The Relationship Between Electrocardiographic Findings and Cardiac Magnetic Resonance Results in Patients with Acute Myocarditis: A Retrospective Analysis
Abstract
1. Introduction
2. Materials and Methods
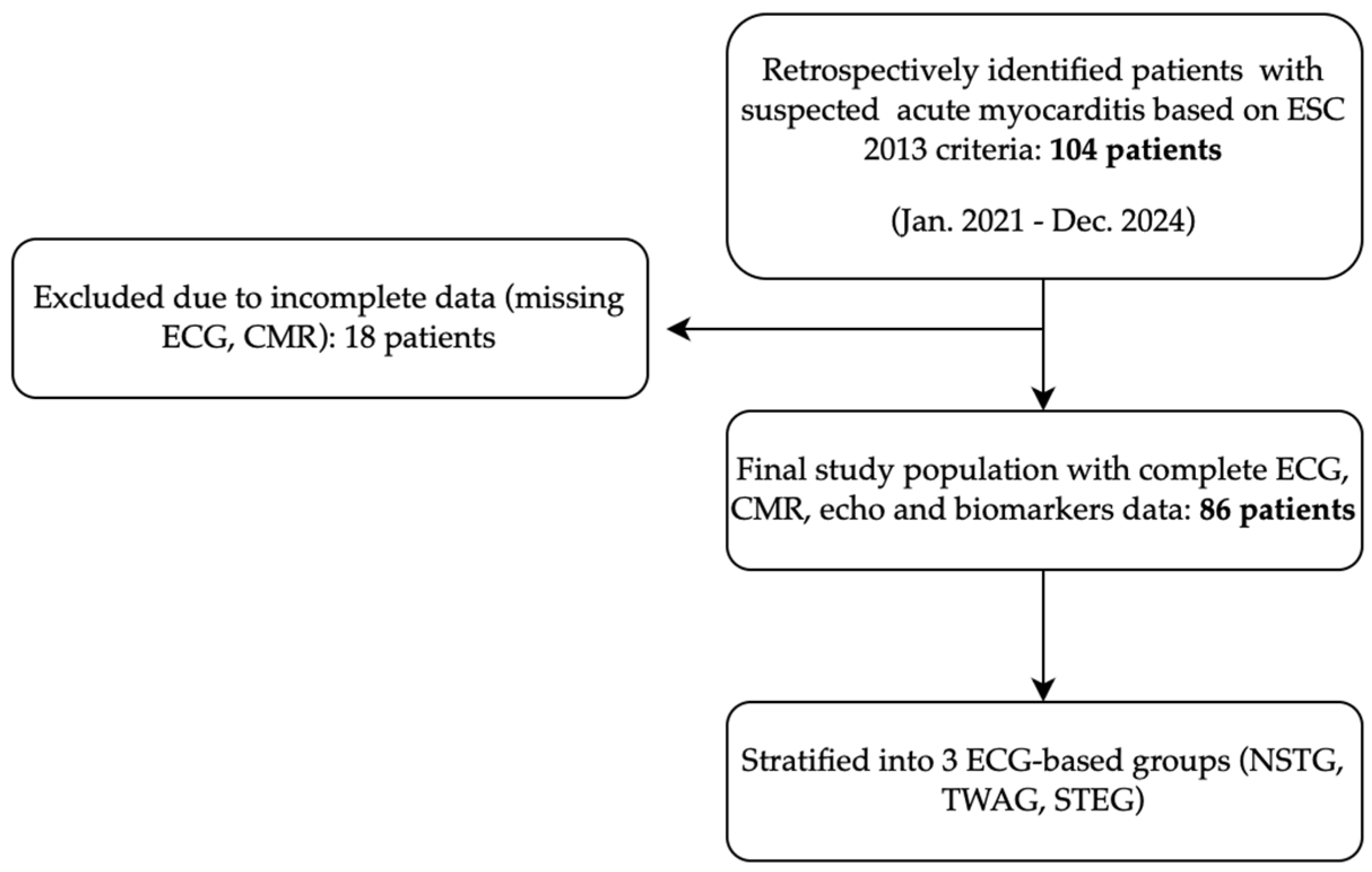
2.1. Study Design
2.2. Participants
2.3. Data Collection
- Demographic characteristics and past medical history;
- Clinical presentation and recent infection prior to hospitalization;
- Laboratory findings: TnI and CRP levels;
- ECGs;
- Imaging findings (TTE and CMR);
- Coronary angiography findings;
- Medication regimens;
- Cardiac-related events during follow-up, like acute heart failure (HF) and heart failure-related therapy up-titration.
