Physiological Ageing of the Lumbar Intervertebral Disc Based on Magnetic Resonance Imaging, a Systematic Literature Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Study Selection
2.2. Risk of Bias and Quality Assessment
2.3. Data Collection
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias and Quality Assessment
3.4. Study Results
3.5. Effect of Age on Degeneration
3.6. Effect of Gender on LIDD
3.7. Effect of Spinal Level on Degeneration
4. Discussion
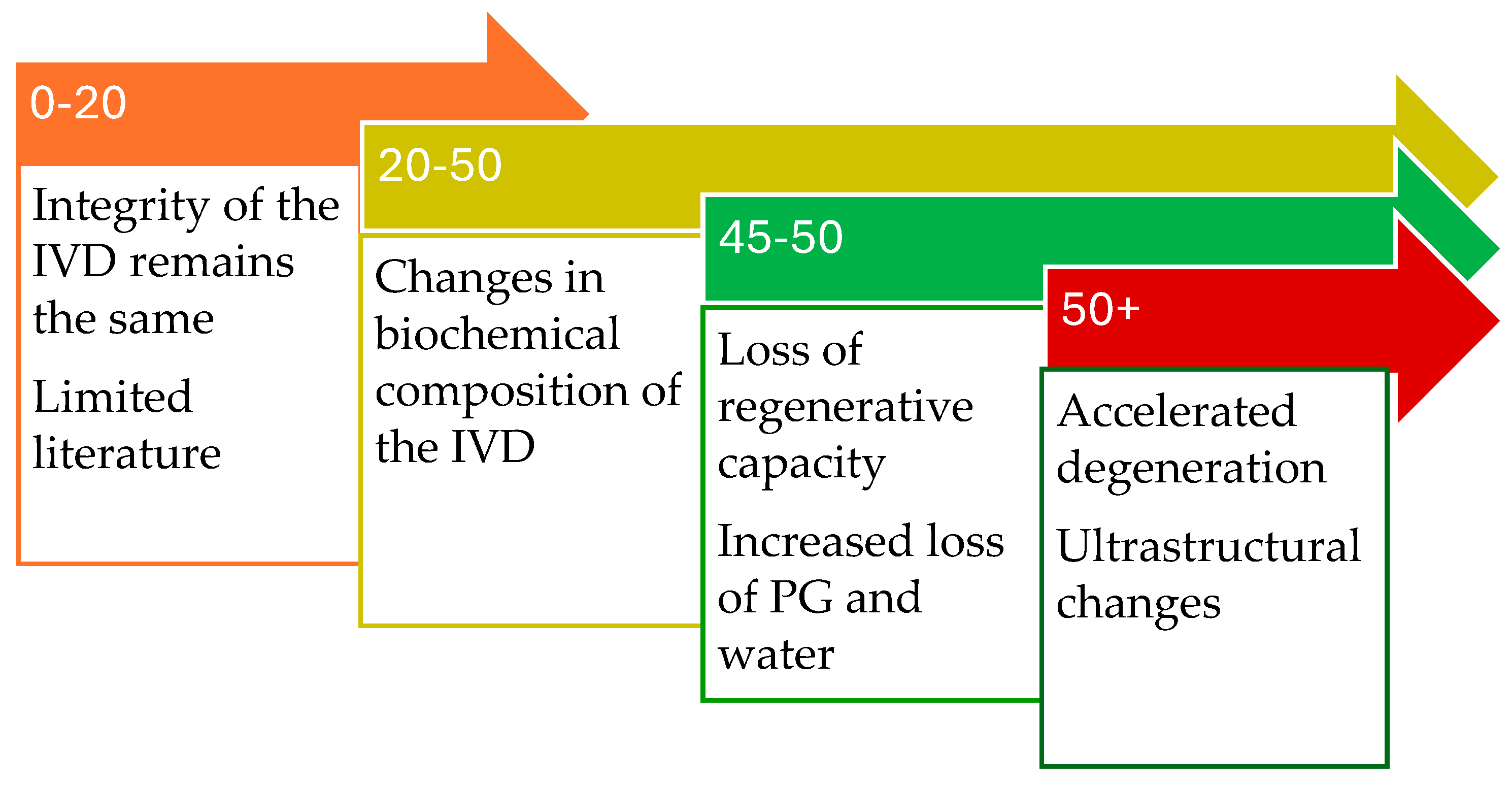
4.1. Zero to Twenty Years
4.2. Twenty to Fifty Years
4.3. Fifty Years
4.4. CEP
4.5. Interindividual Variability
4.6. Location of Degeneration
4.7. Pfirrmann Grading
4.8. Study Heterogeneity
4.9. Limitations
4.10. Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Annulus Fibrosis | AF |
| Apparent diffusion coefficient | ADC |
| Cartilage endplate | CEP |
| Combined task force classification | CTF |
| Disability adjusted life years | DALYs |
| Extracellular matrix | ECM |
| Fractional anisotropy | FA |
| Glycosaminoglycan(s) | GAG(s) |
| Intervertebral disc | IVD |
| Lower backpain | LBP |
| Lumbar intervertebral disc degeneration | LIDD |
| Mean diffusivity | MD |
| Magnetic resonance imaging | MRI |
| Nucleus pulposus | NP |
| Proteoglycan(s) | PG(s) |
| Quality of Life | QoL |
| Risk of bias in non-randomized studies—of interventions | ROBINS-I |
| Years lived with disability | YLD |
References
- DeSai, C.; Reddy, V.; Agarwal, A. Anatomy, Back, Vertebral Column. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- de Schepper, E.I.; Damen, J.; van Meurs, J.B.; Ginai, A.Z.; Popham, M.; Hofman, A.; Koes, B.W.; Bierma-Zeinstra, S.M. The association between lumbar disc degeneration and low back pain: The influence of age, gender, and individual radiographic features. Spine 2010, 35, 531–536. [Google Scholar] [CrossRef]
- Mertimo, T.; Karppinen, J.; Niinimäki, J.; Blanco, R.; Määttä, J.; Kankaanpää, M.; Oura, P. Association of lumbar disc degeneration with low back pain in middle age in the Northern Finland Birth Cohort 1966. BMC Musculoskelet. Disord. 2022, 23, 359. [Google Scholar] [CrossRef]
