Quantitative Analysis of Small Particles Present in Surgical Smoke Generated During Breast Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Devices
2.3. Measurement
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NS | Not significant |
| BMI | Body mass index |
| HR | Hormone receptor |
| HER2 | Human epidermal growth factor receptor 2 |
| Bt | Breast total mastectomy |
| SLNB | Sentinel lymph node biopsy |
| ALND | Axillary lymph node dissection |
| PM2.5 | Particulate matter 2.5 |
References
- Barrett, W.L.; Garber, S.M. Surgical smoke: A review of the literature. Is this just a lot of hot air? Surg. Endosc. 2003, 17, 979–987. [Google Scholar] [CrossRef]
- Fletcher, J.N.; Mew, D.; DesCôteaux, J.G. Dissemination of melanoma cells within electrocautery plume. Am. J. Surg. 1999, 178, 57–59. [Google Scholar] [CrossRef]
- Noduka, C.C.; Poland, N.; Kennedy, M.; Dye, J.; Darzi, A. Does the ultrasonically activated scalpel release viable airborne cancer cells? Surg. Endosc. 1998, 12, 1031–1034. [Google Scholar] [CrossRef]
- Hoye, R.C.; Ketcham, A.S.; Riggle, G.C. The airborne dissemination of viable tumor cells by high energy neodynium laser. Life Sci. 1967, 6, 119–125. [Google Scholar] [CrossRef]
- Champault, G.; Taffinder, N.; Ziol, M.; Riskalla, H.; Catheline, J.M. Cells are present in the smoke created during laparoscopic surgery. Br. J. Surg. 1997, 84, 993–995. [Google Scholar] [CrossRef]
- Ott, D.E.; Moss, E.; Martinez, K. Aerosol exposure from an ultrasonically activated (Harmonic) device. J. Am. Assoc. Gynecol. Laparosc. 1998, 5, 29–32. [Google Scholar] [CrossRef]
- McKinley, I.B., Jr.; Ludlow, M.O. Hazards of laser smoke during endodontic therapy. J. Endod. 1994, 20, 558–559. [Google Scholar] [CrossRef]
- Capizzi, P.J.; Clay, R.P.; Battey, M.J. Microbiologic activity in laser resurfacing plume and debris. Lasers Surg. Med. 1998, 23, 172–174. [Google Scholar] [CrossRef]
- Garden, J.M.; O’Banion, M.K.; Shelnitz, L.S.; Pinski, K.S.; Bakus, A.D.; Reichmann, M.E.; Sundberg, J.P. Papillomavirus in the vapor of carbon dioxide laser-treated verrucae. JAMA 1988, 259, 1199–1202. [Google Scholar] [CrossRef]
- Ferenczy, A.; Bergeron, C.; Richart, R.M. Human papilloma virus DNA in CO2 laser-generated plume of smoke and its consequences to the surgeon. Obstet. Gynecol. 1990, 75, 114–118. [Google Scholar]
