Kleinrensink, G.J.; Jeekel, J.; Lange, J.F. Systemic inflammatory cytokines predict the infectious complications but not prolonged postoperative ileus after colorectal surgery. Mediat. Inflamm. 2018, 2018, 7141342. [Google Scholar] [CrossRef] [PubMed]
- Theodore Armand, T.P.; Nfor, K.A.; Kim, J.I.; Kim, H.C. Applications of artificial intelligence, machine learning, and deep learning in nutrition: A systematic review. Nutrients 2024, 16, 1073. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, S.; Li, Z.; Li, M.; Zhang, Y.; He, M.; Jin, C.; Gao, C.; Gong, J. Insufficient post-operative energy intake is associated with failure of enhanced recovery programs after laparoscopic colorectal cancer surgery: A prospective cohort study. Front. Nutr. 2021, 8, 768067. [Google Scholar] [CrossRef]
- Tan, E.; Chen, H.L.R.; Chok, A.Y.; Tan, I.E.; Zhao, Y.; Lee, R.S.; Ang, K.A.; Au, M.K.H.; Ong, H.S.; Ho, H.S.S.; et al. A reduction in hospital length of stay reduces costs for colorectal surgery: An economic evaluation of the national surgical quality improvement program in singapore. Int. J. Color. Dis. 2023, 38, 257. [Google Scholar] [CrossRef]
- Karanikki, E.; Frountzas, M.; Lidoriki, I.; Kozadinos, A.; Mylonakis, A.; Tsikrikou, I.; Kyriakidou, M.; Toutouza, O.; Koniaris, E.; Theodoropoulos, G.E.; et al. The predictive role of preoperative malnutrition assessment in postoperative outcomes of patients undergoing surgery due to gastrointestinal cancer: A cross-sectional observational study. J. Clin. Med. 2024, 13, 7479. [Google Scholar] [CrossRef]
- Morris, A.M.; Baldwin, L.M.; Matthews, B.; Dominitz, J.A.; Barlow, W.E.; Dobie, S.A.; Billingsley, K.G. Reoperation as a quality indicator in colorectal surgery: A population-based analysis. Ann. Surg. 2007, 245, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Coluzzi, F.; Scerpa, M.S.; Loffredo, C.; Borro, M.; Pergolizzi, J.V.; LeQuang, J.A.; Alessandri, E.; Simmaco, M.; Rocco, M. Opioid use and gut dysbiosis in cancer pain patients. Int. J. Mol. Sci. 2024, 25, 7999. [Google Scholar] [CrossRef]
- Ma, T.; Xue, X.; Tian, H.; Zhou, X.; Wang, J.; Zhao, Z.; Wang, M.; Song, J.; Feng, R.; Li, L.; et al. Effect of the gut microbiota and their metabolites on postoperative intestinal motility and its underlying mechanisms. J. Transl. Med. 2023, 21, 349. [Google Scholar] [CrossRef]
- Iddir, M.; Brito, A.; Dingeo, G.; Del Campo, S.S.F.; Samouda, H.; La Frano, M.R.; Bohn, T. Strengthening the immune system and reducing inflammation and oxidative stress through diet and nutrition: Considerations during the covid-19 crisis. Nutrients 2020, 12, 1562. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Schwarz, J.M.; Montain, S.J.; Rood, J.; Pikosky, M.A.; Castaneda-Sceppa, C.; Glickman, E.; Young, A.J. High protein diet maintains glucose production during exercise-induced energy deficit: A controlled trial. Nutr. Metab. 2011, 8, 26. [Google Scholar] [CrossRef]
- Krezalek, M.A.; Alverdy, J.C. The role of the gut microbiome on the development of surgical site infections. Clin. Colon. Rectal Surg. 2023, 36, 133–137. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, Z.; Guo, J.; Xiong, Z.; Hu, B. Exploring the gut microbiome-postoperative cognitive dysfunction connection: Mechanisms, clinical implications, and future directions. Brain Behav. Immun. Health 2024, 38, 100763. [Google Scholar] [CrossRef]
- Sakuraya, M.; Yamashita, K.; Honda, M.; Niihara, M.; Chuman, M.; Washio, M.; Hosoda, K.; Naitoh, T.; Kumamoto, Y.; Hiki, N. Early administration of postoperative bcaa-enriched ppn may improve lean body mass loss in gastric cancer patients undergoing gastrectomy. Langenbecks Arch. Surg. 2023, 408, 336. [Google Scholar] [CrossRef]
- Cruzat, V.; Rogero, M.M.; Keane, K.N.; Curi, R.; Newsholme, P. Glutamine: Metabolism and immune function, supplementation and clinical translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef]
- Canè, S.; Geiger, R.; Bronte, V. The roles of arginases and arginine in immunity. Nat. Rev. Immunol. 2025, 25, 266–284. [Google Scholar] [CrossRef]