- Berg, A.J.; Ahmadje, U.; Jayanna, H.H.; Trégouët, P.; Sanville, P.; Kapoor, V. The prevalence of lumbar disc degeneration in symptomatic younger patients: A study of MRI scans. J. Clin. Orthop. Trauma 2020, 11, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Waxenbaum, J.A.; Reddy, V.; Futterman, B. Anatomy, Back, Intervertebral Discs. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Ghannam, M.; Jumah, F.; Mansour, S.; Samara, A.; Alkhdour, S.; Alzuabi, M.A.; Aker, L.; Adeeb, N.; Massengale, J.; Oskouian, R.J.; et al. Surgical anatomy, radiological features, and molecular biology of the lumbar intervertebral discs. Clin. Anat. 2017, 30, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Masuda, K.; Thonar, E.J.; An, H.S.; Cs-Szabo, G. Age-related changes in the extracellular matrix of nucleus pulposus and anulus fibrosus of human intervertebral disc. Spine 2009, 34, 10–16. [Google Scholar] [CrossRef]
- Roberts, S.; Urban, J.P.G.; Evans, H.; Eisenstein, S.M. Transport Properties of the Human Cartilage Endplate in Relation to Its Composition and Calcification. Spine 1996, 21, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Vadapalli, R.; Mulukutla, R.; Vadapalli, A.S.; Vedula, R.R. Quantitative Predictive Imaging Biomarkers of Lumbar Intervertebral Disc Degeneration. Asian Spine J. 2019, 13, 527–534. [Google Scholar] [CrossRef]
- Adams, M.A. Biomechanics of back pain. Acupunct. Med. 2004, 22, 178–188. [Google Scholar] [CrossRef]
- Moore, R.J. The vertebral end-plate: What do we know? Eur. Spine J. 2000, 9, 92–96. [Google Scholar] [CrossRef]
- Han, M.; Fields, A.J.; Berg-Johansen, B.; Larson, P.E.Z.; Krug, R.; Lotz, J.C. Cartilage endplate thickness measured by ultrashort echo-time mri associated with disc degeneration. J. Orthop. Res. 2017, 35, E592–E600. [Google Scholar]
- Urban, J.P.G.; Smith, S.; Fairbank, J.C.T. Nutrition of the Intervertebral Disc. Spine 2004, 29, 2700–2709. [Google Scholar] [CrossRef]