- Baggish, M.S.; Poiesz, B.J.; Joret, D.; Williamson, P.; Refai, A. Presence of human immunodeficiency virus DNA in laser smoke. Lasers Surg. Med. 1991, 11, 197–203. [Google Scholar] [CrossRef]
- Taravella, M.J.; Weinberg, A.; May, M.; Stepp, P. Live virus survives excimer laser ablation. Ophthalmology 1999, 106, 1498–1499. [Google Scholar] [CrossRef]
- Kwak, H.D.; Kim, S.H.; Seo, Y.S.; Song, K.J. Detecting hepatitis B virus in surgical smoke emitted during laparoscopic surgery. Occup. Environ. Med. 2016, 73, 857–863. [Google Scholar] [CrossRef]
- Beebe, D.S.; Swica, H.; Carlson, N.; Palahniuk, R.J.; Goodale, R.L. High level of carbon monoxide are produced by electro-cautery of tissue during laparoscopic cholecystectomy. Anesth. Analg. 1993, 77, 330–341. [Google Scholar] [CrossRef]
- Hensman, C.; Baty, D.; Willis, R.G.; Cuschieri, A. Chemical composition of smoke produced by high-frequency electrosurgery in a closed gaseous environment: An in vitro study. Surg. Endosc. 1998, 12, 1017–1019. [Google Scholar] [CrossRef] [PubMed]
- Al Sahaf, O.S.; Vega-Carrascal, I.; Cunningham, F.O.; McGrath, J.P.; Bloomfield, F.J. Chemical composition of smoke produced by high-frequency electrosurgery. Ir. J. Med. Sci. 2007, 176, 229–232. [Google Scholar] [CrossRef]
- Lin, Y.W.; Fan, S.Z.; Chang, K.H.; Huang, C.S.; Tang, C.S. A novel inspection protocol to detect volatile compounds in breast surgery electrocautery smoke. J. Formos. Med. Assoc. 2010, 109, 511–516. [Google Scholar] [CrossRef]
- Fitzgerald, J.E.; Malik, M.; Ahmed, I. A single-blind controlled study of electrocautery and ultrasonic scalpel smoke plumes in laparoscopic surgery. Surg. Endosc. 2012, 26, 337–342. [Google Scholar] [CrossRef]
- Takahashi, H.; Yamasaki, M.; Hirota, M.; Miyazaki, Y.; Moon, J.H.; Souma, Y.; Mori, S.; Doki, Y.; Nakajima, K. Automatic smoke evacuation in laparoscopic surgery: A simplified method for objective evaluation. Surg. Endosc. 2013, 27, 2980–2987. [Google Scholar] [CrossRef]
- Tomita, Y.; Mihashi, S.; Nagata, K.; Ueda, S.; Fujiki, M.; Hirano, M.; Hirohata, T. Mutagenicity of smoke condensates induced by CO2-laser irradiation and electrocauterization. Mutat. Res. 1981, 89, 145–149. [Google Scholar]
