Dual-Task Training Interventions for Cerebral Palsy: A Systematic Review and Meta-Analysis of Effects on Postural Balance and Walking Speed
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Literature Search and Bibliographical Sources
2.3. Study Selection: Inclusion and Exclusion Criteria
2.4. Data Extraction
2.5. Variables
2.6. Assessment of the Methodological Quality, Risk of Bias, and Quality of Evidence
2.7. Statistical Analysis
3. Results
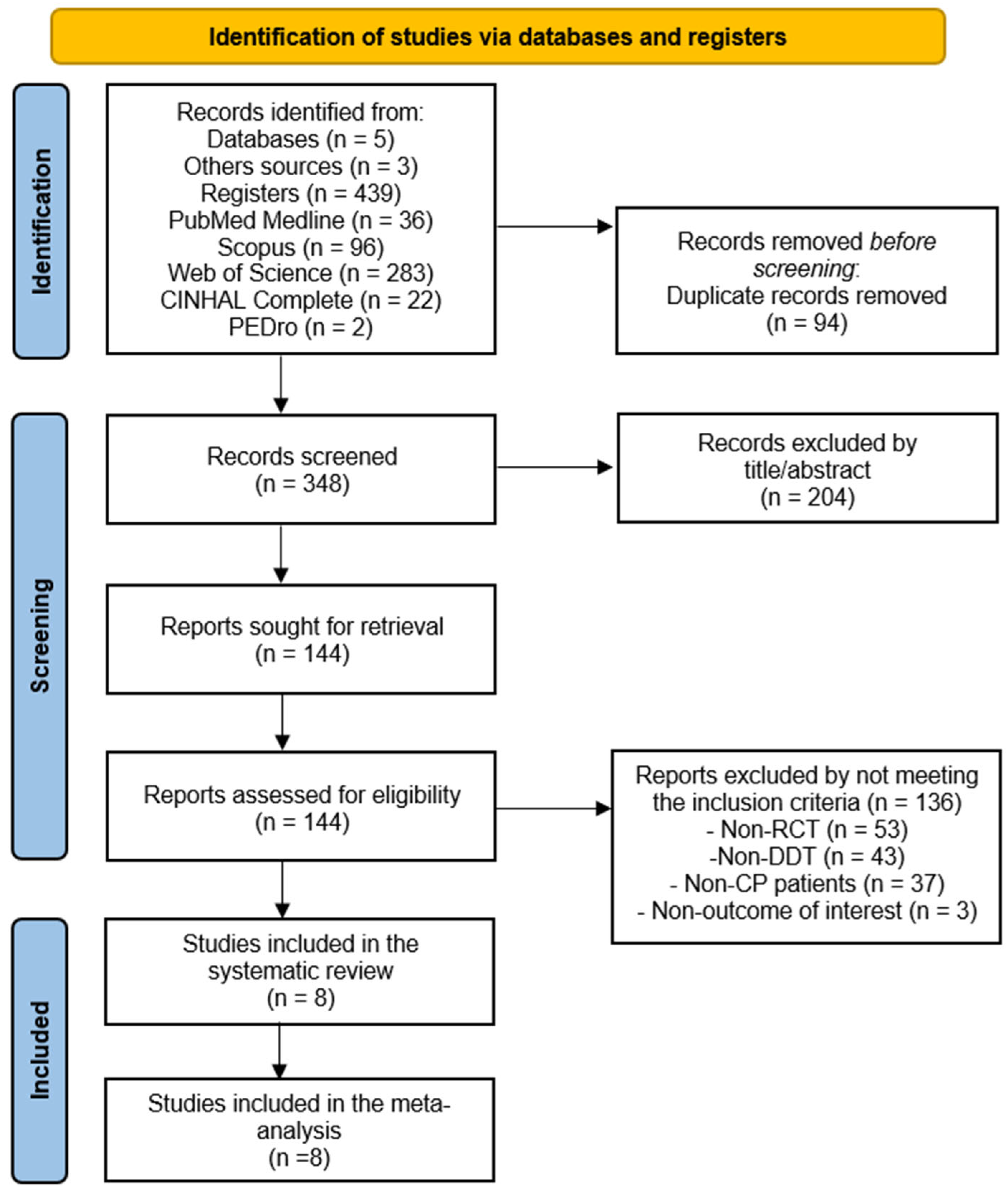
3.1. Study Selection
3.2. Characteristics of the Studies Included in the Review
3.3. Methodological Quality and Risk of Bias of the Studies in the Review
3.4. Meta-Analyses
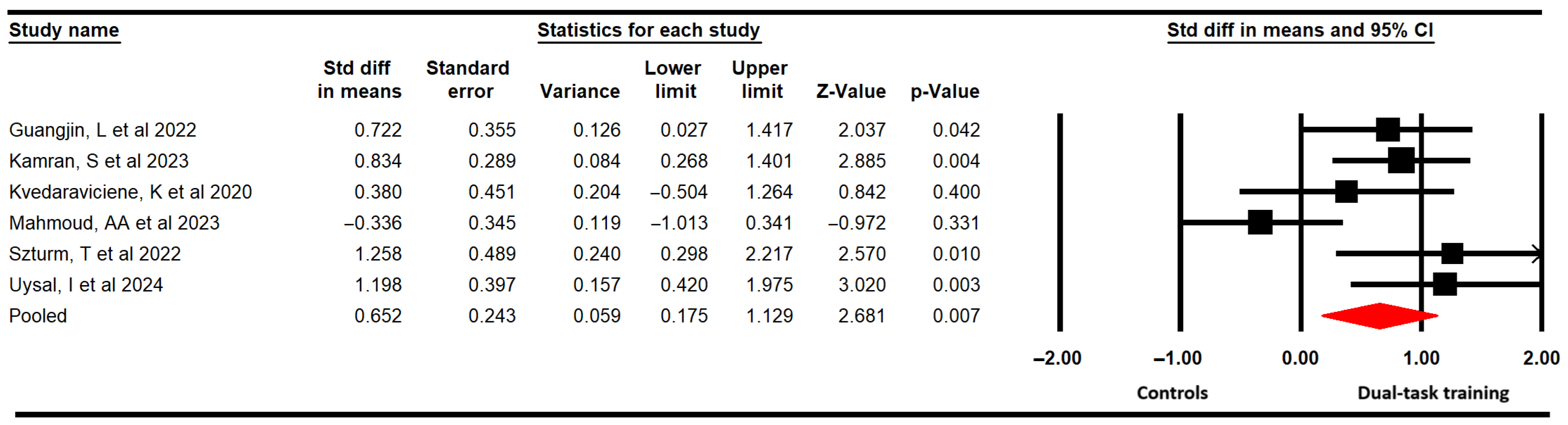
3.4.1. Functional Balance
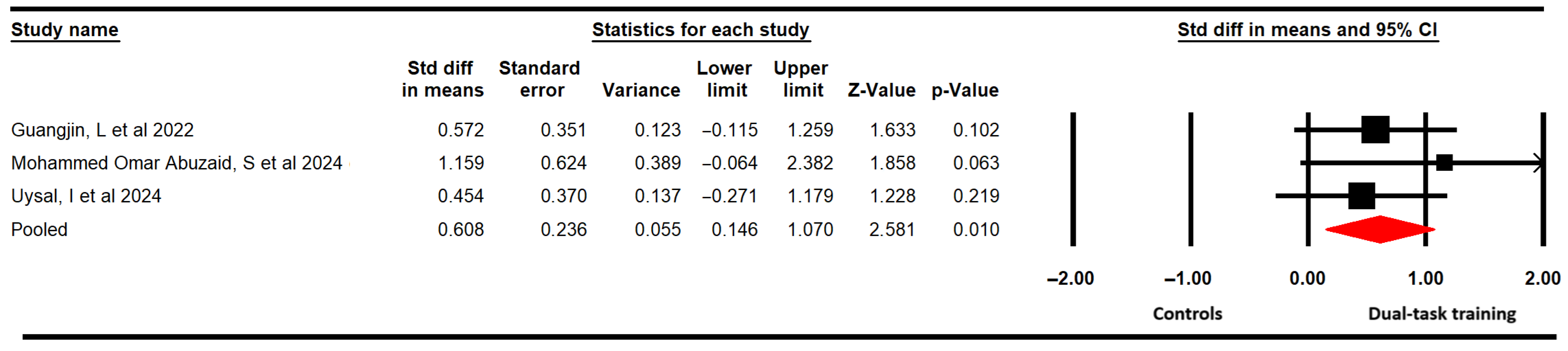
3.4.2. Dynamic Balance
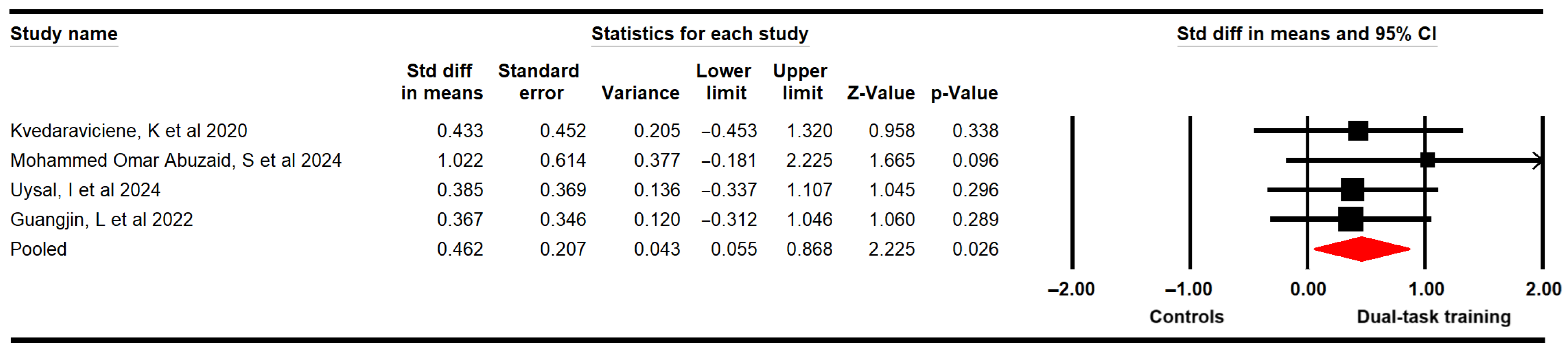
3.4.3. Static Balance
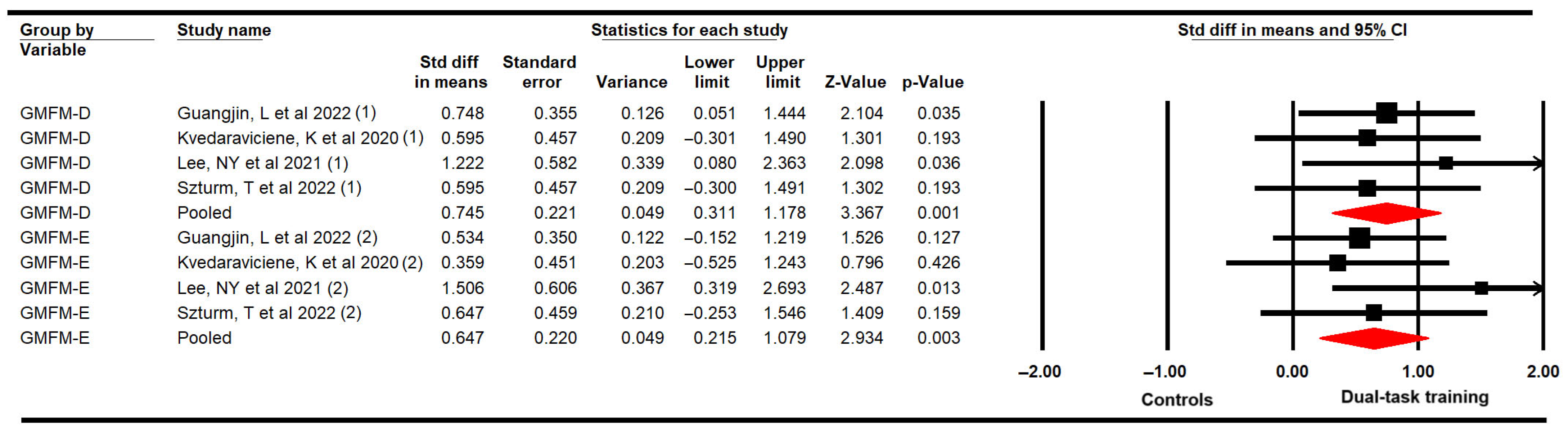
3.4.4. Gross Motor Function Related to Standing Balance and Walking, Running, and Jumping Abilities
3.4.5. Walking Speed
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CP | Cerebral palsy |
| DTT | Dual-task training |
| SRMA | Systematic review and meta-analysis |
| RCTs | Randomized controlled trials |
| SMD | Standardized mean difference |
| 95% CI | 95% confidence interval |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-analyses |
| PEDro | Physiotherapy Evidence Database |
| TUG | Timed Up and Go Test |
| PBS | Pediatric Balance Scale |
| GMFM | Gross Motor Function Measure |
References
- Rosenbaum, P.; Paneth, N.; Leviton, A.; Goldstein, M.; Bax, M. A report: The definition and classification of cerebral palsy April 2006. Dev. Med. Child Neurol. 2007, 49, 8–14. [Google Scholar] [CrossRef]
- Carr, L.J. Definition and classification of cerebral palsy. Dev. Med. Child Neurol. 2007, 47, 508. [Google Scholar] [CrossRef]
- Olusanya, B.O.; Smythe, T.; Ogbo, F.A.; Nair, M.K.C.; Scher, M.; Davis, A.C. Global prevalence of developmental disabilities in children and adolescents: A systematic umbrella review. Front. Public Health 2023, 11, 1122009. [Google Scholar] [CrossRef]
- McIntyre, S.; Goldsmith, S.; Webb, A.; Ehlinger, V.; Hollung, S.J.; McConnell, K.; Arnaud, C.; Smithers-Sheedy, H.; Oskoui, M.; Khandaker, G.; et al. Global prevalence of cerebral palsy: A systematic analysis. Dev. Med. Child Neurol. 2022, 64, 1494–1506. [Google Scholar] [CrossRef]
- Russo, R.N.; Skuza, P.P.; Sandelance, M.; Flett, P. Upper limb impairments, process skills, and outcome in children with unilateral cerebral palsy. Dev. Med. Child Neurol. 2019, 61, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Avery, A.; Bailey, R.; Bell, B.; Coulson, N.; Luke, R.; McLaughlin, J.; Logan, P. The everydayness of falling: Consequences and management for adults with cerebral palsy across the life course. Disabil. Rehabil. 2024, 47, 1534–1542. [Google Scholar] [CrossRef] [PubMed]