- Rajasekaran, S.; Venkatadass, K.; Naresh Babu, J.; Ganesh, K.; Shetty, A.P. Pharmacological enhancement of disc diffusion and differentiation of healthy, ageing and degenerated discs: Results from in-vivo serial post-contrast MRI studies in 365 human lumbar discs. Eur. Spine J. 2008, 17, 626–643. [Google Scholar] [CrossRef]
- Bouhsina, N.; Decante, C.; Hardel, J.-B.; Rouleau, D.; Abadie, J.; Hamel, A.; Le Visage, C.; Lesoeur, J.; Guicheux, J.; Clouet, J.; et al. Comparison of MRI T1, T2, and T2* mapping with histology for assessment of intervertebral disc degeneration in an ovine model. Sci. Rep. 2022, 12, 5398. [Google Scholar] [CrossRef]
- Trattnig, S.; Stelzeneder, D.; Goed, S.; Reissegger, M.; Mamisch, T.C.; Paternostro-Sluga, T.; Weber, M.; Szomolanyi, P.; Welsch, G.H. Lumbar intervertebral disc abnormalities: Comparison of quantitative T2 mapping with conventional MR at 3.0 T. Eur. Radiol. 2010, 20, 2715–2722. [Google Scholar] [CrossRef] [PubMed]
- Adams, A.; Roche, O.; Mazumder, A.; Davagnanam, I.; Mankad, K. Imaging of degenerative lumbar intervertebral discs; linking anatomy, pathology and imaging. Postgrad. Med. J. 2014, 90, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Mwale, F.; Iatridis, J.C.; Antoniou, J. Quantitative MRI as a diagnostic tool of intervertebral disc matrix composition and integrity. Eur. Spine J. 2008, 17, S432–S440. [Google Scholar] [CrossRef]
- Ogon, I.; Takebayashi, T.; Takashima, H.; Morita, T.; Terashima, Y.; Yoshimoto, M.; Yamashita, T. Imaging diagnosis for intervertebral disc. JOR Spine 2020, 3, e1066. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Jeremy Howick, I.C.; Glasziou, P.; Greenhalgh, T.; Heneghan, C.; Liberati, A.; Moschetti, I.; Phillips, B.; Thornton, H.; Goddard, O.; Hodgkinson, M. The Oxford Levels of Evidence 2. Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence (accessed on 7 July 2023).
- Jeremy Howick, I.C.; Glasziou, P.; Greenhalgh, T.; Heneghan, C.; Liberati, A.; Moschetti, I.; Phillips, B.; Thornton, H. Explanation of the 2011 Oxford Centre for Evidence-Based Medicine (OCEBM) Levels of Evidence (Introductory Document). Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence (accessed on 8 July 2023).