2.4. Statistical Analysis
3. Results
3.1. Demographic Characteristics and Past Medical History
3.2. Clinical Presentation and Recent Infection Prior to Hospitalization
3.3. Baseline Findings
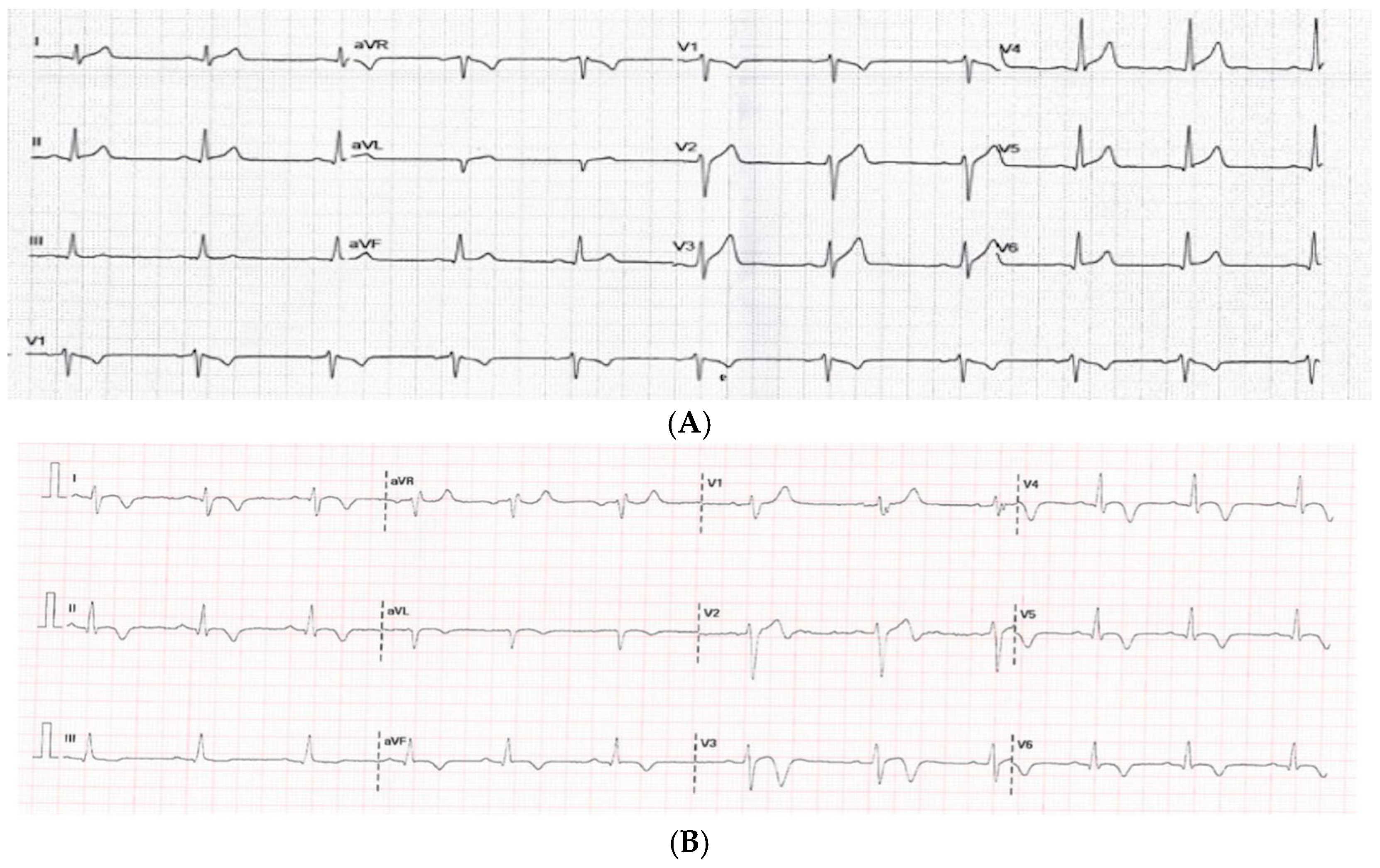
3.3.1. Electrocardiogram
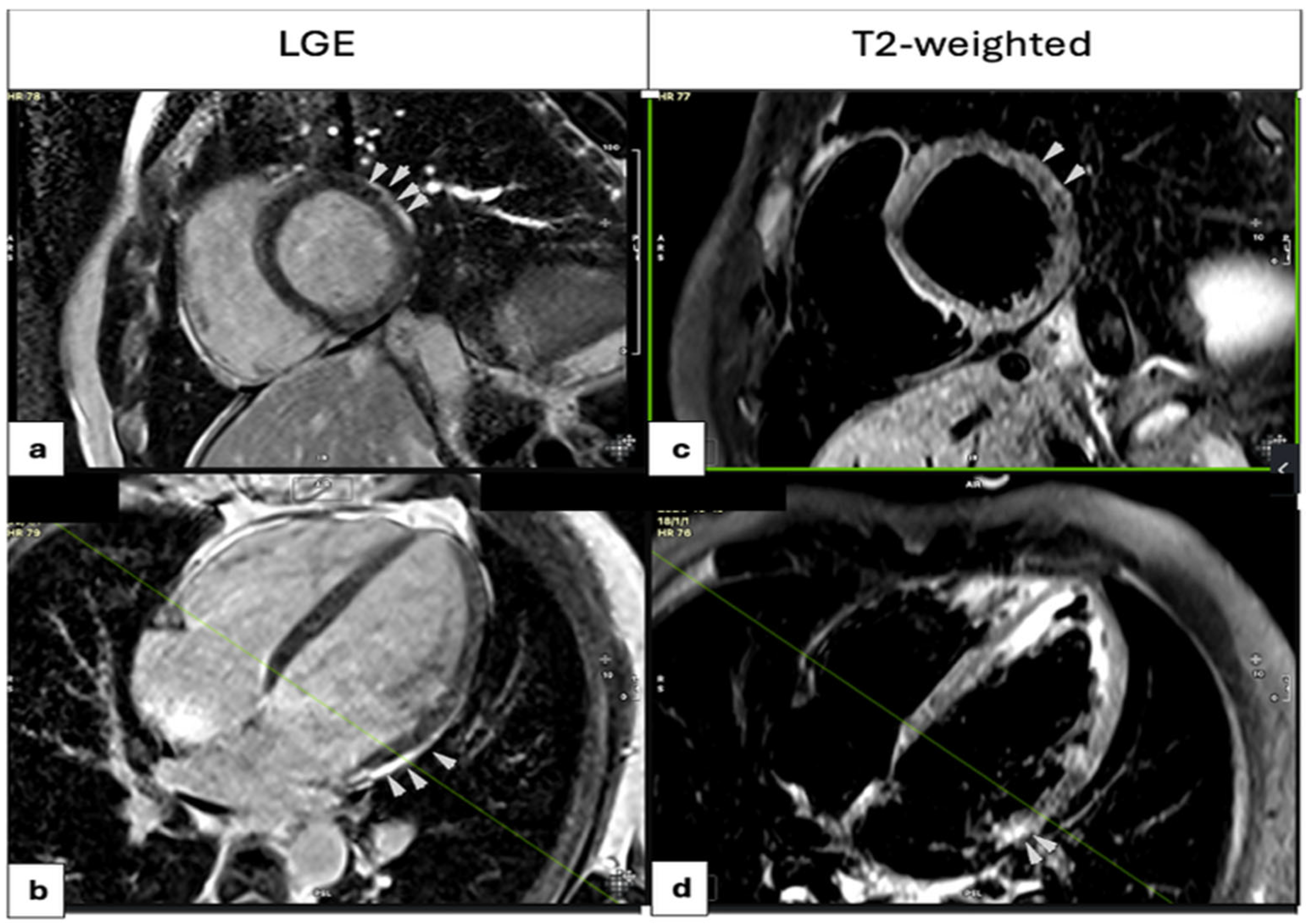
3.3.2. Imaging Modalities
3.3.3. Biomarkers
3.3.4. Comparison Based on ECG Findings on Admission
3.4. Follow-Up
3.4.1. Clinical Outcomes and ECG and Imaging Findings
3.4.2. Therapy
3.5. Correlations
4. Discussion
5. Limitations and Future Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE | Angiotensin-Converting Enzyme |
| AHA | American Heart Association |
| ARB | Angiotensin II Receptor Blocker |
| ASCVD | Atherosclerotic Cardiovascular Disease |
| CI | Confidence Interval |
| CMR | Cardiac Magnetic Resonance |
| CRP | C-Reactive Protein |
| cTnI | Cardiac Troponin I |
| Echo | Echocardiography |
| ECG | Electrocardiogram |
| ECMO | Extracorporeal Membrane Oxygenation |
| ECV | Extracellular Volume |
| ESC | European Society of Cardiology |
| hs-cTn | High-Sensitivity Cardiac Troponin |
| GLS | Global Longitudinal Strain |
| ICU | Intensive Care Unit |
| IQR | Interquartile Range |
| LGE | Late Gadolinium Enhancement |
| LLC | Lake Louise Criteria |
| LVEF | Left Ventricular Ejection Fraction |
| MRA | Mineralocorticoid Receptor Antagonist |
| NSTG | Group with No ST Elevation or T-Wave Abnormalities |
| OR | Odds Ratio |
| RVEF | Right Ventricular Ejection Fraction |
| RWMA | Regional Wall Motion Abnormality |
| SCD | Sudden Cardiac Death |
| STE | ST Elevation |
| STEG | ST Elevation Group |
| T2W | T2-Weighted Images |
| TTE | Transthoracic Echocardiography |
| TWAG | T-Wave Abnormalities Group |
References
- Heymans, S.; Van Linthout, S.; Kraus, S.M.; Cooper, L.T.; Ntusi, N.A.B. Clinical Characteristics and Mechanisms of Acute Myocarditis. Circ. Res. 2024, 135, 397–411. [Google Scholar] [CrossRef]
- Ammirati, E.; Cartella, I.; Varrenti, M.; Selimi, A.; Sormani, P.; Garascia, A.; Palazzini, M. Acute myocarditis: 2024 state of the art. Eur. Hear. J. Suppl. 2025, 27 (Suppl. 1), i56–i60. [Google Scholar] [CrossRef] [PubMed]
- Shams, P.; Kiel, J. Acute Myocarditis; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441847/ (accessed on 1 January 2025).
