Anti-Inflammatory and Antiplatelet Interactions on PAF and ADP Pathways of NSAIDs, Analgesic and Antihypertensive Drugs for Cardioprotection—In Vitro Assessment in Human Platelets
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials, Reagents, and Instrumentation
2.2. Assessment of Antioxidant Activity
2.3. Anti-Inflammatory and Antiplatelet Activity Assessment
2.3.1. Sample Preparation
2.3.2. Platelet Aggregation Assays
- PRP Preparation: Centrifugation at 195× g for 18 min at 24 °C. The PRP supernatant was aspirated using a 1000 μL automatic pipette set to 1 mL, avoiding aspiration of the underlying cellular components. Care was taken to avoid contamination with red blood cells (RBCs) and white blood cells (WBCs), which can induce hemolysis and release confounding biomolecules, compromising assay integrity.
- PPP Preparation: Subsequent centrifugation of remaining PRP at 1465× g for 20 min at 24 °C. Only tubes with clear supernatants (no signs of hemolysis) were used for further analysis.
2.3.3. Sample Loading and Instrument Setup
2.3.4. Dose–Response and Inhibition Assays
2.3.5. Data Analysis
2.4. Drug Combination in a 1:1 Ratio
- Superior performance in the PAF pathway, as indicated by significantly lower IC50 values compared to the ADP pathway.
- Clinical relevance, with an emphasis on commonly used drugs such as Paracetamol, despite its initially high IC50.
- Pharmacological diversity, incorporating NSAIDs, antiplatelets, analgesics, β-blockers, and antihypertensives to assess cross-class synergism.
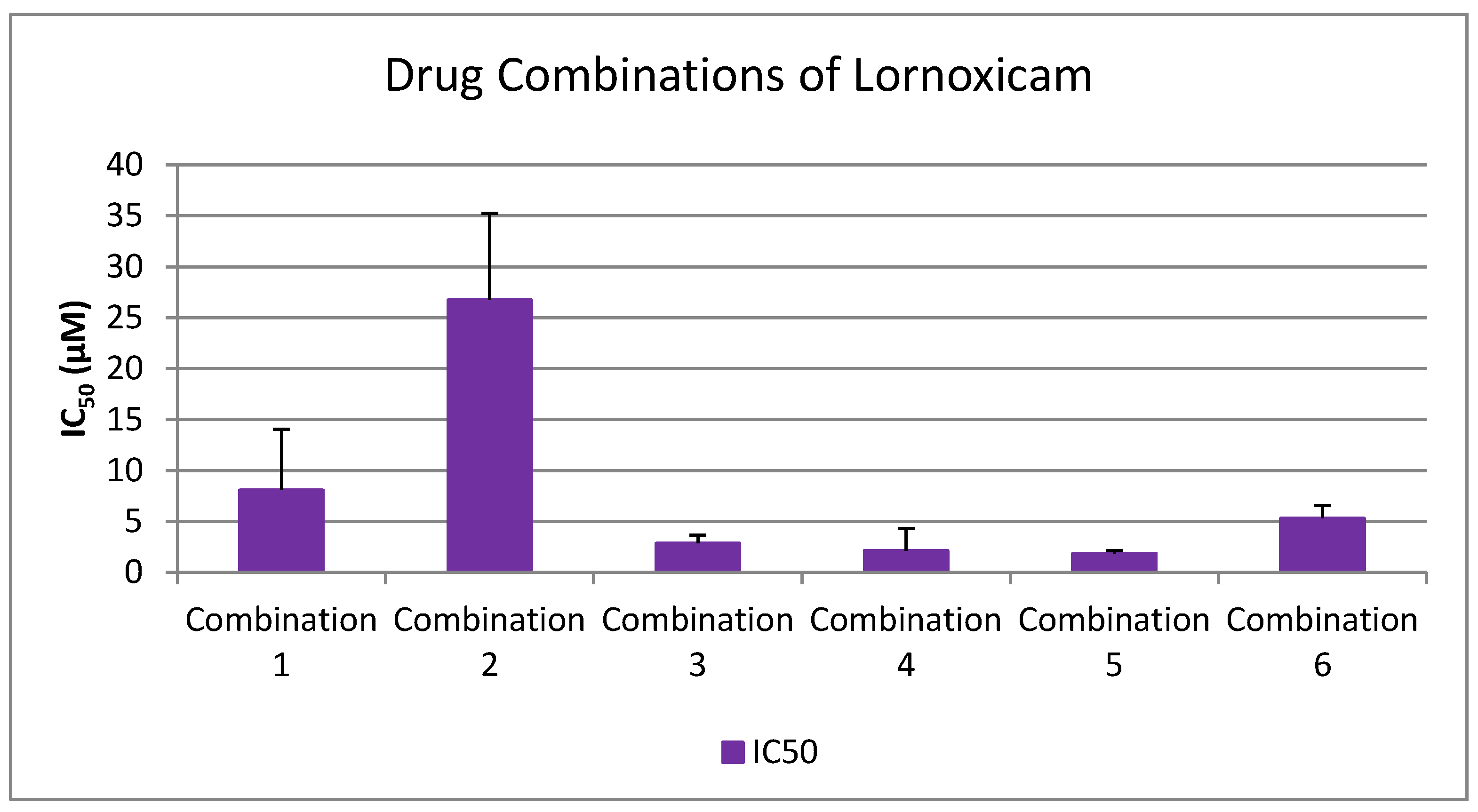
- Lornoxicam + Clopidogrel (1:1)
- Lornoxicam + Paracetamol (1:1)
- Lornoxicam + Clopidogrel + Paracetamol (1:1:1)
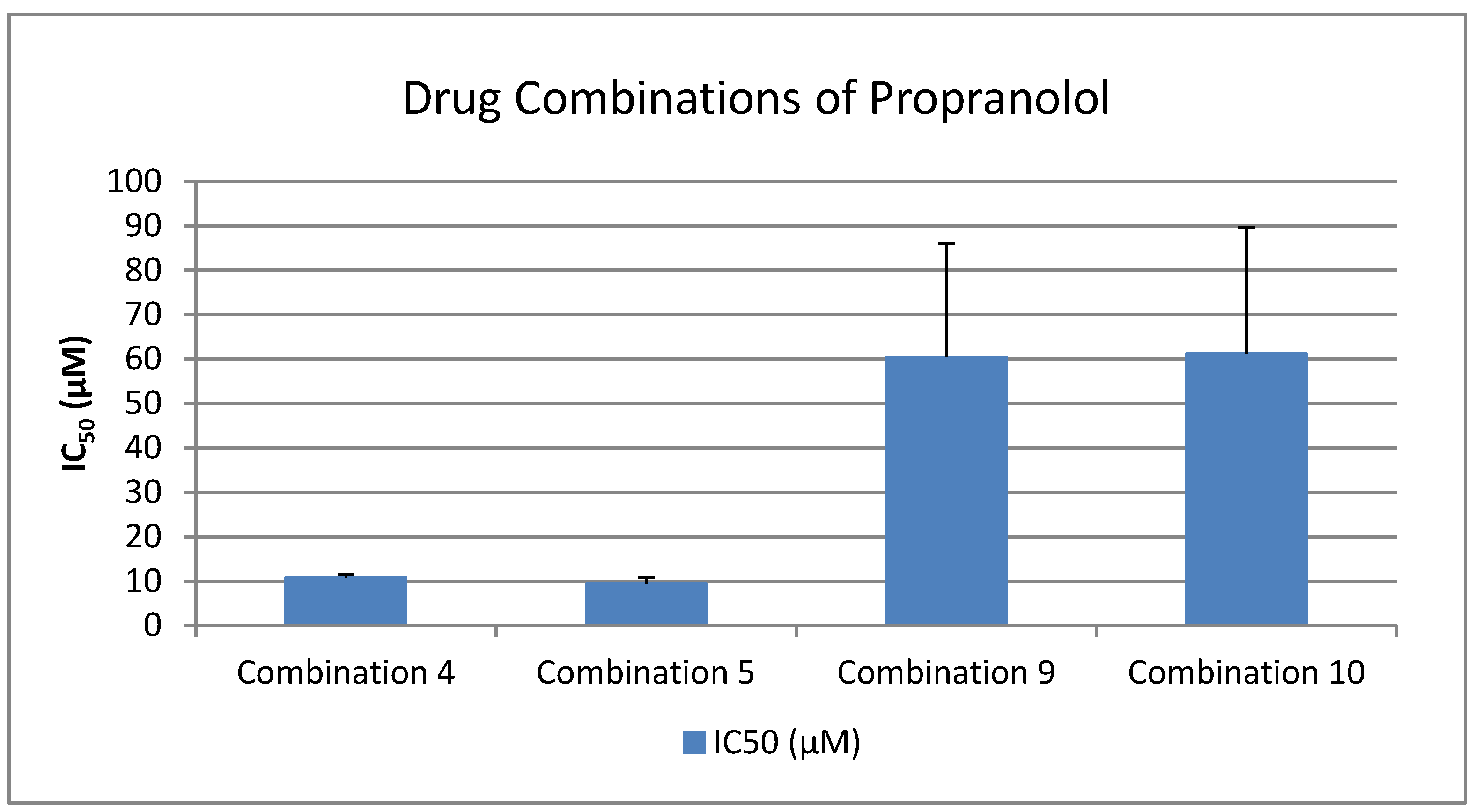
- Lornoxicam + Clopidogrel + Paracetamol + Propranolol + Candesartan (1:1:1:1:1)
- Lornoxicam + Clopidogrel + Paracetamol + Propranolol (1:1:1:1)
- Lornoxicam + Clopidogrel + Paracetamol + Candesartan (1:1:1:1)
- Clopidogrel + Paracetamol (1:1)
- Candesartan + Clopidogrel (1:1)
- Propranolol + Clopidogrel (1:1)