- Hill, D.S.; O’Neill, J.K.; Powell, R.J.; Oliver, D.W. Surgical smoke—A health hazard in the operating theatre: A study to quantify exposure and a survey of the use of smoke extractor systems in UK plastic surgery units. J. Plast. Reconstr. Aesthet. Surg. 2012, 65, 911–916. [Google Scholar] [CrossRef]
- Soleimani, F.; Dobaradaran, S.; De-la-Torre, G.E.; Schmidt, T.C.; Saeedi, R. Content of toxic components of cigarette smoke vs cigarette butts: A comprehensive systematic review. Sci. Total Environ. 2022, 813, 152667. [Google Scholar] [CrossRef]
- Li, Y.; Hecht, S.S. Carcinogenic components of tobacco and tobacco smoke: A 2022 update. Food Chem. Toxicol. 2022, 165, 113179. [Google Scholar] [CrossRef]
- Aghapour, M.; Raee, P.; Moghaddam, S.J.; Hiemstra, P.S.; Heijink, I.H. Airway epithelial barrier dysfunction in chronic obstructive pulmonary disease: Role of cigarette smoke exposure. Am. J. Respir. Cell Mol. Biol. 2018, 58, 157–169. [Google Scholar] [CrossRef]
- Ambrose, J.A.; Barua, R.S. The pathophysiology of cigarette smoking and cardiovascular disease. J. Am. Coll. Cardiol. 2004, 43, 1731–1737. [Google Scholar] [CrossRef]
- Kameyama, H.; Otani, T.; Yamazaki, T.; Iwaya, A.; Uehara, H.; Harada, R.; Hirai, M.; Komatsu, M.; Kubota, A.; Katada, T.; et al. Comparison of surgical smoke between open surgery and laparoscopic surgery for colorectal disease in the COVID-19 era. Surg. Endosc. 2022, 36, 1243–1250. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Okoshi, K.; Hida, K.; Kinoshita, K.; Morishima, T.; Nagai, Y.; Tomizawa, Y.; Yorozuya, K.; Nishida, T.; Matsumoto, H.; Yamato, H. Measurement of particulate matter 2.5 in surgical smoke and its health hazards. Surg. Today 2022, 52, 1341–1347. [Google Scholar] [CrossRef]
- Feng, S.; Gao, D.; Liao, F.; Zhou, F.; Wang, X. The health effects of ambient PM2.5 and potential mechanisms. Ecotoxicol. Environ. Saf. 2016, 128, 67–74. [Google Scholar] [CrossRef]
- Krittanawong, C.; Qadeer, Y.K.; Hayes, R.B.; Wang, Z.; Virani, S.; Thurston, G.D.; Lavie, C.J. PM2.5 and cardiovascular health risks. Curr. Probl. Cardiol. 2023, 48, 101670. [Google Scholar] [CrossRef]