- Li, J.; Fan, H.; Yang, Y.; Huang, Z.; Yuan, Y.; Liang, B. Global burden of myocarditis from 1990 to 2021: Findings from the Global Burden of Disease Study 2021. BMC Cardiovasc. Disord. 2024, 24, 720. [Google Scholar] [CrossRef] [PubMed]
- Lynge, T.H.; Nielsen, T.S.; Winkel, B.G.; Tfelt-Hansen, J.; Banner, J. Sudden cardiac death caused by myocarditis in persons aged 1–49 years: A nationwide study of 14294 deaths in Denmark. Forensic Sci. Res. 2019, 4, 247–256. [Google Scholar] [CrossRef]
- Caforio, A.L.P.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef]
- Hazebroek, M.R.; Everaerts, K.; Heymans, S. Diagnostic approach of myocarditis: Strike the golden mean. Neth. Heart J. 2014, 22, 80–84. [Google Scholar] [CrossRef]
- Friedrich, M.G.; Sechtem, U.; Schulz-Menger, J.; Holmvang, G.; Alakija, P.; Cooper, L.T.; White, J.A.; Abdel-Aty, H.; Gutberlet, M.; Prasad, S.; et al. Cardiovascular Magnetic Resonance in Myocarditis: A JACC White Paper. J. Am. Coll. Cardiol. 2009, 53, 1475–1487. [Google Scholar] [CrossRef]
- Buttà, C.; Zappia, L.; Laterra, G.; Roberto, M. Diagnostic and prognostic role of electrocardiogram in acute myocarditis: A comprehensive review. Ann. Noninvasive Electrocardiol. 2020, 25, e12726. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Hussein, Z.; Jacob, T.; Wedgwood, M.; Tran, P.; Kuehl, M.; Banerjee, P. Myocarditis in the modern era: Analysing the impact of high-sensitivity troponin & C-reactive protein in predicting the extent of myocarditis and LV systolic dysfunction on cardiac magnetic resonance. Eur. Heart J. 2024, 45 (Suppl. 1), ehae666.1989. [Google Scholar]
- Berg, J.; Kottwitz, J.; Baltensperger, N.; Kissel, C.K.; Lovrinovic, M.; Mehra, T.; Scherff, F.; Schmied, C.; Templin, C.; Lüscher, T.F.; et al. Cardiac Magnetic Resonance Imaging in Myocarditis Reveals Persistent Disease Activity Despite Normalization of Cardiac Enzymes and Inflammatory Parameters at 3-Month Follow-Up. Circ. Heart Fail. 2017, 10, e004262. [Google Scholar] [CrossRef] [PubMed]
- Goitein, O.; Sabag, A.; Koperstein, R.; Hamdan, A.; Di Segni, E.; Konen, E.; Matetzky, S. Role of C reactive protein in evaluating the extent of myocardial inflammation in acute myocarditis. J. Cardiovasc. Magn. Reson. 2015, 17, P291. [Google Scholar] [CrossRef]
