Clostridioides difficile Infections: Epidemiological and Laboratory Data from the Internal Medicine Departments of a Tertiary Care Hospital in Athens, Greece, During the Past Decade
Abstract
1. Introduction
2. Materials and Methods
3. Statistical Analysis
4. Results
4.1. Characteristics of the Study Population
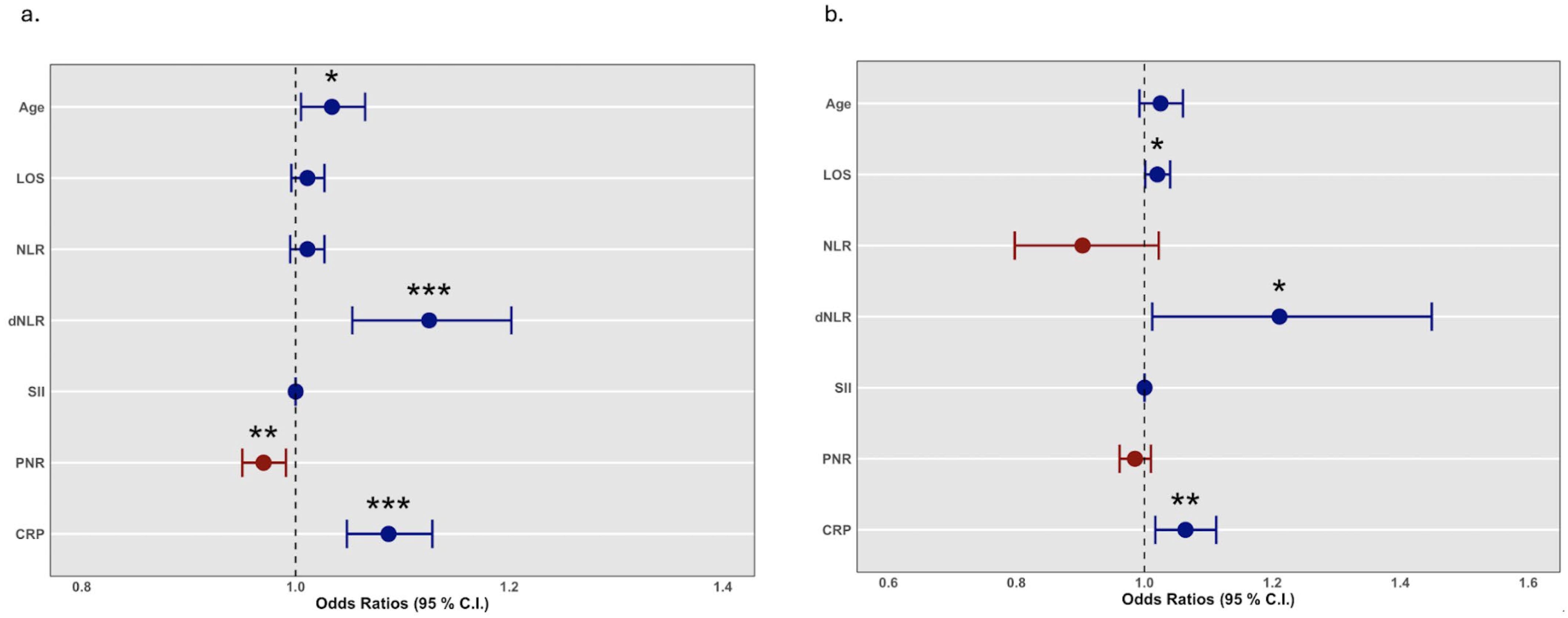
4.2. Logistic Regression Analysis
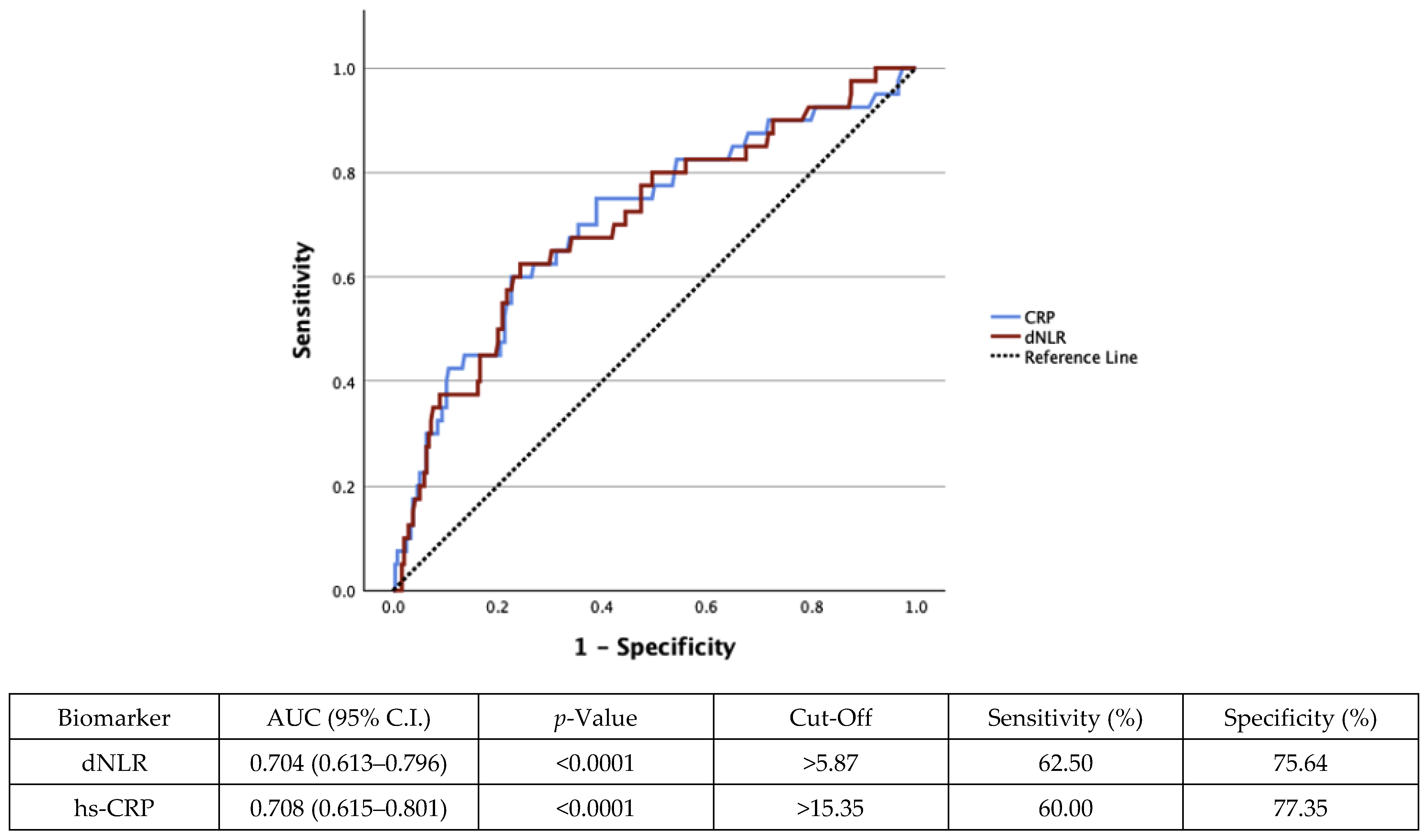
4.3. Receiver Operating Characteristic (ROC) Curves
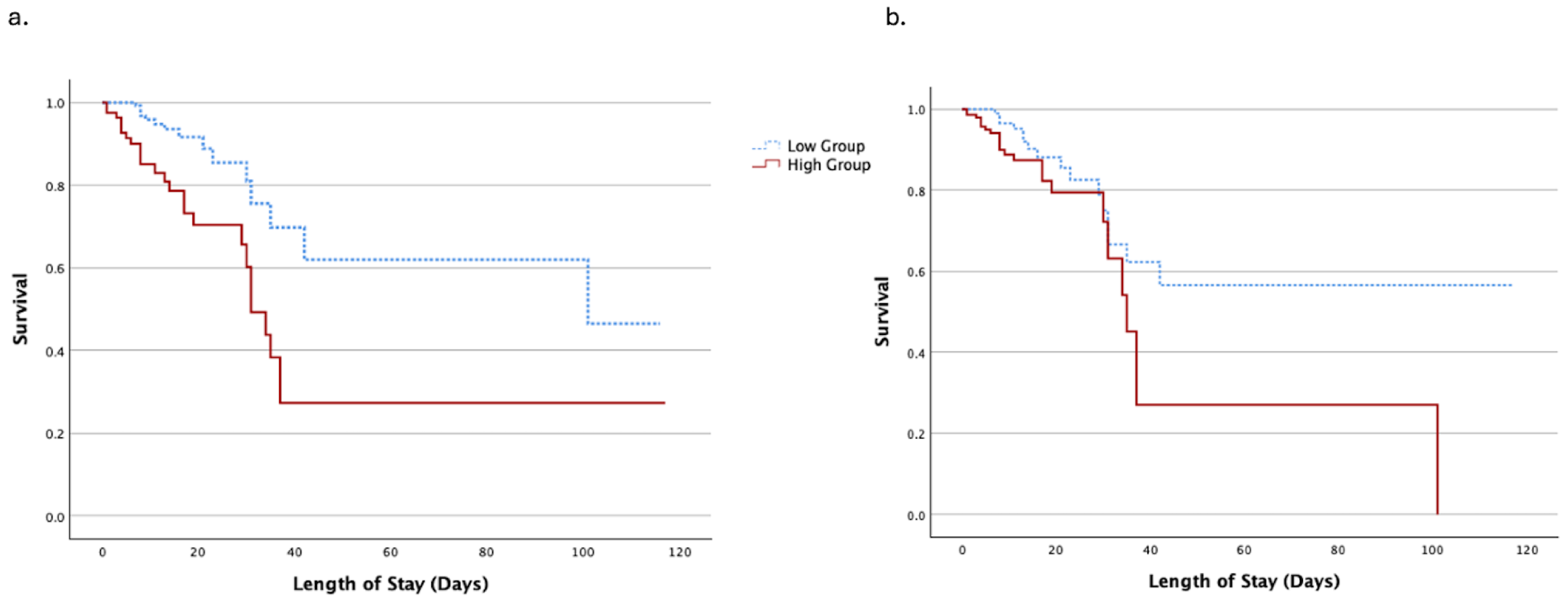
4.4. Survival Analysis
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akorful, R.A.A.; Odoom, A.; Awere-Duodu, A.; Donkor, E.S. The Global Burden of Clostridioides difficile Infections, 2016–2024: A Systematic Review and Meta-Analysis. Infect. Dis. Rep. 2025, 17, 31. [Google Scholar] [CrossRef] [PubMed]
- Angulo, F.J.; Furtado, M.; Gonzalez, E.; Zhang, P.; Kelly, P.H.; Moïsi, J.C. Incidence of public health surveillance-reported Clostridioides difficile infections in thirteen countries worldwide: A narrative review. Anaerobe 2024, 88, 102878. [Google Scholar] [CrossRef] [PubMed]
- Eeuwijk, J.; Ferreira, G.; Yarzabal, J.P.; Robert-Du Ry van Beest Holle, M. A Systematic Literature Review on Risk Factors for and Timing of Clostridioides difficile Infection in the United States. Infect. Dis. Ther. 2024, 13, 273–298. [Google Scholar] [CrossRef] [PubMed]
- Feuerstadt, P.; Theriault, N.; Tillotson, G. The burden of CDI in the United States: A multifactorial challenge. BMC. Infect. Dis. 2023, 23, 132. [Google Scholar] [CrossRef]
- Persson, S.; Nielsen, H.L.; Coia, J.E.; Engberg, J.; Olesen, B.S.; Engsbro, A.L.; Petersen, A.M.; Holt, H.M.; Lemming, L.; Marmolin, E.S.; et al. Sentinel surveillance and epidemiology of Clostridioides difficile in Denmark, 2016 to 2019. Eurosurveillance 2022, 27, 2200244. [Google Scholar] [CrossRef]