- Hayes, R.B.; Lim, C.; Zhang, Y.; Cromar, K.; Shao, Y.; Reynolds, H.R.; Silverman, D.T.; Jones, R.R.; Park, Y.; Jerrett, M.; et al. PM2.5 air pollution and cause-specific cardiovascular disease mortality. Int. J. Epidemiol. 2020, 49, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Raaschou-Nielsen, O.; Andersen, Z.J.; Beelen, R.; Samoli, E.; Stafoggia, M.; Weinmayr, G.; Hoffmann, B.; Fischer, P.; Nieuwenhuijsen, M.J.; Brunekreef, B.; et al. Air pollution and lung cancer incidence in 17 European cohorts: Prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE). Lancet Oncol. 2013, 14, 813–822. [Google Scholar] [CrossRef]
- Chauhan, B.V.S.; Corada, K.; Young, C.; Smallbone, K.L.; Wyche, K.P. Review on Sampling Methods and Health Impacts of Fine (PM2.5, ≤2.5 µm) and Ultrafine (UFP, PM0.1, ≤0.1 µm) Particles. Atmosphere 2024, 15, 572. [Google Scholar] [CrossRef]
- Zhou, L.; Tao, Y.; Su, X.; Chen, X.; Li, L.; Fu, Q.; Xie, J.; Chen, R. Short-Term Associations between Size-Fractioned Particles and Cardiopulmonary Function in COPD Patients: A Panel Study in Shanghai, China, during 2014-2021. Int. J. Environ. Res. Public Health 2022, 19, 12473. [Google Scholar] [CrossRef]
- Meng, X.; Ma, Y.; Chen, R.; Zhou, Z.; Chen, B.; Kan, H. Size-fractionated particle number concentrations and daily mortality in a Chinese city. Environ. Health Perspect. 2013, 121, 1174–1178. [Google Scholar] [CrossRef] [PubMed]
- Okoshi, K.; Kobayashi, K.; Kinoshita, K.; Tomizawa, Y.; Hasegawa, S.; Sakai, Y. Health risks associated with exposure to surgical smoke for surgeons and operation room personnel. Surg. Today 2015, 45, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Spearman, J.; Tsavellas, G.; Nichols, P. Current attitudes and practices towards diathermy smoke. Ann. R. Coll. Surg. Engl. 2007, 89, 162–165. [Google Scholar] [CrossRef]




| Electrosurgical Device Group (n = 20) | Electrosurgical Device with Smoke Evacuator Group (n = 20) | p Value | |
|---|---|---|---|
| Age (y) | 60.5 (45–97) | 70.5 (40–81) | 0.21 |
| Female/male | 20/0 | 20/0 | NS |
| BMI (kg/m2) | 21.28 (15.90–31.48) | 24.08 (17.68–30.37) | 0.41 |
| Stage 0–II/III, IV | 17/3 | 17/3 | NS |
| Subtype HR(+), HER2(−) HER2(+) HR(−), HER2(−) | 11 4 5 | 10 6 4 | 0.84 |
| Preoperative treatment | 5 | 8 | 0.50 |
| Surgical procedure Bt Bt SLNB Bt ALND | 1 14 5 | 3 9 8 | 0.32 |
| Weight of specimen (grams) | 310 (126–1325) | 371 (91–858) | 0.91 |
| Operating Side | Contralateral Side | p Value | |
|---|---|---|---|