- Morgera, T.; Di Lenarda, A.; Dreas, L.; Pinamonti, B.; Humar, F.; Bussani, R.; Silvestri, F.; Chersevani, D.; Camerini, F. Electrocardiography of myocarditis revisited: Clinical and prognostic significance of electrocardiographic changes. Am. Heart J. 1992, 124, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Nucifora, G.; Miani, D.; Di Chiara, A.; Piccoli, G.; Artico, J.; Puppato, M.; Slavich, G.; De Biasio, M.; Gasparini, D.; Proclemer, A. Infarct-Like Acute Myocarditis: Relation Between Electrocardiographic Findings and Myocardial Damage as Assessed by Cardiac Magnetic Resonance Imaging. Clin. Cardiol. 2013, 36, 146–152. [Google Scholar] [CrossRef]
- Di Bella, G.; Florian, A.; Oreto, L.; Napolitano, C.; Todaro, M.C.; Donato, R.; Calamelli, S.; Camastra, G.S.; Zito, C.; Carerj, S.; et al. Electrocardiographic findings and myocardial damage in acute myocarditis detected by cardiac magnetic resonance. Clin. Res. Cardiol. 2012, 101, 617–624. [Google Scholar] [CrossRef]
- Nakashima, H.; Honda, Y.; Katayama, T. Serial Electrocardiographic Findings in Acute Myocarditis. Intern. Med. 1994, 33, 659–666. [Google Scholar] [CrossRef]
- Birnbaum, Y.; Perez Riera, A.R.; Nikus, K. PR depression with multi-lead ST elevation and ST depression in aVR: Is it always acute pericarditis? J. Electrocardiol. 2019, 54, 13–17. [Google Scholar] [CrossRef]
- Chan, T.C.; Brady, W.J.; Pollack, M. Electrocardiographic manifestations: Acute myopericarditis. J. Emerg. Med. 1999, 17, 865–872. [Google Scholar] [CrossRef]
- Ramantauskaitė, G.; Okeke, K.A.; Mizarienė, V. Myocarditis: Differences in Clinical Expression Between Patients with ST-Segment Elevation in Electrocardiogram vs. Patients Without ST-Segment Elevation. J. Pers. Med. 2024, 14, 1057. [Google Scholar] [CrossRef]
- Ginsberg, F.; Parrillo, J.E. Fulminant Myocarditis. Crit. Care Clin. 2013, 29, 465–483. [Google Scholar] [CrossRef]
- Punja, M.; Mark, D.G.; McCoy, J.V.; Javan, R.; Pines, J.M.; Brady, W. Electrocardiographic manifestations of cardiac infectious-inflammatory disorders. Am. J. Emerg. Med. 2010, 28, 364–377. [Google Scholar] [CrossRef]