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Moon, S.M.; Yoder, J.H.; Wright, A.C.; Smith, L.J.; Vresilovic, E.J.; Elliott, D.M. Evaluation of intervertebral disc cartilaginous endplate structure using magnetic resonance imaging. Eur. Spine J. 2013, 22, 1820–1828. [Google Scholar] [CrossRef]
- Haneder, S.; Ong, M.M.; Budjan, J.M.; Schmidt, R.; Konstandin, S.; Morelli, J.N.; Schad, L.R.; Schoenberg, S.O.; Kerl, U.H. 23Na-magnetic resonance imaging of the human lumbar vertebral discs: In vivo measurements at 3.0 T in healthy volunteers and patients with low back pain. Spine J. 2014, 14, 1343–1350. [Google Scholar] [CrossRef]
- Sharma, A.; Walk, R.E.; Tang, S.Y.; Eldaya, R.; Owen, P.J.; Belavy, D.L. Variability of T2-Relaxation Times of Healthy Lumbar Intervertebral Discs is More Homogeneous within an Individual than across Healthy Individuals. AJNR Am. J. Neuroradiol. 2020, 41, 2160–2165. [Google Scholar] [CrossRef]
- Fyllos, A.H.; Arvanitis, D.L.; Karantanas, A.H.; Varitimidis, S.E.; Hantes, M.; Zibis, A.H. Magnetic resonance morphometry of the adult normal lumbar intervertebral space. Surg. Radiol. Anat. 2018, 40, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Machino, M.; Nakashima, H.; Ito, K.; Tsushima, M.; Ando, K.; Kobayashi, K.; Imagama, S. Influence of Age and Gender on Intervertebral Disk Degeneration and Height in the Thoracolumbar Spine. Spine Surg. Relat. Res. 2022, 6, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Kolf, A.-K.; Konieczny, M.; Hesper, T.; Hosalkar, H.; Schleich, C.; Antoch, G.; Krauspe, R.; Bittersohl, B. T2* Mapping of the Adult Intervertebral Lumbar Disc: Normative Data and Analysis of Diurnal Effects. J. Orthop. Res. 2019, 37, 1956–1962. [Google Scholar] [CrossRef]
- Wang, L.; Han, M.; Wong, J.; Zheng, P.; Lazar, A.A.; Krug, R.; Fields, A.J. Evaluation of human cartilage endplate composition using MRI: Spatial variation, association with adjacent disc degeneration, and in vivo repeatability. J. Orthop. Res. 2021, 39, 1470–1478. [Google Scholar] [CrossRef] [PubMed]
- Alkalay, R.; David, H. Diffusion based MR measurements correlates with age-related changes in human intervertebral disks. Clin. Biomech. 2019, 61, 38–45. [Google Scholar] [CrossRef]
- Ellingson, A.M.; Nagel, T.M.; Polly, D.W.; Ellermann, J.; Nuckley, D.J. Quantitative T2* (T2 star) relaxation times predict site specific proteoglycan content and residual mechanics of the intervertebral disc throughout degeneration. J. Orthop. Res. 2014, 32, 1083–1089. [Google Scholar] [CrossRef]
- Johannessen, W.; Auerbach, J.D.; Wheaton, A.J.; Kurji, A.; Borthakur, A.; Reddy, R.; Elliott, D.M. Assessment of human disc degeneration and proteoglycan content using T1rho-weighted magnetic resonance imaging. Spine 2006, 31, 1253–1257. [Google Scholar] [CrossRef]
- Shen, S.; Wang, H.; Zhang, J.; Wang, F.; Liu, S. Diffusion Weighted Imaging, Diffusion Tensor Imaging, and T2* Mapping of Lumbar Intervertebral Disc in Young Healthy Adults. Iran J. Radiol. 2016, 13, e30069. [Google Scholar] [CrossRef]
- Matsumoto, M.; Okada, E.; Toyama, Y.; Fujiwara, H.; Momoshima, S.; Takahata, T. Tandem age-related lumbar and cervical intervertebral disc changes in asymptomatic subjects. Eur. Spine J. 2013, 22, 708–713. [Google Scholar] [CrossRef]