- Propranolol + Clopidogrel + Candesartan (1:1:1)
- Propranolol + Candesartan (1:1)
2.5. Toxicological Assessment of Drug-Induced Platelet Aggregation in PRP
2.6. Desensitization Effects and Intrinsic Platelet Activation
3. Results and Discussion
3.1. Antioxidant Activity Results and Interpretation
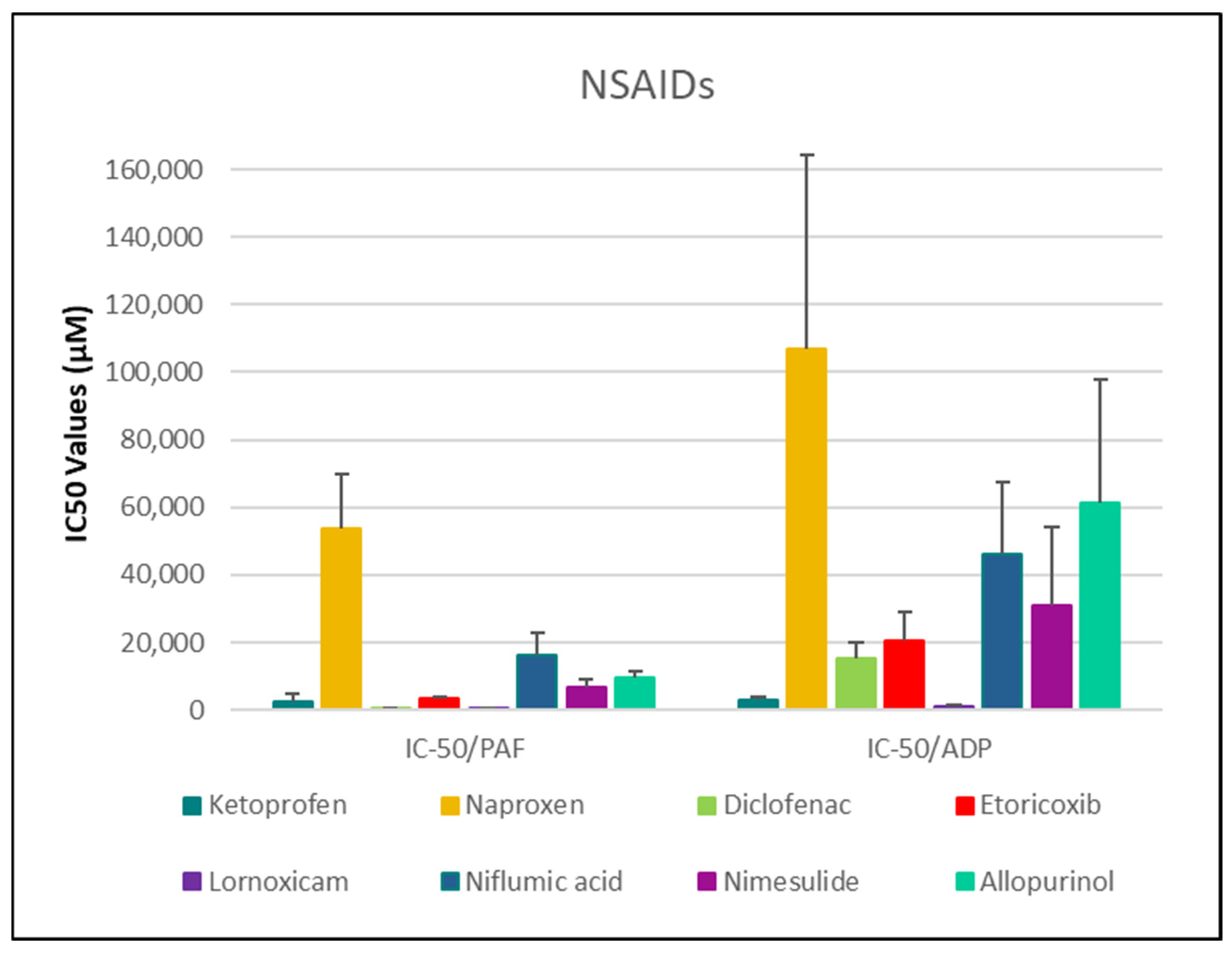
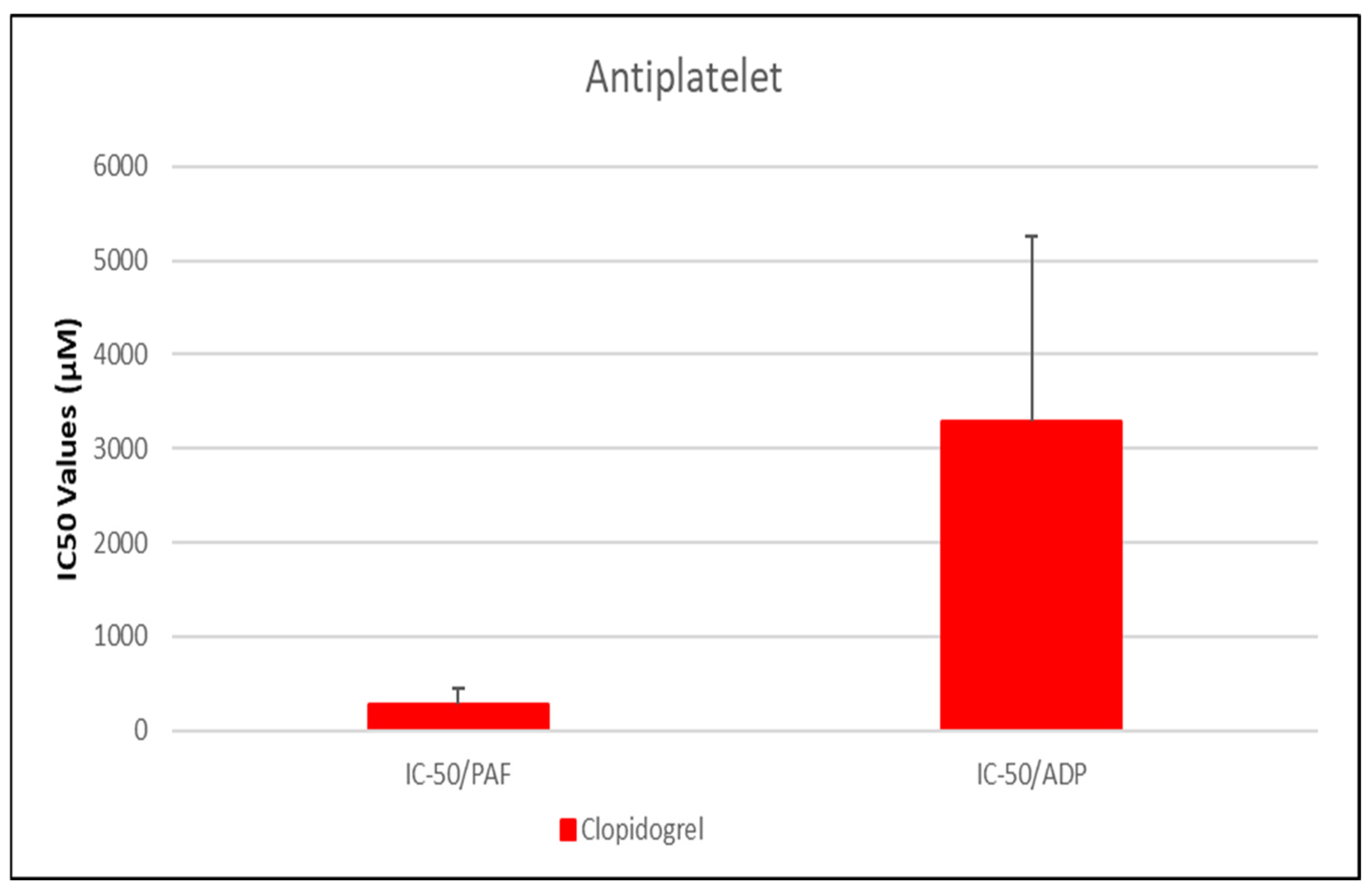
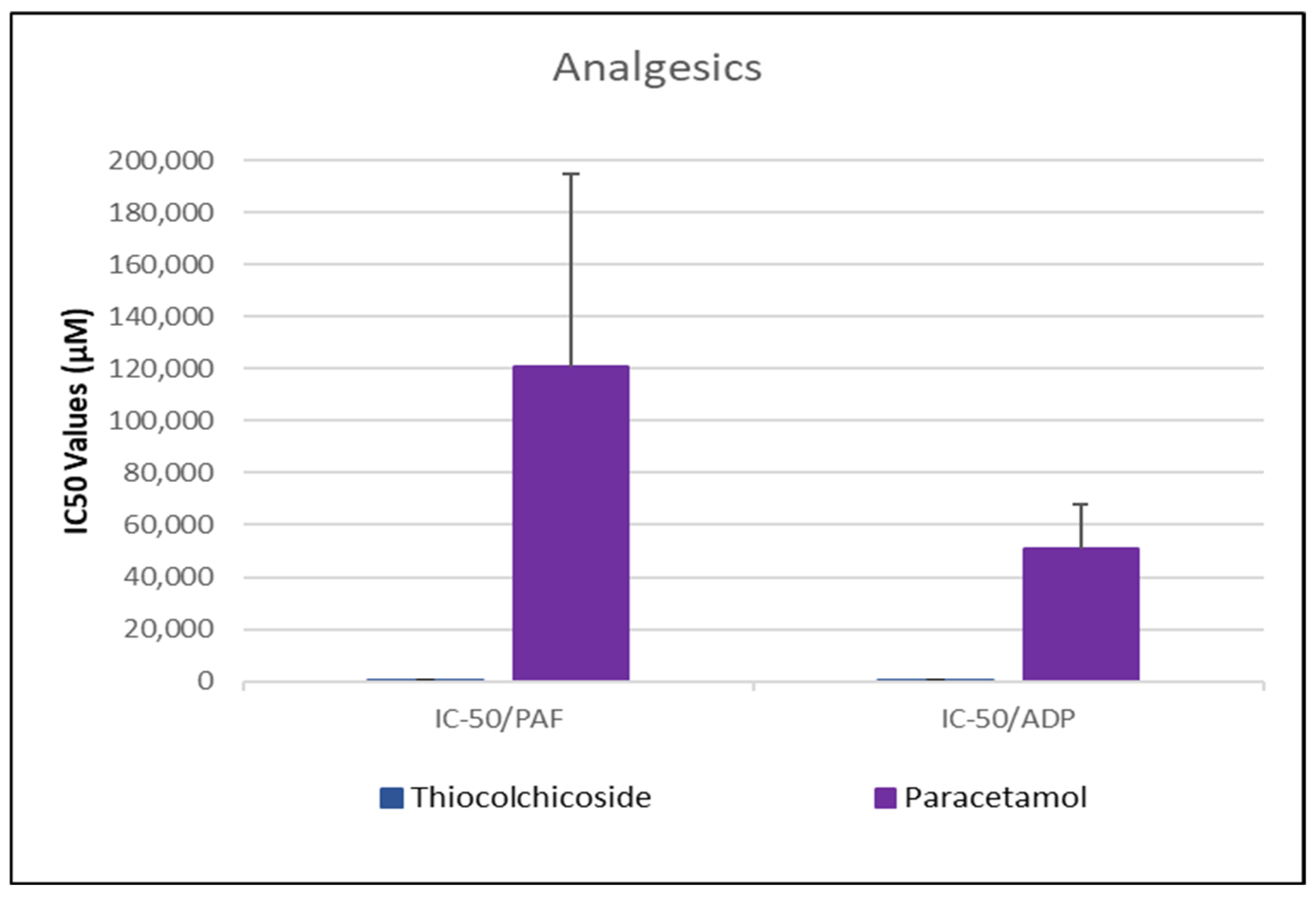
3.2. Anti-Inflammatory and Anti-Platelet Properties of Common Drug Classes from Data Analysis
3.2.1. Data Analysis for ADP and PAF Pathway
| Drug | IC50 PAF (µM) | 95% CI (PAF) | % Aggr. of PAF (Mean ± SD) | IC50 ADP (µM) | 95% CI (ADP) | % Aggr. of ADP (Mean ± SD) |
|---|---|---|---|---|---|---|
| Clopidogrel | 281.01 ± 176.03 * | [113.6–448.4] | 34.3 ± 15.5% | 3291.07 ± 1961.99 | [1684.4–4897.7] | 44.85 ± 19.49% |
| Lornoxicam | 313.99 ± 58.34 * | [252.8–375.2] | 52.92 ± 9.04% | 1276.02 ± 328.66 # | [931.1–1620.9] | 43.49 ± 9.61 |
| Propranolol | 346.66 ± 129.19 * | [233.1–460.2] | 62.36 ± 19.45% | 161.86 ± 90.22 # | [85.7–238.1] | 54.80 ± 25.04% |