- Graham, H.K.; Rosenbaum, P.; Paneth, N.; Dan, B.; Lin, J.-P.; Damiano, D.L.; Becher, J.G.; Gaebler-Spira, D.; Colver, A.; Reddihough, D.S.; et al. Cerebral palsy. Nat. Rev. Dis. Primers 2016, 2, 15082. [Google Scholar] [CrossRef]
- Adıguzel, H.; Elbasan, B. Effects of modified pilates on trunk, postural control, gait and balance in children with cerebral palsy: A single-blinded randomized controlled study. Acta Neurol. Belg. 2022, 122, 903–914. [Google Scholar] [CrossRef]
- Erkek, S.; Çekmece, Ç. Investigation of the Relationship between Sensory-Processing Skills and Motor Functions in Children with Cerebral Palsy. Children 2023, 10, 1723. [Google Scholar] [CrossRef]
- Spomer, A.M.; Conner, B.C.; Schwartz, M.H.; Lerner, Z.F.; Steele, K.M. Audiovisual biofeedback amplifies plantarflexor adaptation during walking among children with cerebral palsy. J. Neuroeng. Rehabil. 2023, 20, 164. [Google Scholar] [CrossRef] [PubMed]
- Dimakopoulos, R.; Syrogiannopoulos, G.; Grivea, I.; Dailiana, Z.; Youroukos, S.; Spinou, A. Kinematic and Temporospatial Changes in Children with Cerebral Palsy during the Initial Stages of Gait Development. Dev. Neurorehabil. 2022, 25, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Jackman, M.; Sakzewski, L.; Morgan, C.; Boyd, R.N.; Brennan, S.E.; Langdon, K.; Toovey, R.A.M.; Greaves, S.; Thorley, M.; Novak, I. Interventions to improve physical function for children and young people with cerebral palsy: International clinical practice guideline. Dev. Med. Child Neurol. 2022, 64, 536–549. [Google Scholar] [CrossRef] [PubMed]
- Carcreff, L.; Gerber, C.N.; Paraschiv-Ionescu, A.; De Coulon, G.; Aminian, K.; Newman, C.J.; Armand, S. Walking Speed of Children and Adolescents with Cerebral Palsy: Laboratory Versus Daily Life. Front. Bioeng. Biotechnol. 2020, 8, 812. [Google Scholar] [CrossRef]
- Araneda, R.; Ebner-Karestinos, D.; Paradis, J.; Klöcker, A.; Saussez, G.; Demas, J.; Bailly, R.; Bouvier, S.; de Tournai, A.C.; Herman, E.; et al. Changes Induced by Early Hand-Arm Bimanual Intensive Therapy Including Lower Extremities in Young Children with Unilateral Cerebral Palsy. JAMA Pediatr. 2024, 178, 19. [Google Scholar] [CrossRef]
- McCoy, S.W.; Palisano, R.; Avery, L.; Jeffries, L.; Fiss, A.L.; Chiarello, L.; Hanna, S. Physical, occupational, and speech therapy for children with cerebral palsy. Dev. Med. Child Neurol. 2020, 62, 140–146. [Google Scholar] [CrossRef]