| Electrosurgical device group (n = 20) | 9220.9 (1251.9–22,780.1) | 5305.5 (69.6–49,788.7) | <0.05 |
| Procedure | Electrosurgical Device Group (n = 20) | Electrosurgical Device with Smoke Evacuator Group (n = 20) | p Value |
|---|---|---|---|
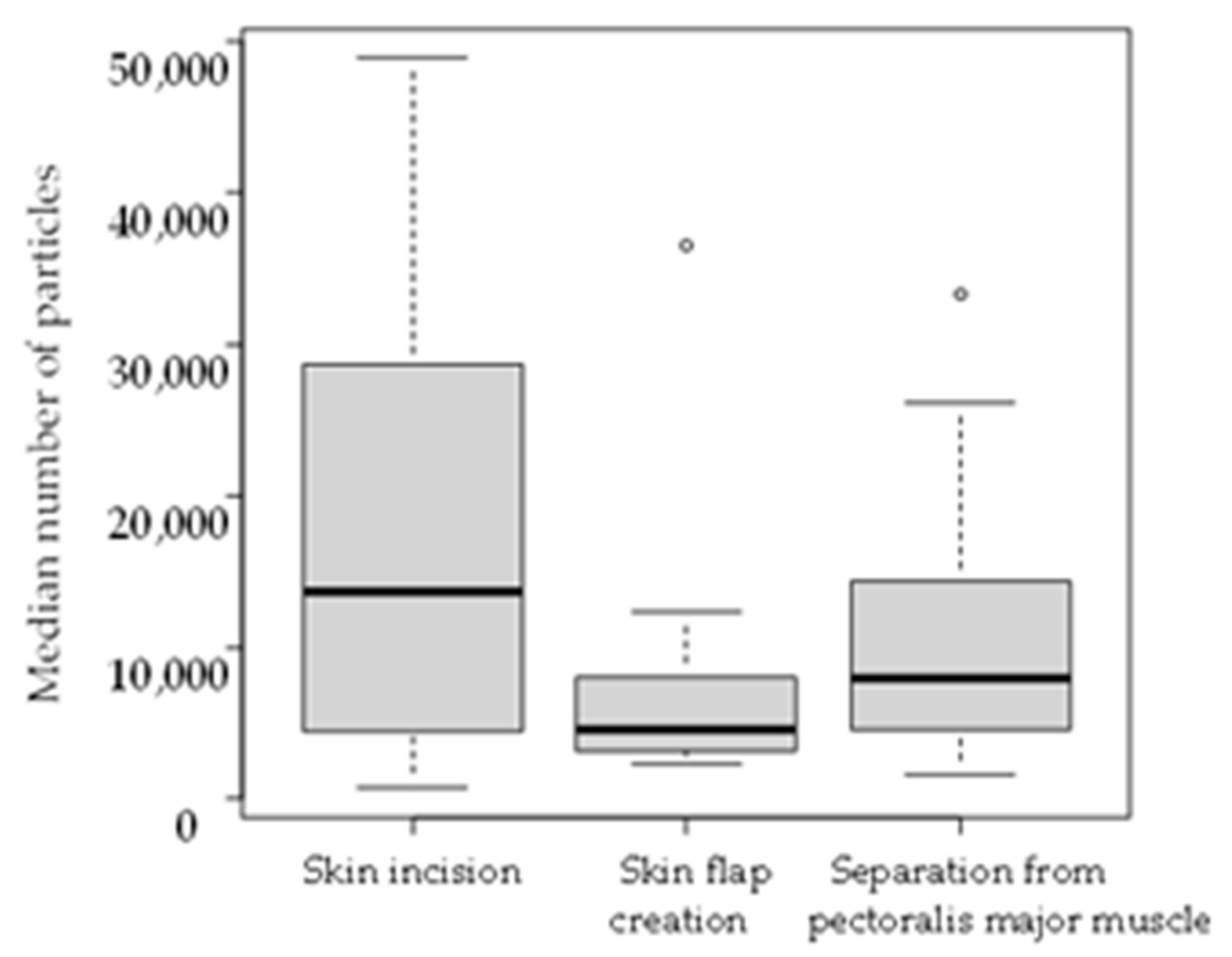
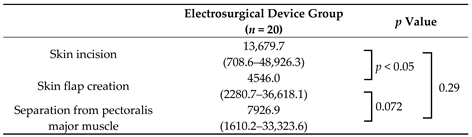
| Skin incision | 13,679.7 (708.6–48,926.3) | 576.3 (39.4–13,345.9) | <0.001 |
| Skin flap creation | 4546.0 (2280.7–36,618.1) | 286.0 (25.4–2162.3) | <0.001 |
| Separation from pectoralis major muscle | 7926.9 (1610.2–33,323.6) | 393.8 (20.6–1601.5) | <0.001 |
| Total procedure | 7254.0 (2749.9–36,923.1) | 445.9 (105.6–3701.2) | <0.001 |
| Correlation Coefficient | p Value | ||
|---|---|---|---|
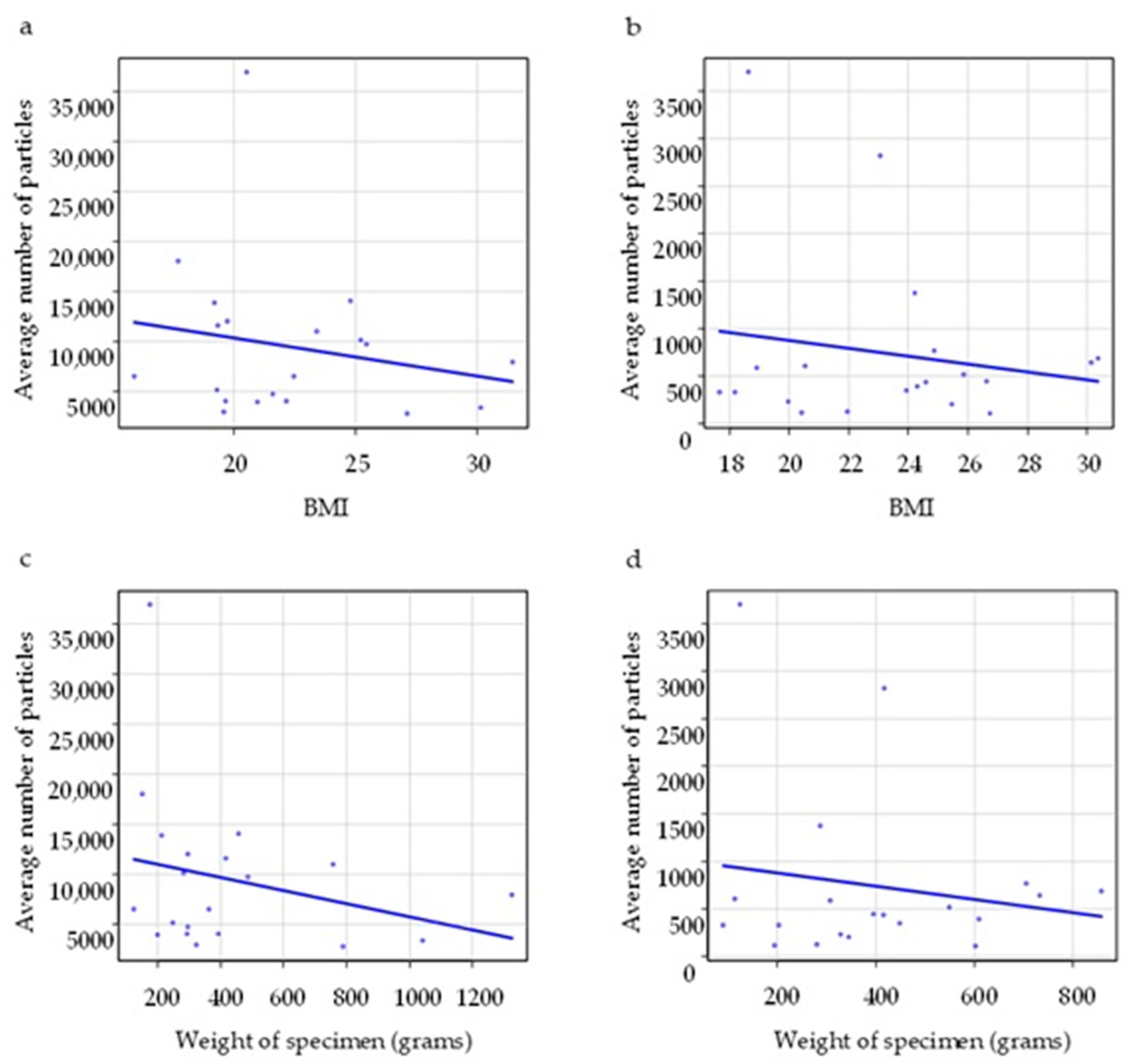
| BMI | Electrosurgical device group (n = 20) | –0.28 | 0.31 |
| Electrosurgical device with smoke evacuator group (n = 20) | 0.11 | 0.64 | |
| Weight of specimen | Electrosurgical device group (n = 20) | –0.26 | 0.26 |
| Electrosurgical device with smoke evacuator group (n = 20) | 0.18 | 0.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hara, M.; Oda, G.; Hayashi, K.; Adachi, M.; Kumaki, Y.; Ishiba, T.; Yamaga, E.; Fujioka, T.; Nakagawa, T.; Mori, H.; et al. Quantitative Analysis of Small Particles Present in Surgical Smoke Generated During Breast Surgery. Medicina 2025, 61, 1422. https://doi.org/10.3390/medicina61081422
Hara M, Oda G, Hayashi K, Adachi M, Kumaki Y, Ishiba T, Yamaga E, Fujioka T, Nakagawa T, Mori H, et al. Quantitative Analysis of Small Particles Present in Surgical Smoke Generated During Breast Surgery. Medicina. 2025; 61(8):1422. https://doi.org/10.3390/medicina61081422
Chicago/Turabian StyleHara, Masatake, Goshi Oda, Kumiko Hayashi, Mio Adachi, Yuichi Kumaki, Toshiyuki Ishiba, Emi Yamaga, Tomoyuki Fujioka, Tsuyoshi Nakagawa, Hiroki Mori, and et al. 2025. "Quantitative Analysis of Small Particles Present in Surgical Smoke Generated During Breast Surgery" Medicina 61, no. 8: 1422. https://doi.org/10.3390/medicina61081422
APA StyleHara, M., Oda, G., Hayashi, K., Adachi, M., Kumaki, Y., Ishiba, T., Yamaga, E., Fujioka, T., Nakagawa, T., Mori, H., & Aruga, T. (2025). Quantitative Analysis of Small Particles Present in Surgical Smoke Generated During Breast Surgery. Medicina, 61(8), 1422. https://doi.org/10.3390/medicina61081422