- Spigaglia, P.; Barbanti, F.; Criscuolo, E.M.; D’Ancona, F. Italian CDI Surveillance Pilot working group. Pilot study of Clostridioides difficile infection (CDI) in hospitals, Italy, September to December 2022. Eurosurveillance 2025, 30, 2400206. [Google Scholar] [CrossRef]
- ECDC. Clostridioides difficile infection. In Annual Epidemiological Report for 2018–2020; European Centre for Disease Prevention and Control: Solna, Sweden, 2024. [Google Scholar]
- Alam, M.Z.; Madan, R. Clostridioides difficile Toxins: Host Cell Interactions and Their Role in Disease Pathogenesis. Toxins 2024, 16, 241. [Google Scholar] [CrossRef]
- Tiecco, G.; De Francesco, M.A.; Lenzi, A.; Pellizzeri, S.; Rossini, F.; Sollima, A.; Signorini, L.; Castelli, F.; Caruso, A.; Quiros-Roldan, E. Clostridioides difficile infections caused by hypervirulent strains: A single-centre real-life study. Eur. J. Clin. Microbiol. Infect. Dis. 2025, 44, 99–107. [Google Scholar] [CrossRef]
- Kelly, C.R.; Fischer, M.; Allegretti, J.R.; LaPlante, K.; Stewart, D.B.; Limketkai, B.N.; Stollman, N.H. ACG clinical guidelines: Prevention, diagnosis, and treatment of Clostridioides difficile infections. Am. J. Gastroenterol. 2021, 116, 1124–1147. [Google Scholar] [CrossRef]
- Arslan, M.; Shabbir, M.U.; Farooq, U.; Bilal, B.; Abbas, S.; Chaudhry, N.; Qasim, M.; Nizamuddin, S. Clinical Characteristics and Outcomes of Clostridioides difficile Infection in Cancer Patients From a Tertiary Care Hospital. Cureus 2025, 17, e77616. [Google Scholar] [CrossRef]
- Olsen, M.A.; Stwalley, D.; Demont, C.; Dubberke, E.R. Clostridium difficile infection increases acute and chronic morbidity and mortality. Infect. Control Hosp. Epidemiol. 2019, 40, 65–71. [Google Scholar] [CrossRef]
- Sayed, A.A. The Preparation of Future Statistically Oriented Physicians: A Single-Center Experience in Saudi Arabia. Medicina 2024, 60, 1694. [Google Scholar] [CrossRef] [PubMed]