- Kachmar, O.O.; Kozyavkina, N.V.; Kushnir, A.D.; Kozyavkina, O.V. Neuroplasticity in rehabilitation of children with cerebral palsy. Int. Neurol. J. 2025, 21, 52–59. [Google Scholar] [CrossRef]
- Okur, E.O.; Arik, M.I.; Okur, I.; Gokpinar, H.H.; Gunel, M.K. Dual-task training effect on gait parameters in children with spastic diplegic cerebral palsy: Preliminary results of a self-controlled study. Gait Posture 2022, 94, 45–50. [Google Scholar] [CrossRef]
- Oliva, H.N.P.; Machado, F.S.M.; Rodrigues, V.D.; Leão, L.L.; Monteiro-Júnior, R.S. The effect of dual-task training on cognition of people with different clinical conditions: An overview of systematic reviews. IBRO Rep. 2020, 9, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Yang, H.; Zhou, Q.; Pan, H. Effects of cognitive motor dual-task training on stroke patients: A RCT-based meta-analysis. J. Clin. Neurosci. 2021, 92, 175–182. [Google Scholar] [CrossRef]
- Freitag, F.; Brucki, S.M.D.; Barbosa, A.F.; Chen, J.; Souza, C.d.O.; Valente, D.F.; Chien, H.F.; Bedeschi, C.; Voos, M.C. Is virtual reality beneficial for dual-task gait training in patients with Parkinson’s disease? A systematic review. Dement. Neuropsychol. 2019, 13, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Plummer-D’aMato, P.; Altmann, L.J.; Saracino, D.; Fox, E.; Behrman, A.L.; Marsiske, M. Interactions between cognitive tasks and gait after stroke: A dual task study. Gait Posture 2008, 27, 683–688. [Google Scholar] [CrossRef]
- Leone, C.; Feys, P.; Moumdjian, L.; D’aMico, E.; Zappia, M.; Patti, F. Cognitive-motor dual-task interference: A systematic review of neural correlates. Neurosci. Biobehav. Rev. 2017, 75, 348–360. [Google Scholar] [CrossRef]
- Lee, Y.; Jung, J.; Kim, H.; Lee, S. Comparison of the Influence of Dual-Task Activities on Prefrontal Activation and Gait Variables in Older Adults with Mild Cognitive Impairment during Straight and Curved Walking. Medicina (B Aires) 2024, 60, 235. [Google Scholar] [CrossRef]
- Cogdell-Brooke, L.S.; Sowden, P.T.; Violante, I.R.; Thompson, H.E. A meta-analysis of functional magnetic resonance imaging studies of divergent thinking using activation likelihood estimation. Hum. Brain Mapp. 2020, 41, 5057–5077. [Google Scholar] [CrossRef]
- Mark, H. Neuroplasticity and rehabilitation. J. Rehabil. Res. Dev. 2005, 42, 17–22. [Google Scholar]
- Kachmar, O.; Mysula, I.; Kushnir, A.; Voloshyn, T.; Matiushenko, O.; Hasiuk, M.; Hordiyevych, M. Changes in motor functions in children with cerebral palsy after the course of intensive neurophysiological rehabilitation: A single-blind study. Int. Neurol. J. 2019, 5–11. [Google Scholar] [CrossRef]