| Diclofenac | 531.79 ± 128.61 * | [396.8–666.8] | 55.46 ± 11.02% | 15,326.30 ± 4948.85 | [10,132.8–20,519.8] | 42.06 ± 7.80% |
| Thiocolchicoside | 621.52 ± 201.86 * | [418.3–824.7] | 52.02 ± 9.33% | 405.94 ± 135.83 # | [275.2–536.7] | 49.10 ± 11.09% |
| Ketoprofen | 2595.78 ± 2046.64 | [1131.7–4059.9] | 67.70 ± 11.13% | 3080.21 ± 661.63 | [2385.9–3774.5] | 40.79 ± 18.69% |
| Metoprolol | 2361.72 ± 360.88 | [1995.3–2728.2] | 44.63 ± 15.49% | 1287.08 ± 472.37 # | [906.3–1667.9] | 25.47 ± 6.68% |
| Atenolol | 6284.80 ± 3008.44 | [3721.1–8848.5] | 45.77 ± 19.91% | 5560.11 ± 3082.81 | [2938.1–8182.1] | 35.33 ± 17.31% |
| Etoricoxib | 3376.45 ± 418.77 | [2937.0–3815.9] | 49.89 ± 5.73% | 20,625.69 ± 8629.95 | [11,569.1–29,682.3] | 59.87 ± 11.01% |
| Candesartan | 3907.52 ± 1299.50 | [1382.6–6432.4] | 66.83 ± 9.94% | 3895.13 ± 2045.13 | [1963.6–5826.7] | 58.43 ± 14.94% |
| Nimesulide | 6894.14 ± 2044.11 | [4962.4–8825.9] | 45.50 ± 13.67%5 | 31,092.82 ± 22,876.90 | [11,056.4–51,129.2] | 56.43 ± 17.16% |