- Boven, A.; Vlieghe, E.; Engstrand, L.; Andersson, F.L.; Callens, S.; Simin, J.; Brusselaers, N. Clostridioides difficile infection-associated cause-specific and all-cause mortality: A population-based cohort study. Clin. Microbiol. Infect. 2023, 29, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- De-la-Rosa-Martinez, D.; Zinser-Peniche, P.; Martin-Onraet, A.; Rivera-Buendía, F.; Vilar-Compte, D. Performance of Clostridioides difficile infection severity scores and risk factors related to 30-day all-cause mortality in patients with cancer. Support Care Cancer 2023, 31, 187. [Google Scholar] [CrossRef] [PubMed]
- Drobnik, J.; Pobrotyn, P.; Belovičová, M.; Madziarska, K.; Trocha, M.; Baran, M. Mortality in Clostridioides difficile infection among patients hospitalized at the university clinical hospital in Wroclaw, Poland—A 3-year observational study. BMC. Infect. Dis. 2024, 24, 625. [Google Scholar] [CrossRef]
- Tydeman, F.; Craine, N.; Kavanagh, K.; Adams, H.; Reynolds, R.; McClure, V.; Hughes, H.; Hickman, M.; Robertson, C. Incidence of Clostridioides difficile infection (CDI) related to antibiotic prescribing by GP surgeries in Wales. J. Antimicrob. Chemother. 2021, 76, 2437–2445. [Google Scholar] [CrossRef]
- Scarlata, G.G.M.; Quirino, A.; Costache, C.; Toc, D.A.; Marascio, N.; Pantanella, M.; Leucuta, D.C.; Ismaiel, A.; Dumitrascu, D.L.; Abenavoli, L. Clostridioides difficile Infection: Use of Inflammatory Biomarkers and Hemogram-Derived Ratios to Predict Mortality Risk in Hospitalized Patients. Antibiotics 2024, 13, 769. [Google Scholar] [CrossRef]
- Asaduzzaman, M.D.; Romel Bhuia, M.; Nazmul Alam, Z.; Zabed Jillul Bari, M.; Ferdousi, T. Significance of hemogram-derived ratios for predicting in-hospital mortality in COVID-19: A multicenter study. Health Sci. Rep. 2022, 5, e663. [Google Scholar] [CrossRef]