- Kar, A.; Ghosh, P.; Patra, P.; Chini, D.S.; Nath, A.K.; Saha, J.K.; Patra, B.C. Omega-3 fatty acids mediated cellular signaling and its regulation in human health. Clin. Nutr. Open Sci. 2023, 52, 72–86. [Google Scholar] [CrossRef]
- Shahidin; Wang, Y.; Wu, Y.; Chen, T.; Wu, X.; Yuan, W.; Zhu, Q.; Wang, X.; Zi, C. Selenium and selenoproteins: Mechanisms, health functions, and emerging applications. Molecules 2025, 30, 437. [Google Scholar] [CrossRef] [PubMed]
- Franceschilli, M.; Siragusa, L.; Usai, V.; Dhimolea, S.; Pirozzi, B.; Sibio, S.; Di Carlo, S. Immunonutrition reduces complications rate and length of stay after laparoscopic total gastrectomy: A single unit retrospective study. Discov. Oncol. 2022, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Berlana, D. Parenteral nutrition overview. Nutrients 2022, 14, 4480. [Google Scholar] [CrossRef]
- Mohsen, G.; Stroemer, A.; Mayr, A.; Kunsorg, A.; Stoppe, C.; Wittmann, M.; Velten, M. Effects of omega-3 fatty acids on postoperative inflammatory response: A systematic review and meta-analysis. Nutrients 2023, 15, 3414. [Google Scholar] [CrossRef] [PubMed]
- Bjelakovic, G.; Nikolova, D.; Gluud, L.L.; Simonetti, R.G.; Gluud, C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst. Rev. 2012, 2012, Cd007176. [Google Scholar] [CrossRef] [PubMed]
- Bidgood, E.; Huang, J.; Murphy, E.; Prentice, R.; Hede, B.; Russell, D. Peripheral parenteral nutrition: A retrospective observational study to evaluate utility and complications. Nutr. Clin. Pract. 2024, 40, 942–949. [Google Scholar] [CrossRef]
- Bujang, M.A.; Baharum, N. Guidelines of the minimum sample size requirements for kappa agreement test. Epidemiol. Biostat. Public Health 2017, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Ragan, B.G.; Park, J.H. Issues in outcomes research: An overview of randomization techniques for clinical trials. J. Athl. Train. 2008, 43, 215–221. [Google Scholar] [CrossRef]
- Tomàs, M.M.; Juan, E.P.; Cerdá, S.M.A. Complicaciones de la nutrición parenteral periférica. Observación clínica de 2 casos. [Complications of peripheral parenteral nutrition. Clinical observations of 2 cases]. Enferm. Intensiv. 2014, 25, 30–34. [Google Scholar] [CrossRef]
- Boléo-Tomé, C.; Monteiro-Grillo, I.; Camilo, M.; Ravasco, P. Validation of the malnutrition universal screening tool (must) in cancer. Br. J. Nutr. 2012, 108, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The clavien-dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Cavallaro, P.; Bordeianou, L. Implementation of an eras pathway in colorectal surgery. Clin. Colon. Rectal Surg. 2019, 32, 102–108. [Google Scholar] [CrossRef]
- Danielis, M.; Lorenzoni, G.; Cavaliere, L.; Ruffolo, M.; Peressoni, L.; De Monte, A.; Muzzi, R.; Beltrame, F.; Gregori, D. Optimizing protein intake and nitrogen balance (opinib) in adult critically ill patients: A study protocol for a randomized controlled trial. JMIR Res. Protoc. 2017, 6, e78. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C.P.; Grimberg, A. Insulin-like growth factor-i is a marker for the nutritional state. Pediatr. Endocrinol. Rev. 2015, 13, 499–511. [Google Scholar] [PubMed]
- Storz, M.A.; Müller, A.; Niederreiter, L.; Zimmermann-Klemd, A.M.; Suarez-Alvarez, M.; Kowarschik, S.; Strittmatter, M.; Schlachter, E.; Pasluosta, C.; Huber, R.; et al. A cross-sectional study of nutritional status in healthy, young, physically-active german omnivores, vegetarians and vegans reveals adequate vitamin b(12) status in supplemented vegans. Ann. Med. 2023, 55, 2269969. [Google Scholar] [CrossRef]
- Hernando, V.-U.; Andry, M.-M.; Virginia, P.-F.M.; Valentina, A. Vitamin d nutritional status in the adult population in colombia–an analytical cross-sectional study. Heliyon 2020, 6, e03479. [Google Scholar] [CrossRef]
- Cabellos Olivares, M.; Martínez, M.L.; Torralba, M.; Fraile, J.R.R.; Martínez, J.C.A. C-reactive protein as a marker of the surgical stress reduction within an eras protocol (enhanced recovery after surgery) in colorectal surgery: A prospective cohort study. J. Surg. Oncol. 2018, 117, 717–724. [Google Scholar] [CrossRef]