- Van Criekinge, T.; Sahu, U.; Bhatt, T. Effect of Explicit Prioritization on Dual Tasks During Standing and Walking in People with Neurologic and Neurocognitive Disorders: A Systematic Review and Meta-analysis. Arch. Phys. Med. Rehabil. 2024, 105, 2166–2183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ma, J.; Liu, X.; Jin, A.; Wang, K.; Yin, X. Cognitive-motor dual-task training on gait and balance in stroke patients: Meta-analytic report and trial sequential analysis of randomized clinical trials. J. Neuroeng. Rehabil. 2024, 21, 227. [Google Scholar] [CrossRef]
- García-López, H.; Castillo-Pintor, M.d.L.Á.; Castro-Sánchez, A.M.; Lara-Palomo, I.C.; Obrero-Gaitán, E.; Cortés-Pérez, I. Efficacy of Dual-Task Training in Patients with Parkinson’s Disease: A Systematic Review with Meta-Analysis. Mov. Disord. Clin. Pract. 2023, 10, 1268–1284. [Google Scholar] [CrossRef]
- Roostaei, M.; Raji, P.; Morone, G.; Razi, B.; Khademi-Kalantari, K. The effect of dual-task conditions on gait and balance performance in children with cerebral palsy: A systematic review and meta-analysis of observational studies. J. Bodyw. Mov. Ther. 2021, 26, 448–462. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Wiley Blackwell & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef]
- Mehrdad, A.-B.; Ali, J. Population, Intervention, Comparison, Outcomes and Study (PICOS) design as a framework to formulate eligibility criteria in systematic reviews. Emerg. Med. J. 2020, 37, 387. [Google Scholar] [CrossRef] [PubMed]
- Jacob, C. A Coefficient of Agreement for Nominal Scales. Educ. Psychol. Meas. 1960, 20, 37–46. [Google Scholar] [CrossRef]
- Landis, J.; Richard, K.G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159. [Google Scholar] [CrossRef]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef]
- Gonzalez, G.Z.; Moseley, A.M.; Maher, C.G.; Nascimento, D.P.; Costa, L.d.C.M.; Costa, L.O. Methodologic Quality and Statistical Reporting of Physical Therapy Randomized Controlled Trials Relevant to Musculoskeletal Conditions. Arch. Phys. Med. Rehabil. 2018, 99, 129–136. [Google Scholar] [CrossRef]
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004, 328, 1490. [Google Scholar] [CrossRef]
- Meader, N.; King, K.; Llewellyn, A.; Norman, G.; Brown, J.; Rodgers, M.; Moe-Byrne, T.; Higgins, J.P.; Sowden, A.; Stewart, G. A checklist designed to aid consistency and reproducibility of GRADE assessments: Development and pilot validation. Syst. Rev. 2014, 3, 82. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Hedges, L.; Higgins, J.; Rothstein, H. Comprehensive Meta-Analysis Software, version 4; BioStat: Englewood, NJ, USA, 2023.