- Alagbe, A.E.; Pedroso, G.A.; de Oliveira, B.B.; da Costa, E.; Maia, G.A.F.; Piellusch, B.F.; Domingues Costa Jorge, S.E.; Costa, F.F.; Modena, J.L.P.; Schreiber, A.Z. Hemograms and serial hemogram-derived ratios in survivors and non-survivors of COVID-19 in Campinas, Brazil. Hematol. Transfus. Cell Ther. 2024, 46, 14–21. [Google Scholar] [CrossRef]
- Segalo, S.; Kiseljakovic, E.; Papic, E.; Joguncic, A.; Pasic, A.; Sahinagic, M.; Lepara, O.; Sporisevic, L. The Role of Hemogram-derived Ratios in COVID-19 Severity Stratification in a Primary Healthcare Facility. Acta Inform. Med. 2023, 31, 41–47. [Google Scholar] [CrossRef]
- Velazquez, S.; Madurga, R.; Castellano, J.M.; Rodriguez-Pascual, J.; de Aguiar Diaz Obregon, S.R.; Jimeno, S.; Montero, J.I.; Wichner, P.S.V.; López-Escobar, A. Hemogram-derived ratios as prognostic markers of ICU admission in COVID-19. BMC Emerg. Med. 2021, 21, 89. [Google Scholar] [CrossRef]
- Ghobadi, H.; Mohammadshahi, J.; Javaheri, N.; Fouladi, N.; Mirzazadeh, Y.; Aslani, M.R. Role of leukocytes and systemic inflammation indexes (NLR, PLR, MLP, dNLR, NLPR, AISI, SIR-I, and SII) on admission predicts in-hospital mortality in non-elderly and elderly COVID-19 patients. Front. Med. 2022, 9, 916453. [Google Scholar] [CrossRef]
- Barbosa-Martins, J.; Mendonça, J.; Carvalho, C.; Sarmento, H.; Mota, P.; Coutinho, C.; Cotter, J. Clostridium difficile Severity and Outcome at a North of Portugal Healthcare Facility. Acta Med. Port. 2022, 35, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Sabo, C.M.; Leucuta, D.C.; Simiraș, C.; Deac, I.Ș.; Ismaiel, A.; Dumitrascu, D.L. Hemogram-Derived Ratios in the Prognosis of Acute Diverticulitis. Medicina 2023, 59, 1523. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, M.G.; Sherwin, J.C.; Gkrania-Klotsas, E. Risk factors for mortality in Clostridium difficile infection in the general hospital population: A systematic review. J. Hosp. Infect. 2012, 82, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Asaoka, M.; Horita, Y.; Wachino, C.; Kondo, S.; Hotta, Y.; Kataoka, T.; Sanagawa, A.; Hayakawa, T.; Nakamura, A.; Kimura, K. Clinical Usefulness of the “MN Criteria”—The Clostridioides difficile Infection Severity Scoring System—In the Japanese Setting. Intern. Med. 2023, 62, 59–67. [Google Scholar] [CrossRef]