- Zhang, Z.; Chan, Q.; Anthony, M.P.; Samartzis, D.; Cheung, K.M.; Khong, P.L.; Kim, M. Age-related diffusion patterns in human lumbar intervertebral discs: A pilot study in asymptomatic subjects. Magn. Reson. Imaging 2012, 30, 181–188. [Google Scholar] [CrossRef]
- Gübitz, R.; Lange, T.; Gosheger, G.; Heindel, W.; Allkemper, T.; Stehling, C.; Gerss, J.; Kanthak, C.; Schulte, T.L. Influence of Age, BMI, Gender and Lumbar Level on T1ρ Magnetic Resonance Imaging of Lumbar Discs in Healthy Asymptomatic Adults. Rofo 2018, 190, 144–151. [Google Scholar] [CrossRef]
- Pfirrmann, C.W.; Metzdorf, A.; Elfering, A.; Hodler, J.; Boos, N. Effect of aging and degeneration on disc volume and shape: A quantitative study in asymptomatic volunteers. J. Orthop. Res. 2006, 24, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Menezes-Reis, R.; Salmon, C.E.; Bonugli, G.P.; Mazoroski, D.; Tamashiro, M.H.; Savarese, L.G.; Nogueira-Barbosa, M.H. Lumbar intervertebral discs T2 relaxometry and T1ρ relaxometry correlation with age in asymptomatic young adults. Quant. Imaging Med. Surg. 2016, 6, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, J.; Demers, C.N.; Beaudoin, G.; Goswami, T.; Mwale, F.; Aebi, M.; Alini, M. Apparent diffusion coefficient of intervertebral discs related to matrix composition and integrity. Magn. Reson. Imaging 2004, 22, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.M.; Johannessen, W.; Yoder, J.H.; Wheaton, A.J.; Vresilovic, E.J.; Borthakur, A.; Elliott, D.M. Noninvasive quantification of human nucleus pulposus pressure with use of T1p-weighted magnetic resonance imaging. J. Bone Jt. Surg. 2008, 90, 796–802. [Google Scholar] [CrossRef]
- Wei, Z.; Lombardi, A.F.; Lee, R.R.; Wallace, M.; Masuda, K.; Chang, E.Y.; Du, J.; Bydder, G.M.; Yang, W.; Ma, Y.J. Comprehensive assessment of in vivo lumbar spine intervertebral discs using a 3D adiabatic T(1ρ) prepared ultrashort echo time (UTE-Adiab-T(1ρ)) pulse sequence. Quant. Imaging Med. Surg. 2022, 12, 269–280. [Google Scholar] [CrossRef]
- Schleich, C.; Mueller-Lutz, A.; Eichner, M.; Schmitt, B.; Matuschke, F.; Bittersohl, B.; Zilkens, C.; Wittsack, H.-J.; Antoch, G.; Miese, F. Glycosaminoglycan Chemical Exchange Saturation Transfer of Lumbar Intervertebral Discs in Healthy Volunteers. Spine 2016, 41, 146–152. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, X.; Wang, Y.; Zhao, J.; Zhang, X.; Gao, Y.; Li, S. Assessment of apparent diffusion coefficient in lumbar intervertebral disc degeneration. Eur. Spine J. 2014, 23, 1830–1836. [Google Scholar] [CrossRef]
- Delucca, J.F.; Peloquin, J.M.; Smith, L.J.; Wright, A.C.; Vresilovic, E.J.; Elliott, D.M. MRI quantification of human spine cartilage endplate geometry: Comparison with age, degeneration, level, and disc geometry. J. Orthop. Res. 2016, 34, 1410–1417. [Google Scholar] [CrossRef]
- Niu, G.; Yang, J.; Wang, R.; Dang, S.; Wu, E.X.; Guo, Y. MR imaging assessment of lumbar intervertebral disk degeneration and age-related changes: Apparent diffusion coefficient versus T2 quantitation. AJNR Am. J. Neuroradiol. 2011, 32, 1617–1623. [Google Scholar] [CrossRef]
- Wang, W.; Hou, J.; Lv, D.; Liang, W.; Jiang, X.; Han, H.; Quan, X. Multimodal quantitative magnetic resonance imaging for lumbar intervertebral disc degeneration. Exp. Ther. Med. 2017, 14, 2078–2084. [Google Scholar] [CrossRef][Green Version]