- Prenner, S.B.; Shah, S.J.; Goldberger, J.J.; Sauer, A.J. Repolarization Heterogeneity: Beyond the QT Interval. J. Am. Heart Assoc. 2016, 5, e003607. [Google Scholar] [CrossRef] [PubMed]
- De Lazzari, M.; Zorzi, A.; Baritussio, A.; Siciliano, M.; Migliore, F.; Susana, A.; Giorgi, B.; Lacognata, C.; Iliceto, S.; Marra, M.P.; et al. Relationship between T-wave inversion and transmural myocardial edema as evidenced by cardiac magnetic resonance in patients with clinically suspected acute myocarditis: Clinical and prognostic implications. J. Electrocardiol. 2016, 49, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Zareba, K.M.; Truong, V.T.; Mazur, W.; Smart, S.M.; Xia, X.; Couderc, J.; Raman, S.V. T-wave and its association with myocardial fibrosis on cardiovascular magnetic resonance examination. Ann. Noninvasive Electrocardiol. 2021, 26, e12819. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, P.; Piazza, I.; Grosu, A.; Brambilla, P.; Sironi, S.; Senni, M. QRS fragmentation as a possible new marker of fibrosis in patients with myocarditis. Eur. J. Heart Fail. 2019, 21, 614–622. [Google Scholar] [CrossRef]
- Ruberg, F.L.; Baggish, A.L.; Hays, A.G.; Jerosch-Herold, M.; Kim, J.; Ordovas, K.G.; Reddy, G.; Shenoy, C.; Weinsaft, J.W.; Woodard, P.K. Utilization of Cardiovascular Magnetic Resonance Imaging for Resumption of Athletic Activities Following COVID-19 Infection: An Expert Consensus Document on Behalf of the American Heart Association Council on Cardiovascular Radiology and Intervention Leadership and Endorsed by the Society for Cardiovascular Magnetic Resonance. Circ. Cardiovasc. Imaging 2023, 16, e014106. [Google Scholar] [CrossRef]
- Luetkens, J.A.; Homsi, R.; Dabir, D.; Kuetting, D.L.; Marx, C.; Doerner, J.; Schlesinger-Irsch, U.; Andrié, R.; Sprinkart, A.M.; Schmeel, F.C.; et al. Comprehensive Cardiac Magnetic Resonance for Short-Term Follow-Up in Acute Myocarditis. J. Am. Heart Assoc. 2016, 5, e003603. [Google Scholar] [CrossRef]
- Polte, C.L.; Bobbio, E.; Bollano, E.; Bergh, N.; Polte, C.; Himmelman, J.; Lagerstrand, K.M.; Gao, S.A. Cardiovascular Magnetic Resonance in Myocarditis. Diagnostics 2022, 12, 399. [Google Scholar] [CrossRef]