- Carratala, J.; Roson, B.; Fernandez-Sabe, N.; Shaw, E.; del Rio, O.; Rivera, A. Factors associated with complications and mortality in adult patients hospitalized for infectious cellulitis. Eur. J. Clin. Microbiol. Infect. Dis. 2003, 22, 151–157. [Google Scholar] [CrossRef]
- Lee, C.S.; Min, I.S.; Hwang, J.H.; Kwon, K.S.; Lee, H.B. Clinical significance of hypoalbuminemia in outcome of patients with scrub typhus. BMC Infect. Dis. 2010, 10, 216. [Google Scholar] [CrossRef]
- Wiedermann, C.J. Hypoalbuminemia as Surrogate and Culprit of Infections. Int. J. Mol. Sci. 2021, 22, 4496. [Google Scholar] [CrossRef]
- Stămăteanu, L.O.; Miftode, I.L.; Pleşca, C.E.; Hurmuzache, M.E.; Manciuc, D.C.; Leca, D.; Miftode, E.G. Healthcare-Associated Clostridioides difficile Infection: A Hospital-Based Retrospective Study in North Eastern Romania. Microorganisms 2025, 13, 1377. [Google Scholar] [CrossRef]
- Medaglia, A.A.; Mancuso, A.; Albano, C.; Zinna, G.; Pipitò, L.; Calà, C.; Immordino, R.; Rubino, R.; Bonura, S.; Canino, B.; et al. Clostridioides difficile Infection in an Italian Tertiary Care University Hospital: A Retrospective Analysis. Antibiotics 2023, 12, 837. [Google Scholar] [CrossRef] [PubMed]
- Markovska, R.; Dimitrov, G.; Gergova, R.; Boyanova, L. Clostridioides difficile, a New “Superbug”. Microorganisms. 2023, 11, 845. [Google Scholar] [CrossRef]
- Ignuta, F.; Vlad, A.; Cerbulescu, T.; Loredana, S.; Bratosin, F.; Rosca, O.; Stelea, L.; Nistor, D. Evaluation of Inflammatory Markers and Clinical Outcomes in COVID-19 Patients with Concurrent Clostridioides difficile Infection: A Comparative Cohort Analysis. Biomedicines 2025, 13, 111. [Google Scholar] [CrossRef] [PubMed]