- Filippi, C.G.; Duncan, C.T.; Watts, R.; Nickerson, J.P.; Gonyea, J.V.; Hipko, S.G.; Andrews, T. In vivo quantification of T1ρ in lumbar spine disk spaces at 3 T using parallel transmission MRI. AJR Am. J. Roentgenol. 2013, 201, W110–W116. [Google Scholar] [CrossRef]
- Zobel, B.B.; Vadala, G.; Del Vescovo, R.; Battisti, S.; Martina, F.M.; Stellato, L.; Leoncini, E.; Borthakur, A.; Denaro, V. T1 rho Magnetic resonance imaging quantification of early lumbar intervertebral disc degeneration in healthy young adults. Spine 2012, 37, 1224–1230. [Google Scholar] [CrossRef]
- Auerbach, J.D.; Johannessen, W.; Borthakur, A.; Wheaton, A.J.; Dolinskas, C.A.; Balderston, R.A.; Reddy, R.; Elliott, D.M. In vivo quantification of human lumbar disc degeneration using T 1rho-weighted magnetic resonance imaging. Eur. Spine J. 2006, 15, S338–S344. [Google Scholar] [CrossRef]
- Wang, C.; Witschey, W.; Goldberg, A.; Elliott, M.; Borthakur, A.; Reddy, R. Magnetization transfer ratio mapping of intervertebral disc degeneration. Magn. Reson. Med. 2010, 64, 1520–1528. [Google Scholar] [CrossRef]
- Akeda, K.; Ohishi, K.; Takegami, N.; Sudo, T.; Yamada, J.; Fujiwara, T.; Niimi, R.; Matsumoto, T.; Nishimura, Y.; Ogura, T.; et al. Platelet-Rich Plasma Releasate versus Corticosteroid for the Treatment of Discogenic Low Back Pain: A Double-Blind Randomized Controlled Trial. J. Clin. Med. 2022, 11, 304. [Google Scholar] [CrossRef] [PubMed]
- Ashinsky, B.G.; Gullbrand, S.E.; Wang, C.; Bonnevie, E.D.; Han, L.; Mauck, R.L.; Smith, H.E. Degeneration alters structure-function relationships at multiple length-scales and across interfaces in human intervertebral discs. J. Anat. 2020, 238, 986–998. [Google Scholar] [CrossRef] [PubMed]
- Bae, W.C.; Statum, S.; Zhang, Z.; Yamaguchi, T.; Wolfson, T.; Gamst, A.C.; Du, J.; Bydder, G.M.; Masuda, K.; Chung, C.B. Morphology of the cartilaginous endplates in human intervertebral disks with ultrashort echo time MR imaging. Radiology 2013, 266, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Niinimaki, J.L.; Parviainen, O.; Ruohonen, J.; Ojala, R.; Kurunlahti, M.; Karppinen, J.; Tervonen, O.; Nieminen, M.T. In vivo quantification of delayed gadolinium enhancement in the nucleus pulposus of human intervertebral disc. J. Magn. Reson. Imaging 2006, 24, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Beattie, P.F.; Morgan, P.S.; Peters, D. Diffusion-weighted magnetic resonance imaging of normal and degenerative lumbar intervertebral discs: A new method to potentially quantify the physiologic effect of physical therapy intervention. J. Orthop. Sports Phys. Ther. 2008, 38, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Muftuler, L.T.; Jarman, J.P.; Yu, H.J.; Gardner, V.O.; Maiman, D.J.; Arpinar, V.E. Association between intervertebral disc degeneration and endplate perfusion studied by DCE-MRI. Eur. Spine J. 2014, 24, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Jamaludin, A.; Kadir, T.; Zisserman, A.; McCall, I.; Williams, F.M.K.; Lang, H.; Buchanan, E.; Urban, J.P.G.; Fairbank, J.C.T. ISSLS PRIZE in Clinical Science 2023: Comparison of degenerative MRI features of the intervertebral disc between those with and without chronic low back pain. An exploratory study of two large female populations using automated annotation. Eur. Spine J. 2023, 32, 1504–1516. [Google Scholar] [CrossRef]