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: New York, NY, USA, 1977. [Google Scholar]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials. 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Kinney, A.R.; Eakman, A.M.; Graham, J.E. Novel Effect Size Interpretation Guidelines and an Evaluation of Statistical Power in Rehabilitation Research. Arch. Phys. Med. Rehabil. 2020, 101, 2219–2226. [Google Scholar] [CrossRef]
- Gerta, R.; Guido, S. Beyond the forest plot: The drapery plot. Res. Synth. Methods 2021, 12, 13–19. [Google Scholar] [CrossRef]
- Jaeschke, R.; Singer, J.; Guyatt, G.H. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin. Trials. 1989, 10, 407–415. [Google Scholar] [CrossRef]
- Man-Son-Hing, M.; Laupacis, A.; O’rOurke, K.; Molnar, F.J.; Mahon, J.; Chan, K.B.Y.; Wells, G. Determination of the clinical importance of study results. J. Gen. Intern. Med. 2002, 17, 469–476. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Egger, M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J. Clin. Epidemiol. 2001, 54, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. Br. Med. J. 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef]
- Shi, L.; Lin, L.; Stefano, O. The trim-and-fill method for publication bias: Practical guidelines and recommendations based on a large database of meta-analyses. Medicine 2019, 98, e15987. [Google Scholar] [CrossRef]
- Rothman, K.J.; Greenland, S.; Lash, T.L. Modern Epidemiology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008. [Google Scholar]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Julian, H.; Simon, T.; Douglas, A. Statistical heterogeneity in systematic reviews of clinical trials: A critical appraisal of guidelines and practice. J. Health Serv. Res. Policy 2002, 7, 51–61. [Google Scholar]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Amira, A.M.; Hanna, S.; Kamal, E.S.; Eltalawy, H.A. Effectiveness of Vestibular Versus Dual-Task Training on Balance in Children with Diplegic Cerebral Palsy. Med. J. Cairo Univ. 2023, 91, 1013–1019. [Google Scholar]
- Kamran, S.; Saghir, M.; Maqbool, S.; Amjad, A.; Thomas, J.; Meerab. Comparative effects of motor and cognitive dual task gait training on Balance, Spasticity and quality of life in patients with Diplegic Cerebral Palsy. Al-Qantara 2023, 9, 103–116. [Google Scholar]
- Kvedaravičienė, K.; Solianik, R. Effect of Dual Task Training on Balance and Functional Mobility in Children with Cerebral Palsy. Reabil. Moksl. Slauga Kineziter. Ergoter. 2020, 1, 45–53. [Google Scholar] [CrossRef]
- Lee, N.-Y.; Lee, E.-J.; Kwon, H.-Y. The effects of dual-task training on balance and gross motor function in children with spastic diplegia. J. Exerc. Rehabil. 2021, 17, 21–27. [Google Scholar] [CrossRef]
- Abuzaid, S.M.O. Effects of motor and cognitive dual tasks on walking and balance in children with diparetic cerebral palsy. Appl. Neuropsychol. Child 2024, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Szturm, T.; Parmar, S.T.; Mehta, K.; Shetty, D.R.; Kanitkar, A.; Eskicioglu, R.; Gaonkar, N. Game-Based Dual-Task Exercise Program for Children with Cerebral Palsy: Blending Balance, Visuomotor and Cognitive Training: Feasibility Randomized Control Trial. Sensors 2022, 22, 761. [Google Scholar] [CrossRef] [PubMed]
- Uysal, I.; Özden, F.; Tümtürk, I.; İmErci, A. The effectiveness of dual task exercise training on balance, mobility, physical performance, and quality of life in children with cerebral palsy: A single-blind randomized controlled trial. Ir. J. Med. Sci. 2024, 193, 813–821. [Google Scholar] [CrossRef]
- Luo, G.; Yu, X.; Sun, L.; Yuan, A. Effects of dual-task treadmill training on motor function of children with spastic hemiplegic cerebral palsy. Chin. J. Appl. Clin. Pediatr. 2022, 37, 1167–1171. [Google Scholar]
- Chen, C.-L.; Shen, I.-H.; Chen, C.-Y.; Wu, C.-Y.; Liu, W.-Y.; Chung, C.-Y. Validity, responsiveness, minimal detectable change, and minimal clinically important change of Pediatric Balance Scale in children with cerebral palsy. Res. Dev. Disabil. 2013, 34, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Yogev-Seligmann, G.; Hausdorff, J.M.; Giladi, N. Do we always prioritize balance when walking? Towards an integrated model of task prioritization. Mov. Disord. 2012, 27, 765–770. [Google Scholar] [CrossRef]
- Hassani, S.; Krzak, J.J.; Johnson, B.; Flanagan, A.; Gorton, G.; Bagley, A.; Õunpuu, S.; Romness, M.; Tylkowski, C.; Oeffinger, D. One-Minute Walk and modified Timed Up and Go tests in children with cerebral palsy: Performance and minimum clinically important differences. Dev. Med. Child Neurol. 2014, 56, 482–489. [Google Scholar] [CrossRef]
- Cinnera, A.M.; Bisirri, A.; Leone, E.; Morone, G.; Gaeta, A. Effect of dual-task training on balance in patients with multiple sclerosis: A systematic review and meta-analysis. Clin. Rehabil. 2021, 35, 1399–1412. [Google Scholar] [CrossRef]
- Storm, F.A.; Petrarca, M.; Beretta, E.; Strazzer, S.; Piccinini, L.; Maghini, C.; Panzeri, D.; Corbetta, C.; Morganti, R.; Reni, G.; et al. Minimum Clinically Important Difference of Gross Motor Function and Gait Endurance in Children with Motor Impairment: A Comparison of Distribution-Based Approaches. Biomed. Res. Int. 2020, 2020, 2794036. [Google Scholar] [CrossRef]