- Madden, G.R.; Boone, R.H.; Lee, E.; Sifri, C.D.; Petri, W.A., Jr. Predicting Clostridioides difficile infection outcomes with explainable machine learning. EBioMedicine 2024, 106, 105244. [Google Scholar] [CrossRef] [PubMed]
- Morado, F.; Nanda, N.A. Review of Therapies for Clostridioides difficile Infection. Antibiotics 2025, 14, 17. [Google Scholar] [CrossRef] [PubMed]
| Survivors (n = 234) | Non-Survivors (n = 40) | p-Value | |
|---|---|---|---|
| Age (years), (mean ± SD) | 81 (72–87) | 86 (76–92) | 0.026 * |
| Sex, n (%) | 0.733 | ||
| Male | 108 (46.2) | 17 (42.5) | |
| Female | 126 (53.8) | 23 (57.5) | |
| Comorbidities, n (%) | 138 (59.0) | 28 (70.0) | 0.222 |
| Cancer, n (%) | 47 (20.2) | 8 (20.0) | >0.999 |
| Diabetes, n (%) | 62 (26.5) | 14 (35.0) | 0.339 |
| Heart failure, n (%) | 37 (15.9) | 17 (42.5) | <0.001 *** |
| Liver insufficiency, n (%) | 10 (4.3) | 3 (7.5) | 0.414 |
| Renal failure, n (%) | 46 (19.7) | 8 (20.0) | >0.999 |
| Respiratory failure, n (%) | 28 (12.1) | 8 (20.0) | 0.205 |
| Length of stay (median, IQR) | 10 (6–17) | 14 (8–31) | 0.045 * |
| Laboratory data (median, IQR) | |||
| White blood cells (103/μL) | 11.00 (6.87–14.31) | 12.79 (9.10–22.55) | 0.012 * |
| Neutrophils (103/μL) | 8.33 (5.07–12.05) | 10.06 (7.39–20.81) | 0.004 ** |
| Lymphocytes (103/μL) | 1.29 (0.90–1.80) | 1.17 (0.82–1.41) | 0.078 |
| Monocytes (103/μL) | 0.63 (0.45–0.94) | 0.54 (0.41–0.97) | 0.345 |
| Eosinophiles (103/μL) | 0.06 (0.02–0.13) | 0.04 (0.01–0.16) | 0.184 |
| Basophiles (103/μL) | 0.03 (0.02–0.05) | 0.04 (0.01–0.08) | 0.457 |
| Platelets (103/μL) | 252.50 (178.80–323.50) | 255.00 (156.30–360.80) | 0.695 |
| Hematocrit (HCT) (%) | 32.35 (28.68–36.50) | 30.20 (25.20–34.38) | 0.024 * |
| Hemoglobin (Hgb) (g/dL) | 10.80 (9.30–12.00) | 10.05 (8.50–11.48) | 0.012 * |
| NLR | 5.66 (3.31–10.45) | 12.48 (4.97–23.35) | <0.001 *** |
| dNLR | 3.24 (2.15–5.85) | 6.39 (3.48–11.70) | <0.001 *** |