| Niflumic acid | 16,240.62 ± 6528.41 | [10,092.6–22,388.6] | 58.39 ± 16.27% | 45,947.12 ± 21,504.17 | [23,983.2–67,911.1] | 43.52 ± 16.78% |
| Sotalol | 9901.80 ± 4750.39 | [5879.6–13,923.9] | 47.57 ± 20.22% | 10,661.67 ± 3755.12 | [7027.8–14,295.5] | 24.90 ± 9.32% |
| Allopurinol | 9634.41 ± 2110.18 | [7187.1–12,081.8] | 47.43 ± 9.71% | 61,368.99 ± 36,384.12 | [22,955.6–99,782.3] | 46.06 ± 14.92% |
| Valsartan | 13,572.57 ± 5513.50 | [4550.9–22,594.2] | 74.40 ± 11.54% | 11,172.65 ± 5076.32 | [3690.6–18,654.7] | 54.16 ± 18.24% |
| Naproxen | 53,914.04 ± 15,901.04 | [39,208.0–68,620.0] | 68.05 ± 6.61% | 106,864.36 ± 57,249.36 | [46,784.8–166,943.9] | 63.18 ± 8.74% |
| Paracetamol | 121,042.32 ± 73,625.26 | [52,537.9–189,546.7] | 61.51 ± 10.50% | 50,720.23 ± 17,357.19 | [34,694.5–66,745.9] | 55.29 ± 15.53% |
- NSAIDs and clopidogrel generally favor the inhibition against the PAF pathway, supporting an anti-PAF specificity and thus a more profound anti-inflammatory efficacy, followed by dual anti-platelet roles.