- Moreau, N.G.; Bodkin, A.W.; Bjornson, K.; Hobbs, A.; Soileau, M.; Lahasky, K. Effectiveness of Rehabilitation Interventions to Improve Gait Speed in Children With Cerebral Palsy: Systematic Review and Meta-analysis. Phys. Ther. 2016, 96, 1938–1954. [Google Scholar] [CrossRef]
- Bherer, L.; Kramer, A.F.; Peterson, M.S.; Colcombe, S.; Erickson, K.; Becic, E. Transfer Effects in Task-Set Cost and Dual-Task Cost After Dual-Task Training in Older and Younger Adults: Further Evidence for Cognitive Plasticity in Attentional Control in Late Adulthood. Exp. Aging Res. 2008, 34, 188–219. [Google Scholar] [CrossRef] [PubMed]
- Johansson, H.; Folkerts, A.-K.; Hammarström, I.; Kalbe, E.; Leavy, B. Effects of motor–cognitive training on dual-task performance in people with Parkinson’s disease: A systematic review and meta-analysis. J. Neurol. 2023, 270, 2890–2907. [Google Scholar] [CrossRef]
- Houwink, A.; Aarts, P.B.; Geurts, A.C.; Steenbergen, B. A neurocognitive perspective on developmental disregard in children with hemiplegic cerebral palsy. Res. Dev. Disabil. 2011, 32, 2157–2163. [Google Scholar] [CrossRef]
- Kayabinar, B.; Alemdaroğlu-Gürbüz, I.; Yilmaz, Ö. The effects of virtual reality augmented robot-assisted gait training on dual-task performance and functional measures in chronic stroke: A randomized controlled single-blind trial. Eur. J. Phys. Rehabil. Med. 2021, 57, 227–237. [Google Scholar] [CrossRef] [PubMed]
- McPhee, A.M.; Cheung, T.C.K.; Schmuckler, M.A. Dual-task interference as a function of varying motor and cognitive demands. Front. Psychol. 2022, 13, 952245. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Zhao, Y.; Luo, H.; Xie, L.; Hu, H.; Sun, C. Comparative effectiveness of different dual task mode interventions on cognitive function in older adults with mild cognitive impairment or dementia: A systematic review and network meta-analysis. Aging Clin. Exp. Res. 2025, 37, 139. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liu, N. Effectiveness of cognitive-motor dual task training in preventing falls in community older adults: A meta-analysis and systematic review. Geriatr. Nurs. 2025, 64, 103366. [Google Scholar] [CrossRef]
- Armijo-Olivo, S.; Saltaji, H.; da Costa, B.R.; Fuentes, J.; Ha, C.; Cummings, G.G. What is the influence of randomisation sequence generation and allocation concealment on treatment effects of physical therapy trials? A meta-epidemiological study. BMJ Open 2015, 5, e008562. [Google Scholar] [CrossRef] [PubMed]
- Armijo-Olivo, S.; Fuentes, J.; da Costa, B.R.; Saltaji, H.; Ha, C.; Cummings, G.G. Blinding in Physical Therapy Trials and Its Association with Treatment Effects. Am. J. Phys. Med. Rehabil. 2017, 96, 34–44. [Google Scholar] [CrossRef] [PubMed]

| Study | DTT Group | Control Group | Outcome and Test | Qualitative Findings in Individual Studies | ||||
|---|---|---|---|---|---|---|---|---|
| Sample Characteristics (n, Age, Sex) | Specific Task Comparisons | DTT Intervention Characteristics | Sample Characteristics (n, Age, Sex Ratio) | Control Intervention Characteristics | Intra-Group Differences | Inter-Group Differences | ||
| Guangjin, L et al. 2022 (China) Single-blinded RCT Setting: Quindao Women and Children’s Hospital Funding: Yes. Quindao Medical Science Guidance Program Project (2020-WJZD130) | 18 children Mean age: 4.5 ± 0.4 Sex: 6G:12B GMFCS: I-II | Motor–cognitive dual task vs. motor single task | Walking on a treadmill while performing a series of five distinct motor and cognitive tasks DTT type: Motor and cognitive dual task Application: 20 sessions, for 4 weeks, 5 per week, and 50 min per session | 16 children Mean age: 4.6 ± 0.5 Sex: 6G:10B GMFCS: I-II | Conventional therapy (treadmill training) Control type: motor single-task Application: 20 sessions, for 4 weeks, 5 per week, and 50 min per session | Functional balance (PBS) | Statistically significant improvement in both groups | Non-statistically significant differences between groups (p > 0.05) |
| Dynamic balance (TUG) | Statistically significant improvement in both groups | Statistically significant differences favor DTT groups (p = 0.028) | ||||||
| Walking speed (MSWT) | Statistically significant improvement in both groups | Non-statistically significant differences between groups (p > 0.05) | ||||||
| Gross motor function (GMFM D-E) | Statistically significant improvement in both groups | Non-statistically significant differences between groups (p > 0.05) | ||||||
| Kamran, S et al. 2023 (Pakistan) Single-blinded RCT Setting: Physiotherapy Department of Allama Iqbal Memorial Hospital Funding: NR | 26 children Mean age: 8.6 ± 1.9 Sex: 11B:15B GMFCS: II-III | Motor–cognitive dual-task vs. motor single task | Walking on a treadmill while performing a series of five distinct motor or cognitive tasks (lasting 3 min) DTT type: Motor and cognitive dual task Application: 8 weeks and 15 min per session. Session per week not reported | 26 children Mean age: 8.5 ± 2 Sex: 14B:12B GMFCS: II-III | Conventional therapy (exercises for balance and gait improvement) Control type: Motor single task Application: 8 weeks and 15 min per session. Session per week and minutes NR | Functional balance (PBS) | Statistically significant improvement in both groups | Statistically significant differences favor DTT groups (p = 0.001) |