| SII | 1494.00 (653.2–2923) | 1992.00 (870.1–7381) | 0.019 * |
| PNR | 28.28 (17.68–48.26) | 21.77 (10.81–33.18) | 0.001 ** |
| PLR | 188.20 (117.20–270.10) | 193.3 (139.3–342.4) | 0.359 |
| hs-CRP (mg/L) | 8.20 (4.08–14.43) | 16.50 (8.83–24.95) | <0.001 *** |
| Albumin (g/dL) | 3.20 (2.90–3.60) | 2.50 (2.00–2.90) | <0.001 *** |
| Total bilirubin (mg/dL) | 0.52 (0.39–0.75) | 0.51 (0.33–0.71) | 0.301 |
| Direct bilirubin (mg/dL) | 0.23 (0.17–0.35) | 0.27 (0.17–0.39) | 0.582 |
| Total protein (g/dL) | 5.80 (5.30–6.26) | 5.20 (4.70–5.80) | <0.001 *** |
| Glucose (mg/dL) | 96.00 (78.75–117.00) | 100.50 (80.00–135.00) | 0.538 |
| Troponin (ng/mL) | 32.00 (14.00–63.50) | 47.00 (17.25–100.50) | 0.353 |
| Urea (mg/dL) | 38.00 (25.00–65.75) | 57.50 (29.75–103.30) | 0.023 * |
| Creatinine (mg/dL) | 0.99 (0.70–1.50) | 0.95 (0.60–2.00) | 0.830 |
| Aspartate aminotransferase (U/L) | 18.00 (13.50–27.00) | 16.00 (13.00–28.50) | 0.379 |
| Alanine transaminase (U/L) | 12.00 (8.00–20.00) | 10.00 (6.00–20.00) | 0.137 |
| Alkaline phosphatase (U/L) | 75.50 (56.00–97.75) | 91.50 (67.00–134.00) | 0.007 ** |
| Gamma-glutamyltransferase (U/L) | 21.00 (13.00–34.00) | 19.00 (12.25–50.00) | 0.583 |
| Lactate dehydrogenase (U/L) | 211.00 (167.00–265.00) | 250.50 (206.80–322.80) | 0.005 ** |
| Creatine phosphokinase (U/L) | 50.50 (27.00–94.75) | 35.00 (23.00–74.00) | 0.170 |
| eGFR (ml/min/1.73 m2) | 71.00 (36.00–94.00) | 70.50 (30.00–93.75) | 0.654 |
| Variable | Univariate Model | Multivariate Model | ||||
|---|---|---|---|---|---|---|
| O.R. | 95% C.I. | p-Value | O.R. | 95% C.I. | p-Value | |
| Age | 1.034 | 1.005–1.065 | 0.023 * | 1.025 | 0.992–1.060 | 0.141 |
| LOS | 1.011 | 0.996–1.027 | 0.162 | 1.020 | 1.001–1.040 | 0.038 * |
| NLR | 1.011 | 0.995–1.027 | 0.176 | 0.903 | 0.797–1.022 | 0.108 |
| dNLR | 1.125 | 1.053–1.202 | <0.001 *** | 1.211 | 1.012–1.449 | 0.036 * |
| SII | 1.000 | 1.000–1.000 | 0.230 | 1.000 | 1.000–1.000 | 0.289 |