- Monney, P.A.; Sekhri, N.; Burchell, T.; Knight, C.; Davies, C.; Deaner, A.; Sheaf, M.; Baithun, S.; Petersen, S.; Wragg, A.; et al. Acute myocarditis presenting as acute coronary syndrome: Role of early cardiac magnetic resonance in its diagnosis. Heart 2011, 97, 1312–1318. [Google Scholar] [CrossRef]
- Gulati, A.; Jabbour, A.; Ismail, T.F.; Guha, K.; Khwaja, J.; Raza, S.; Morarji, K.; Brown, T.D.H.; Ismail, N.A.; Dweck, M.R.; et al. Association of Fibrosis with Mortality and Sudden Cardiac Death in Patients with Nonischemic Dilated Cardiomyopathy. JAMA 2013, 309, 896. [Google Scholar] [CrossRef]
- Ismail, T.F.; Jabbour, A.; Gulati, A.; Mallorie, A.; Raza, S.; Cowling, T.E.; Das, B.; Khwaja, J.; Alpendurada, F.D.; Wage, R.; et al. Role of late gadolinium enhancement cardiovascular magnetic resonance in the risk stratification of hypertrophic cardiomyopathy. Heart 2014, 100, 1851–1858. [Google Scholar] [CrossRef]
- Georgiopoulos, G.; Figliozzi, S.; Sanguineti, F.; Aquaro, G.D.; Di Bella, G.; Stamatelopoulos, K.; Chiribiri, A.; Garot, J.; Masci, P.G.; Ismail, T.F. Prognostic Impact of Late Gadolinium Enhancement by Cardiovascular Magnetic Resonance in Myocarditis: A Systematic Review and Meta-Analysis. Circ. Cardiovasc. Imaging 2021, 14, e011492. [Google Scholar] [CrossRef]
| Sex | Male, n | 75 (87.2%) |
| Female, n | 11 (12.8%) | |
| Age | 26 (20–33.25) | |
| Comorbidities— ASCVD risk factors | Smoking, n | 26 (30.2%) |
| Obesity, n | 3 (3.5%) | |
| Hypertension, n | 3 (3.5%) | |
| Dyslipidemia, n | 6 (7%) | |
| Family history of ASCVD, n | 7 (8.1%) | |
| Diabetes, n | 0 (0%) | |
| Other comorbidities | Prior myocarditis, n | 10 (11.6%) |
| Autoimmune disease, n | 5 (5.8%) | |
| Congenital heart disease, n | 3 (3.5%) |
| Variable | NSTG (n = 27) | TWA (n = 24) | STEG (n = 35) | p-Value |
|---|---|---|---|---|
| Age (years) | 26 (19–34) | 28.5 (21.3–34) | 25 (20–32) | 0.77 |
| TnI admission (ng/mL) | 1.8 (0.6–3.7) | 1.8 (0.9–4.6) | 3.0 (1.1–9.0) | 0.066 |
| TnI peak (ng/mL) | 3.7 (1.4–9.0) | 4.3 (1.5–7.6) | 7.1 (4.6–19.3) | 0.005 |
| TnI discharge (ng/mL) | 0.15 (0.04–0.80) | 0.08 (0.03–0.34) | 0.09 (0.03–0.29) | 0.709 |
| CRP admission (mg/L) | 27.3 (6.4–88.6) | 47.8 (6.8–80.5) | 62.2 (26.4–92.3) | 0.215 |
| CRP peak (mg/L) | 27.3 (7.0–86.6) | 52.8 (27.9–108.4) | 65.7 (36.2–102) | 0.18 |