| Kvedaravičienė, K et al. 2020 (Lithuanian) Single-blinded RCT Setting: Lithuanian Sport University Funding: No | 10 children Mean age: 10.4 ± 1.2 Sex: NR GMFCS: I-II | Motor–motor dual task vs. motor single task | Walking or standing on an unstable surfacer while tasking other motor task DTT type: Motor and motor dual-task Application: 15 sessions, for 3 weeks, 5 per week and 40 min per session | 10 children Mean age: 10.4 ± 1.2 Sex: NR GMFCS: I-II | Conventional therapy (basic physiotherapy approach) Control type: motor single-task Application: 15 sessions, for 3 weeks, 5 per week and 40 min per session | Functional balance (PBS) | Statistically significant improvement in both groups | Statistically significant differences favor DTT groups (p < 0.05) |
| Walking speed (1MWT) | Statistically significant improvement in both groups | Statistically significant differences favor DTT groups (p < 0.05) | ||||||
| Gross motor function (GMFM D-E) | Statistically significant improvement in both groups | NR | ||||||
| Lee, NY et al. 2021 (South Korea) Single-blinded RCT Setting: Pediatric physical therapy center Funding: No | 7 children Mean age: 9.4 ± 2.1 Sex: 3G:4B GMFCS: I-III | Motor–cognitive dual task vs. motor single task | Task performance of controlling balance on an unstable support surface accompanied with other motor task DTT type: Motor and cognitive dual-task Application: 16 sessions, for 8 weeks, 2 per week and 30 min per session | 7 children; Mean age: 9.4 ± 2.3 9.42 ± 2.29 years; Sex: 4G:3B GMFCS: I-III | Neurodevelopmental treatment Control type: motor single-task Application: 16 sessions, for 8 weeks, 2 per week and 30 min per session | Gross motor function (GMFM D-E) | Statistically significant differences favor DTT groups (p < 0.05) | Statistically significant difference favor DTT groups (p < 0.05) |
| Mahmoud, A et al. 2023 (Egypt) Single-blinded RCT Setting: Clinic of faculty of physical therapy Funding: NR | 17 children Mean age: 7.7 ± 2.2 Sex: 7G:10B GMFCS: I-II | Motor–motor dual task vs. motor single task | Walking on balance board while performing a motor task. DTT type: Motor and motor dual task Application: 24 sessions, for 8 weeks, 3 per week and 30 min per session | 17 children Mean age: 7.6 ± 1.7 Sex: 10G:7B GMFCS: I-II | Vestibular training (balance and walking traditional exercises) Control type: Motor single-task Application: 24 sessions, for 8 weeks, 3 per week, and 30 min per session | Functional balance (PBS) | Statistically significant improvement in both groups | Non-statistically significant differences between groups (p = 0.33) |
| Static balance (EO and EC) | Statistically significant improvement in both groups | Non-statistically significant differences between groups (p > 0.05) | ||||||
| Mohammed Omar Abuzaid, S et al. 2024 (Saudi Arabia) Single-blinded RCT Setting: Taiba Educational City Funding: NR | 6 children Mean age: 9.3 ± 1.4 Sex: NR GMFCS: I-II | Motor–cognitive dual task vs. no task | A dual-task paradigm involving a motor task and a cognitive task requiring participants to name animals. DTT type: Motor and cognitive dual task Application: 16 sessions, for 8 weeks, 2 per week, and 30 min per session | 6 children Mean age: 9.3 ± 1.4 Sex: NR GMFCS: I-II | Usual care (did not receive intervention) Control type: No task | Dynamic balance (TUG) | Statistically significant improvement in both groups | Statistically significant differences favor DTT groups (p = 0.001) |
| Walking speed (10MWT) | Statistically significant improvement in both groups | Statistically significant differences favor DTT groups (p = 0.001) | ||||||
| Szturm, T et al. 2022 (Canada) Single-blinded RCT Setting: Physiotherapy Outpatient Department of SMD College of Medical Sciences Funding: No | 10 children Mean age: 6.3 ± 2.3 Sex: 3G:7M GMFCS: I-III | Motor–cognitive dual task vs. motor single task | A dual-task paradigm involving a motor task requiring balance exercises and a cognitive task utilizing interactive videogames DTT type: Motor and cognitive dual task Application: 36 sessions, for 12 weeks, 3 per week, and 45 min per session | 10 children Mean age: 6.3 ± 2.3 Sex: 3G:7M GMFCS: I-III | Conventional therapy (balance exercise program) Control type: Motor single task Application: 16 sessions, for 12 weeks, 3 per week, and 45 min per session | Functional balance (PBS) | Statistically significant improvement in both groups | Statistically significant differences favor DTT groups (p < 0.05) |
| Static balance (EO and EC) | Statistically significant improvement in both groups | Statistically significant differences favor DTT groups (p = 0.03) | ||||||
| Gross motor function (GMFM D-E) | NR | Non-statistically significant differences between groups (p > 0.05) | ||||||