| PNR | 0.970 | 0.950–0.991 | 0.004 ** | 0.985 | 0.961–1.010 | 0.234 |
| hs-CRP | 1.087 | 1.048–1.128 | <0.001 *** | 1.064 | 1.017–1.112 | 0.007 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kounatidis, D.; Jahaj, E.; Geladari, E.V.; Papachristodoulou, K.; Panagopoulos, F.; Marakomichelakis, G.; Papastamopoulos, V.; Sevastianos, V.; Vallianou, N.G. Clostridioides difficile Infections: Epidemiological and Laboratory Data from the Internal Medicine Departments of a Tertiary Care Hospital in Athens, Greece, During the Past Decade. Medicina 2025, 61, 1416. https://doi.org/10.3390/medicina61081416
Kounatidis D, Jahaj E, Geladari EV, Papachristodoulou K, Panagopoulos F, Marakomichelakis G, Papastamopoulos V, Sevastianos V, Vallianou NG. Clostridioides difficile Infections: Epidemiological and Laboratory Data from the Internal Medicine Departments of a Tertiary Care Hospital in Athens, Greece, During the Past Decade. Medicina. 2025; 61(8):1416. https://doi.org/10.3390/medicina61081416
Chicago/Turabian StyleKounatidis, Dimitris, Edison Jahaj, Eleni V. Geladari, Kyriaki Papachristodoulou, Fotis Panagopoulos, Georgios Marakomichelakis, Vasileios Papastamopoulos, Vasilios Sevastianos, and Natalia G. Vallianou. 2025. "Clostridioides difficile Infections: Epidemiological and Laboratory Data from the Internal Medicine Departments of a Tertiary Care Hospital in Athens, Greece, During the Past Decade" Medicina 61, no. 8: 1416. https://doi.org/10.3390/medicina61081416
APA StyleKounatidis, D., Jahaj, E., Geladari, E. V., Papachristodoulou, K., Panagopoulos, F., Marakomichelakis, G., Papastamopoulos, V., Sevastianos, V., & Vallianou, N. G. (2025). Clostridioides difficile Infections: Epidemiological and Laboratory Data from the Internal Medicine Departments of a Tertiary Care Hospital in Athens, Greece, During the Past Decade. Medicina, 61(8), 1416. https://doi.org/10.3390/medicina61081416