- Veenhof, A.A.; Vlug, M.S.; van der Pas, M.H.; Sietses, C.; van der Peet, D.L.; de Lange-de Klerk, E.S.; Bonjer, H.J.; Bemelman, W.A.; Cuesta, M.A. Surgical stress response and postoperative immune function after laparoscopy or open surgery with fast track or standard perioperative care: A randomized trial. Ann. Surg. 2012, 255, 216–221. [Google Scholar] [CrossRef]
- Beilin, B.; Shavit, Y.; Dekeyser, F.; Itzik, A.; Weidenfeld, J. The involvement of glucocorticoids and interleukin-1 in the regulation of brain prostaglandin production in response to surgical stress. Neuroimmunomodulation 2006, 13, 36–42. [Google Scholar] [CrossRef]
- Veenhof, A.A.; Sietses, C.; von Blomberg, B.M.; van Hoogstraten, I.M.; Pas, M.H.V.; Meijerink, W.J.; Peet, D.L.V.; Tol, M.P.V.; Bonjer, H.J.; Cuesta, M.A. The surgical stress response and postoperative immune function after laparoscopic or conventional total mesorectal excision in rectal cancer: A randomized trial. Int. J. Color. Dis. 2011, 26, 53–59. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, Y.; Liu, J.; Teng, Y.; Ou, M.; Hao, X. Predictive value of perioperative procalcitonin, c reactive protein and high-sensitivity c reactive protein for the risk of postoperative complications after non-cardiac surgery in elderly patients: A nested case–control study. BMJ Open 2023, 13, e071464. [Google Scholar] [CrossRef]
- Colomina, M.J.; Méndez, E.; Sabate, A. Altered fibrinolysis during and after surgery. Semin. Thromb. Hemost. 2021, 47, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, G.L. Metabolic considerations in management of surgical patients. Surg. Clin. North. Am. 2011, 91, 467–480. [Google Scholar] [CrossRef] [PubMed]

| Author; Year | No of Patients | Type of Study | Study Population | Intervention | Outcomes |
|---|---|---|---|---|---|
| Gys; 1990 [33] | 20 patients | Randomized Controlled Trial | Patients undergoing colorectal resection | 2L PPN daily from POD 1 to 6, regardless of oral intake | Improved nitrogen balance during first 5 postoperative days |
| Liu; 2013 [34] | 40 patients | Retrospective Cohort Study | Malnourished patients post-colorectal resection | PPN + oral feeding during first 5 postoperative days | Higher postoperative albumin, earlier mobilization, shorter hospital stay |
| Sánchez-Guillén; 2021 [35] | 158 patients | Randomized Controlled Trial | Colorectal cancer patients undergoing resection | Early PPN administration during immediate postoperative period | Lower postoperative morbidity, reduced severity of complications, 28% benefit in ERAS non-adherent patients |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frountzas, M.; Stefanoudakis, D.; Mela, E.; Theodorou, P.; Theodoropoulos, G.E.; Theodorou, D.; Toutouzas, K.G. Peripheral Parenteral Nutrition and Personalized Nutritional Approach After Colorectal Resection Surgery: A Comprehensive Review of Current Evidence. Medicina 2025, 61, 1459. https://doi.org/10.3390/medicina61081459
Frountzas M, Stefanoudakis D, Mela E, Theodorou P, Theodoropoulos GE, Theodorou D, Toutouzas KG. Peripheral Parenteral Nutrition and Personalized Nutritional Approach After Colorectal Resection Surgery: A Comprehensive Review of Current Evidence. Medicina. 2025; 61(8):1459. https://doi.org/10.3390/medicina61081459
Chicago/Turabian StyleFrountzas, Maximos, Dimitrios Stefanoudakis, Evgenia Mela, Panagiotis Theodorou, George E. Theodoropoulos, Dimitrios Theodorou, and Konstantinos G. Toutouzas. 2025. "Peripheral Parenteral Nutrition and Personalized Nutritional Approach After Colorectal Resection Surgery: A Comprehensive Review of Current Evidence" Medicina 61, no. 8: 1459. https://doi.org/10.3390/medicina61081459
APA StyleFrountzas, M., Stefanoudakis, D., Mela, E., Theodorou, P., Theodoropoulos, G. E., Theodorou, D., & Toutouzas, K. G. (2025). Peripheral Parenteral Nutrition and Personalized Nutritional Approach After Colorectal Resection Surgery: A Comprehensive Review of Current Evidence. Medicina, 61(8), 1459. https://doi.org/10.3390/medicina61081459