| Uysal, I et al. 2024 (Turkey) Single-blinded RCT Setting: Private Son Atilim Special Education and Rehabilitation Center. Funding: NR | 15 children Mean age: 9.8 ± 2.6 Sex: 5G:10B GMFCS: I-II | Motor–cognitive dual task vs. motor single task | A dual-task paradigm involving a motor task requiring balance, walking, and training exercises added to a cognitive task DTT type: Motor and cognitive dual task Application: 36 sessions, for 12 weeks, 3 per week, and 30 min per session | 15 children; Mean age: 9.7 ± 2.8 Sex: 5G:10B GMFCS: I-II | Conventional therapy (lower limb physical exercise) Control type: Motor single task Application: 36 sessions, for 12 weeks, 3 per week, and 30 min per session | Functional balance (PBS) | Statistically significant improvement in both groups | Statistically significant differences favor DTT groups (p < 0.001) |
| Dynamic balance (TUG) | Statistically significant differences in DTT group | Statistically significant differences favor DTT groups (p < 0.001) | ||||||
| Walking speed (3-MBWT) | Statistically significant improvement in both groups | Statistically significant differences favor DTT groups (p < 0.001) | ||||||
| Static balance (EO and EC) | Statistically significant differences in DTT group | Statistically significant differences favor DTT groups (p < 0.001) | ||||||
| Study | PEDro Items | Total | Quality | Biases | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| i1 | i2 | i3 | i4 | i5 | i6 | i7 | i8 | i9 | i10 | i11 | ||||
| Guangjin, L et al. 2022 | Y | Y | Y | Y | N | N | Y | Y | N | Y | Y | 7/10 | Good | Performance |
| Kamran, S et al. 2023 | Y | Y | Y | Y | N | N | Y | Y | N | Y | Y | 7/10 | Good | Performance |
| Kvedaravičienė, K et al. 2020 | Y | Y | N | Y | N | N | Y | Y | N | Y | Y | 6/10 | Good | Selection and performance |
| Lee, NY et al. 2021 | Y | Y | N | Y | N | N | Y | Y | N | Y | Y | 6/10 | Good | Selection and performance |
| Mohammed Omar Abuzaid, S et al. 2024 | Y | Y | Y | Y | N | N | Y | Y | N | Y | Y | 7/10 | Good | Performance |
| Mahmoud, A et al. 2023 | Y | Y | Y | Y | N | N | Y | Y | N | Y | Y | 7/10 | Good | Performance |
| Szturm, T et al. 2022 * | Y | Y | Y | Y | N | N | Y | Y | Y | Y | Y | 8/10 | Good | Performance |
| Uysal, I et al. 2024 * | N | Y | N | Y | N | N | Y | Y | Y | Y | Y | 7/10 | Good | Selection and performance |
| Variable | Findings Summary | Quality Evidence (GRADE) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect Size | Heterogeneity | Publication Bias | ||||||||||||||
| K | N | Ns | SMD [95% CI] | p | Q (df) | I2 (p) | Egger p | Trim and Fill | Risk of Bias | Inc | Ind | Imp | Pub Bias | Evidence Strength | ||
| Adj SMD | % var | |||||||||||||||
| Functional balance | 6 | 190 | 31.7 | 0.65 [0.18 to 0.13] | 0.007 | 4.92 (5) | 0% (0.43) | 0.71 | 0.65 | 0% | Medium | No | No | Yes | No | Low |
| Dynamic balance | 3 | 76 | 25.3 | 0.61 [0.15 to 1.1] | 0.01 | 0.96 (2) | 0% (0.62) | 0.31 | 0.61 | 0% | Medium | No | No | Yes | No | Low |
| Static balance EC | 3 | 84 | 28 | 0.46 [0.02 to 0.9] | 0.039 | 5 (2) | 50% (0.08) | 0.43 | 0.46 | 0% | Medium | Yes | No | Yes | No | Very low |
| Static balance EO | 3 | 84 | 28 | 0.42 [−0.03 to 0.87] | 0.069 | 12.4 (2) | 73.8% (<0.01) | 0.32 | 0.42 | 0% | Medium | Yes | No | Yes | No | Very low |
| GMFM standing | 4 | 88 | 22 | 0.75 [0.31 to 1.18] | 0.001 | 0.89 (3) | 0% (0.83) | 0.23 | 0.75 | 0% | Medium | No | No | Yes | No | Low |
| GMFM walking, running, jumping | 4 | 88 | 22 | 0.65 [0.22 to 1.08] | 0.003 | 2.53 (3) | 0% (0.47) | 0.11 | 0.52 | 14% | Medium | No | No | Yes | Yes | Very low |
| Walking speed | 4 | 96 | 24 | 0.46 [0.06 to 0.87] | 0.026 | 0.96 (3) | 0% (0.81) | 0.03 | 0.52 | 13% | Medium | No | No | Yes | Yes | Very low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortés-Pérez, I.; Castillo-Pintor, M.d.l.Á.; Barrionuevo-Berzosa, R.; Piñar-Lara, M.; Obrero-Gaitán, E.; García-López, H. Dual-Task Training Interventions for Cerebral Palsy: A Systematic Review and Meta-Analysis of Effects on Postural Balance and Walking Speed. Medicina 2025, 61, 1415. https://doi.org/10.3390/medicina61081415
Cortés-Pérez I, Castillo-Pintor MdlÁ, Barrionuevo-Berzosa R, Piñar-Lara M, Obrero-Gaitán E, García-López H. Dual-Task Training Interventions for Cerebral Palsy: A Systematic Review and Meta-Analysis of Effects on Postural Balance and Walking Speed. Medicina. 2025; 61(8):1415. https://doi.org/10.3390/medicina61081415
Chicago/Turabian StyleCortés-Pérez, Irene, María de los Ángeles Castillo-Pintor, Rocío Barrionuevo-Berzosa, Marina Piñar-Lara, Esteban Obrero-Gaitán, and Héctor García-López. 2025. "Dual-Task Training Interventions for Cerebral Palsy: A Systematic Review and Meta-Analysis of Effects on Postural Balance and Walking Speed" Medicina 61, no. 8: 1415. https://doi.org/10.3390/medicina61081415
APA StyleCortés-Pérez, I., Castillo-Pintor, M. d. l. Á., Barrionuevo-Berzosa, R., Piñar-Lara, M., Obrero-Gaitán, E., & García-López, H. (2025). Dual-Task Training Interventions for Cerebral Palsy: A Systematic Review and Meta-Analysis of Effects on Postural Balance and Walking Speed. Medicina, 61(8), 1415. https://doi.org/10.3390/medicina61081415