| CRP discharge (mg/L) | 6.6 (2.3–15.0) | 10.8 (5.0–18.8) | 10.2 (6.0–18.5) | 0.332 |
| LVEF—Echo (%) | 60 (58–60) | 57.5 (51.3–60) | 57 (50–60) | 0.09 |
| GLS (%) | −18.3 (−19.3 to −17.4) | −18.2 (−19.6 to −13.0) | −18.0 (−20.7 to −15.0) | 0.915 |
| LVEF—CMR (%) | 58 (55–60) | 58 (53–60) | 60 (57–60) | 0.537 |
| RVEF—CMR (%) | 54.5 (52–59.8) | 53.5 (49.8–57.8) | 54.5 (50–56.5) | 0.785 |
| T2W (Edema) (n) | 15 (57.7%) | 15 (65.2%) | 31 (91.2%) | 0.008 |
| LGE (n) | 17 (65.4%) | 17 (77.3%) | 31 (91.2%) | 0.049 |
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| CRP | 1.4 (1.05–2.05) | 0.041 | 1.11 (1.02–1.76) | 0.473 |
| TWA | 4.11 (1.21–10.77) | <0.001 | 3.15 (1.078–9.19) | 0.036 |
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| CRP | 1.26 (1.02–1.98) | 0.032 | 1.22 (1.05–2.25) | 0.446 |
| TWA | 5.55 (1.99–15.78) | <0.001 | 3.93 (1.256–12.276) | 0.019 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyriakou, M.; Kadoglou, N.P.E.; Sokratous, S.; Khattab, E.; Eftychiou, C.; Myrianthefs, M.M. The Relationship Between Electrocardiographic Findings and Cardiac Magnetic Resonance Results in Patients with Acute Myocarditis: A Retrospective Analysis. Medicina 2025, 61, 1444. https://doi.org/10.3390/medicina61081444
Kyriakou M, Kadoglou NPE, Sokratous S, Khattab E, Eftychiou C, Myrianthefs MM. The Relationship Between Electrocardiographic Findings and Cardiac Magnetic Resonance Results in Patients with Acute Myocarditis: A Retrospective Analysis. Medicina. 2025; 61(8):1444. https://doi.org/10.3390/medicina61081444
Chicago/Turabian StyleKyriakou, Michaela, Nikolaos P. E. Kadoglou, Stefanos Sokratous, Elina Khattab, Christos Eftychiou, and Michael M. Myrianthefs. 2025. "The Relationship Between Electrocardiographic Findings and Cardiac Magnetic Resonance Results in Patients with Acute Myocarditis: A Retrospective Analysis" Medicina 61, no. 8: 1444. https://doi.org/10.3390/medicina61081444
APA StyleKyriakou, M., Kadoglou, N. P. E., Sokratous, S., Khattab, E., Eftychiou, C., & Myrianthefs, M. M. (2025). The Relationship Between Electrocardiographic Findings and Cardiac Magnetic Resonance Results in Patients with Acute Myocarditis: A Retrospective Analysis. Medicina, 61(8), 1444. https://doi.org/10.3390/medicina61081444