- Yoon, M.A.; Hong, S.-J.; Kang, C.H.; Ahn, K.-S.; Kim, B.H. T1rho and T2 mapping of lumbar intervertebral disc: Correlation with degeneration and morphologic changes in different disc regions. Magn. Reson. Imaging 2016, 34, 932–939. [Google Scholar] [CrossRef]
- Yu, H.J.; Bahri, S.; Gardner, V.; Muftuler, L.T. In vivo quantification of lumbar disc degeneration: Assessment of ADC value using a degenerative scoring system based on Pfirrmann framework. Eur. Spine J. 2015, 24, 2442–2448. [Google Scholar] [CrossRef]

| Author (Year) | Age | Results | Statistical Methodology |
|---|---|---|---|
| Alkalay et al. (2018) [31] | 37–81 years | Independent of region, age was negatively associated with mean apparent diffusion coefficient (ADC) (r = −0.58, p < 0.001). | Linear correlation coefficient |
| Antoniou et al. (2004) [40] | 11–77 years (mean 48) |
| Linear correlation coefficient |
| DeLucca et al. (2016) [45] | 42–75 years (mean 60.9 ± 10.2) | The CEP anterior/posterior thickness and CEP average thickness decreased with age (r = −0.40, p < 0.01; r = −0.44, p < 0.01). | Multiple regression |
| Filippi et al. (2013) [48] | 21–60 years | Statistically significant moderate negative correlation between average T1ρ values and age (r = 0.686, p < 0.01) | Spearman |
| Fyllos et al. (2018) [27] | 18–54 years (mean 33.08) | Age was a significant coefficient for LVDD (p < 0.001). | Multiple regression |
| Gübitz et al. (2018) [37] | 20–80 years | The effect of age on T1ρ per level was moderate to strong (L1/2 = −0.650; L2/3 = −0.698; L3/4 = −0.786; L4/5 = −0.770; L5/S1 = −0.589). | Pearson |
| Haneder et al. (2014) [25] | 21–60 years (mean 29.2 ± 8.5) | Age had no or only a weak correlation to 23 Nanorm for all anatomic levels L1/2-L5/S1 (0.007 < R2 < 0.202). | Pearson |
| Johannessen et al. (2006) [33] | 15–81 years (mean 51.6) | Strong correlation between T1ρ and age (r = −0.76, p < 0.01). | Linear correlation coefficient |
| Matsumoto et al. (2013) [35] | Mean age 48.0 ± 13.4 | A decrease in disc signal intensity was significantly associated with an increase in age (odds ratio (OR) = 4.2; 95% confidence interval (CI) 1.2–14.9; p = 0.024). | McNemar’s test |
| Menezes-Reis et al. (2016) [39] | 20–40 years (mean 27.1 ± 4.8) | Negative correlation between age and T2 relaxation time (r = −0.30, p < 0.0001) | Spearman |
| Nguyen et al. (2008) [41] | 15–79 years (mean 51.8) | Negative correlation between T1ρ relaxation time and age (r = −0.84, p < 0.05). | Pearson |
| Niu et al. (2011) [46] | 21–73 years (mean 40) | T2 exhibited a more significant inverse correlation with age than ADC (r = −0.77, p < 0.01; r = −0.37, p < 0.01). | Pearson |
| Pfirrmann et al. (2006) [38] | 20–78 years | Age had a significant effect on disc height and disc volume (both p < 0.01). | Multilevel regression analysis |
| Schleich et al. (2016) [43] | 21–49 years (mean 31 ± 8) | Significant correlation between age and morphological Pfirrmann classification, and age and CTF classification (r = 0.3175, p < 0.0001; r = 0.2476, p < 0.0001). | Pearson |
| Vadapalli et al. (2019) [9] | 29–69 years | Strong correlation between fractional anisotropy (FA) (in the NP) and age (R2 = 0.6143). | Pearson |
| Wang et al. (2017) [47] | 20–76 years (mean 34.2 ± 14.0) | T1ρ, T2 and ADC values decrease with the increase of age (r = −0.349; r = −0.594; r = −0.387; all p < 0.01). | Spearman |