- Analgesics and β-blockers leaned toward anti-ADP selectivity, suggesting a more antiplatelet antithrombotic—targeted effect.
- Antihypertensives exhibited balanced activity across both pathways, with slight variation depending on the specific compound.
3.2.2. Anti-Inflammatory and Anti-Platelet Properties of Common Drug Classes: Literature Evidence and Experimental Findings
NSAIDs—Diclofenac, Naproxen, Ketoprofen, Lornoxicam, Etoricoxib, Niflumic Acid, Nimesulide, and Allopurinol
- I.
- Diclofenac
- II.
- Naproxen
- III.
- Ketoprofen
- IV.
- Lornoxicam
- V.
- Etoricoxib
- VI.
- Niflumic Acid
- VII.
- Nimesulide
- VIII.
- Allopurinol
- IX.
- Antiplatelet Agent—Clopidogrel as a Positive Control
Analgesics—Thiocolchicoside and Paracetamol
- I.
- Paracetamol
- II.
- Thiocolchicoside
β-blockers—Atenolol, Propranolol, Metoprolol, and Sotalol
- I.
- Atenolol
- II.
- Propranolol
- III.
- Metoprolol
- IV.
- Sotalol
Other Antihypertensives—Candesartan and Valsartan
- I.
- Candesartan
- II.
- Valsartan
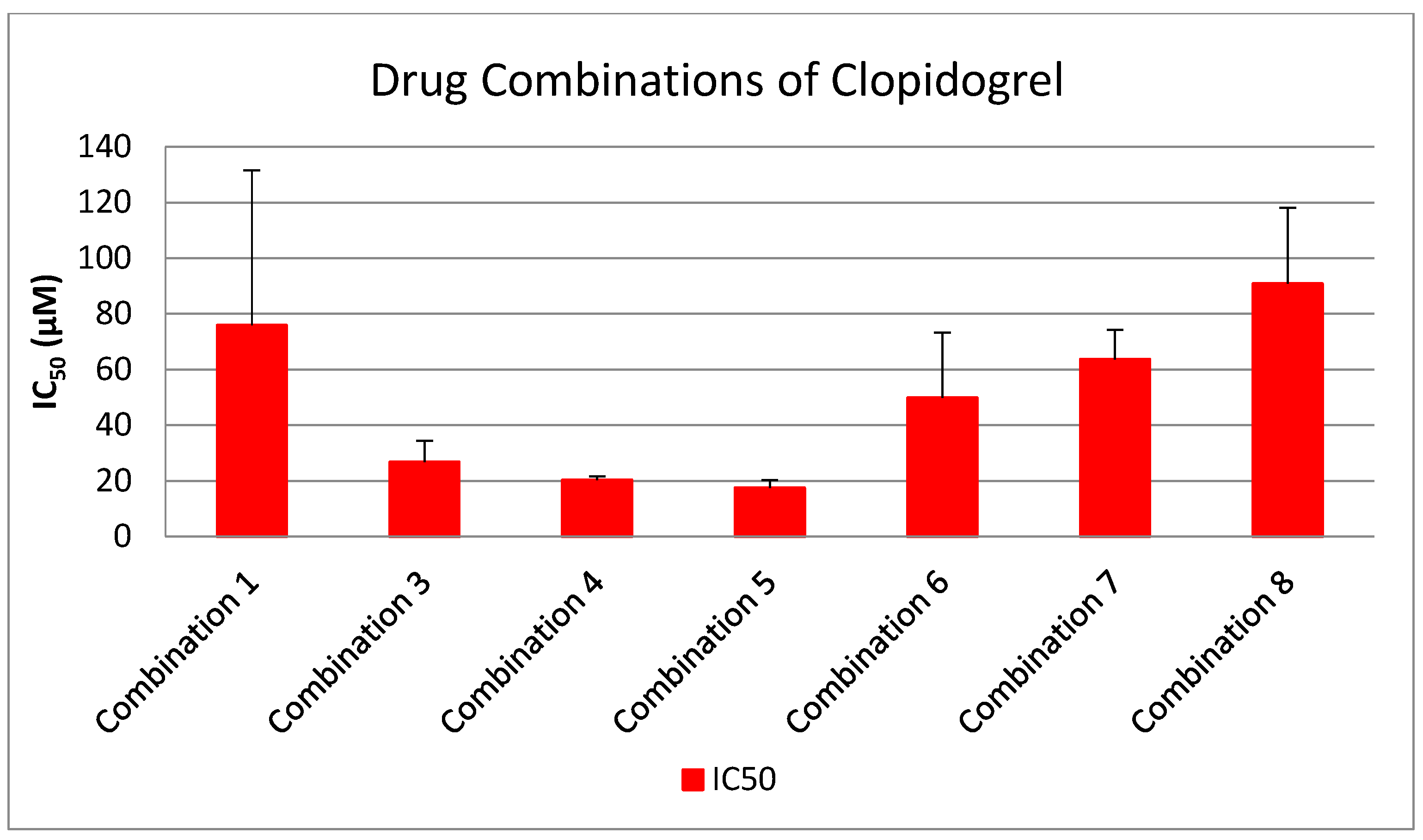
3.3. Evaluation of Synergistic Interactions on Platelet Reactivity of Drug Combinations (1:1 Ratio)
- I.