| Wang et al. (2021) [30] | 25–73 years (mean 36.9 ± 10.9) | Age is inversely associated with both mean T1ρ values in the NP and mean T2* values in the central CEP (r = −0.72, p < 0.001; r = −0.45, p = 0.032). | Pearson |
| Wei et al. (2022) [42] | 25–71 years (mean 43 ± 16) |
| Spearman |
| Zhang et al. (2012) [36] | 25–67 years (mean 46.8 ± 16) | Age had a negative correlation with mean diffusivity (MD) and moderately positive correlation with FA (r = −0.72, p < 0.001; r = 0.45, p < 0.001). | Spearman |
| Zhang et al. (2014) [44] | 20–59 years (mean 41 ± 12) | Age had an inverse correlation with ADC for all spinal levels (L1/2 r = −0.381; L2/3 r = −0.518; L3/4 r = −0.537; L4/5 r = −0.576; L5/S1 r = −0.604; all p < 0.001). | Spearman |
| Author (Year) | Age | Results |
|---|---|---|
| Antoniou et al. (2004) [40] | 11–77 years (mean 48) (subgroup analysis: 0–20; 21–40; 41–60; 61–80) | The ADC showed a significant decrease with older age when comparing different age groups in the NP and in the anterior AF, except no significant difference was found between age 0–20 and 21–40 as well as 41–60 and 61–80 (p < 0.002 0–20 compared with ages 41–60 and 61–80, p < 0.01 with ages 41–60 and 61–80). |
| Filippi et al. (2013) [48] | 21–60 years (analysis per decade) |
|
| Gübitz et al. (2018) [37] | 20–80 years (subgroup analysis: A: 20–39; B: 40–59; C: 60–80) | Significant differences between groups A and C and groups B and C in T1p values (p = 0.0008; p = 0.0149). |
| Machino et al. (2022) [28] | Mean age 49.6 ± 16.5 (analysis per decade) |
|
| Vadapalli et al. (2019) [9] | 29–69 years (subgroup analysis: A = <30; B = 30–50; C = >50) |
|
| Wang et al. (2021) [30] | 25–73 years (mean 36.9 ± 10.9) (subgroup analysis: <50; 51–60; >60) | NP T1ρ values were significantly correlated with CEP T2* values (r = 0.71, p = 0.047) in the youngest age group. |
| Wang et al. (2017) [47] | 20–76 years (mean 34.2 ± 14.0) (analysis per decade) |
|
| Zhang et al. (2012) [44] | 25–67 years (mean 46.8 ± 16) (subgroup analysis: 25–48; 53–67) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Vries, M.H.E.; Caelers, I.J.M.H.; van Hemert, W.L.W.; Boselie, T.F.M.; van Santbrink, H. Physiological Ageing of the Lumbar Intervertebral Disc Based on Magnetic Resonance Imaging, a Systematic Literature Review. Medicina 2025, 61, 1430. https://doi.org/10.3390/medicina61081430
de Vries MHE, Caelers IJMH, van Hemert WLW, Boselie TFM, van Santbrink H. Physiological Ageing of the Lumbar Intervertebral Disc Based on Magnetic Resonance Imaging, a Systematic Literature Review. Medicina. 2025; 61(8):1430. https://doi.org/10.3390/medicina61081430
Chicago/Turabian Stylede Vries, Max H. E., Inge J. M. H. Caelers, Wouter L. W. van Hemert, Toon F. M. Boselie, and Henk van Santbrink. 2025. "Physiological Ageing of the Lumbar Intervertebral Disc Based on Magnetic Resonance Imaging, a Systematic Literature Review" Medicina 61, no. 8: 1430. https://doi.org/10.3390/medicina61081430
APA Stylede Vries, M. H. E., Caelers, I. J. M. H., van Hemert, W. L. W., Boselie, T. F. M., & van Santbrink, H. (2025). Physiological Ageing of the Lumbar Intervertebral Disc Based on Magnetic Resonance Imaging, a Systematic Literature Review. Medicina, 61(8), 1430. https://doi.org/10.3390/medicina61081430