- Inhibition Potency of Clopidogrel vs. Multi-Drug Combinations
- II.
- Inhibition Potency of Lornoxicam vs. Multi-Drug Combinations
- III.
- Inhibition Potency of Propranolol vs. Multi-Drug Combinations
- IV.
- Inhibition Potency of Paracetamol vs. Multi-Drug Combinations
- V.
- Inhibition Potency of Candesartan vs. Multi-Drug Combinations
Clinical Relevance and Translational Implications
3.4. Results of Toxicological Assessment of Drug-Induced Platelet Aggregation in PRP
3.5. Results of Desensitization Effects and Intrinsic Platelet Activation
3.6. Limitations
4. Conclusions
- NSAIDs (e.g., lornoxicam, diclofenac) and clopidogrel showed preferential inhibition of the PAF pathway, supporting their dual antiplatelet and anti-inflammatory effects.
- Analgesics and β-blockers (e.g., thiocolchicoside, propranolol) were more selective for the ADP pathway, aligning with a primarily thrombotic-targeted mechanism.
- Antihypertensives (e.g., candesartan, valsartan) demonstrated more balanced activity across both pathways, though efficacy varied among individual agents.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics—2018 Update: A Report from the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L. A Comprehensive Assessment of Mortality and Disability from Diesease, Injuries and Risk Factors in 1990 and Projected to 2020. Glob. Burd. Dis. 1994, 1, 41. [Google Scholar]
- World Health Organization. The World Health Report 2002: Reducing Risks, Promoting Healthy Life; World Health Organization: Geneva, Switzerland, 2002; ISBN 978-92-4-156207-2. [Google Scholar]
- Kapuku, G.K.; Kop, W.J. Classification of Cardiovascular Diseases: Epidemiology, Diagnosis, and Treatment. In Handbook of Cardiovascular Behavioral Medicine; Waldstein, S.R., Kop, W.J., Suarez, E.C., Lovallo, W.R., Katzel, L.I., Eds.; Springer: New York, NY, USA, 2022; pp. 45–80. ISBN 978-0-387-85960-6. [Google Scholar]
- Berenson, G.S.; Wattigney, W.A.; Tracy, R.E.; Newman, W.P.; Srinivasan, S.R.; Webber, L.S.; Dalferes, E.R.; Strong, J.P. Atherosclerosis of the aorta and coronary arteries and cardiovascular risk factors in persons aged 6 to 30 years and studied at necropsy (The Bogalusa Heart Study). Am. J. Cardiol. 1992, 70, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Passacquale, G.; Sharma, P.; Perera, D.; Ferro, A. Antiplatelet therapy in cardiovascular disease: Current status and future directions. Br. J. Clin. Pharmacol. 2022, 88, 2686–2699. [Google Scholar] [CrossRef]
- Kakarla, R.; Vinjavarapu, L.A.; Krishnamurthy, S. Diet and Nutraceuticals for treatment and prevention of primary and secondary stroke: Emphasis on nutritional antiplatelet and antithrombotic agents. Neurochem. Int. 2024, 179, 105823. [Google Scholar] [CrossRef]
- Kaiser, R.; Escaig, R.; Nicolai, L. Hemostasis without clot formation: How platelets guard the vasculature in inflammation, infection, and malignancy. Blood 2023, 142, 1413–1425. [Google Scholar] [CrossRef]
- Scridon, A. Platelets and Their Role in Hemostasis and Thrombosis—From Physiology to Pathophysiology and Therapeutic Implications. Int. J. Mol. Sci. 2022, 23, 12772. [Google Scholar] [CrossRef]
- Mussbacher, M.; Kral-Pointner, J.B.; Salzmann, M.; Schrottmaier, W.C.; Assinger, A. Mechanisms of Hemostasis: Contributions of Platelets, Coagulation Factors, and the Vessel Wall. In Fundamentals of Vascular Biology; Springer International Publishing: Cham, Switzerland, 2019; pp. 145–169. [Google Scholar] [CrossRef]
- Brass, L. Understanding and Evaluating Platelet Function. Hematology 2010, 2010, 387–396. [Google Scholar] [CrossRef]
- Varon, D.; Spectre, G. Antiplatelet agents. Hematology 2009, 2009, 267–272. [Google Scholar] [CrossRef]
- Born, G.; Patrono, C. Antiplatelet drugs. Br. J. Pharmacol. 2006, 147, S241–S251. [Google Scholar] [CrossRef]
- Altman, R.; Carreras, L.; Diaz, R.; Figueroa, E.; Paolasso, E.; Parodi, J.; Cade, J.; Donnan, G.; Eadie, M.; Gavaghan, T.; et al. Collaborative overview of randomised trials of antiplatelet therapy-I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ 1994, 308, 81–100. [Google Scholar]
- Lees, K.R.; Bath, P.M.W.; Naylor, A.R. Secondary prevention of transient ischaemic attack and stroke. BMJ 2000, 320, 991–994. [Google Scholar] [CrossRef] [PubMed]
- CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996, 348, 1329–1339. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Büttner, H.J.; Petersen, J.; Roskamm, H. A randomized comparison of clopidogrel and aspirin versus ticlopidine and aspirin after the placement of coronary-artery stents. Circulation 2000, 101, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Kolansky, D.M.; Klugherz, B.D.; Curran, S.C.; Herrmann, H.C.; Magness, K.; Wilensky, R.L.; Hirshfeld, J.W. Combination therapy with clopidogrel and aspirin after coronary stenting. Cathet. Cardiovasc. Intervent. 2000, 50, 276–279. [Google Scholar] [CrossRef]
- Steinmeyer, J. Pharmacological basis for the therapy of pain and inflammation with nonsteroidal anti-inflammatory drugs. Arthritis Res. Ther. 2000, 2, 379. [Google Scholar] [CrossRef]
- Rayan, S.A.; George, R.F.; Said, M.F. Insight on development of oxazole and imidazole derivatives as COX inhibitors with anti-inflammatory effects. J. Mol. Struct. 2025, 1321, 140148. [Google Scholar] [CrossRef]
- Salvo, F.; Fourrier-Réglat, A.; Bazin, F.; Robinson, P.; Riera-Guardia, N.; Haag, M.; Caputi, A.; Moore, N.; Sturkenboom, M.; Pariente, A.; et al. Cardiovascular and Gastrointestinal Safety of NSAIDs: A Systematic Review of Meta-Analyses of Randomized Clinical Trials. Clin. Pharmacol. Ther. 2011, 89, 855–866. [Google Scholar] [CrossRef]
- Driver, B.; Marks, D.C.; Wal, D.E. van der Not all (N)SAID and done: Effects of nonsteroidal anti-inflammatory drugs and paracetamol intake on platelets. Res. Pract. Thromb. Haemost. 2020, 4, 36–45. [Google Scholar] [CrossRef]
- Kreutz, R.; Algharably, E.A. el-Hady Antihypertensive Drugs. In Encyclopedia of Molecular Pharmacology; Offermanns, S., Rosenthal, W., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 165–174. ISBN 978-3-030-57401-7. [Google Scholar]
- Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: Results of the HOPE study and MICRO-HOPE substudy. Lancet 2000, 355, 253–259. [CrossRef]
- Verdecchia, P.; Sleight, P.; Mancia, G.; Fagard, R.; Trimarco, B.; Schmieder, R.E.; Kim, J.-H.; Jennings, G.; Jansky, P.; Chen, J.-H.; et al. Effects of Telmisartan, Ramipril, and Their Combination on Left Ventricular Hypertrophy in Individuals at High Vascular Risk in the Ongoing Telmisartan Alone and in Combination With Ramipril Global End Point Trial and the Telmisartan Randomized Assessment Study in ACE Intolerant Subjects With Cardiovascular Disease. Circulation 2009, 120, 1380–1389. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [PubMed]
- O’Gara, P.T.; Kushner, F.G.; Ascheim, D.D.; Casey, D.E.; Chung, M.K.; de Lemos, J.A.; Ettinger, S.M.; Fang, J.C.; Fesmire, F.M.; Franklin, B.A.; et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013, 127, e362–e425. [Google Scholar] [CrossRef] [PubMed]
- Roffi, M.; Patrono, C.; Collet, J.-P.; Mueller, C.; Valgimigli, M.; Andreotti, F.; Bax, J.J.; Borger, M.A.; Brotons, C.; Chew, D.P.; et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 267–315. [Google Scholar] [CrossRef]
- Amsterdam, E.A.; Wenger, N.K.; Brindis, R.G.; Casey, D.E.; Ganiats, T.G.; Holmes, D.R.; Jaffe, A.S.; Jneid, H.; Kelly, R.F.; Kontos, M.C.; et al. 2014 AHA/ACC Guideline for the Management of Patients with Non–ST-Elevation Acute Coronary Syndromes. Circulation 2014, 130, e344–e426. [Google Scholar] [CrossRef]
- McMurray, J.; Solomon, S.; Pieper, K.; Reed, S.; Rouleau, J.; Velazquez, E.; White, H.; Howlett, J.; Swedberg, K.; Maggioni, A.; et al. The Effect of Valsartan, Captopril, or Both on Atherosclerotic Events After Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2006, 47, 726–733. [Google Scholar] [CrossRef]
- Dickstein, K.; Kjekshus, J. Effects of losartan and captopril on mortality and morbidity in high-risk patients after acute myocardial infarction: The OPTIMAAL randomised trial. Lancet 2002, 360, 752–760. [Google Scholar] [CrossRef]
- Gottlieb, S.S.; McCarter, R.J.; Vogel, R.A. Effect of beta-blockade on mortality among high-risk and low-risk patients after myocardial infarction. N. Engl. J. Med. 1998, 339, 489–497. [Google Scholar] [CrossRef]
- Hawkins, N.M.; Huang, Z.; Pieper, K.S.; Solomon, S.D.; Kober, L.; Velazquez, E.J.; Swedberg, K.; Pfeffer, M.A.; McMurray, J.J.V.; Maggioni, A.P.; et al. Chronic obstructive pulmonary disease is an independent predictor of death but not atherosclerotic events in patients with myocardial infarction: Analysis of the Valsartan in Acute Myocardial Infarction Trial (VALIANT). Eur. J. Heart Fail. 2009, 11, 292–298. [Google Scholar] [CrossRef]
- MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in-Congestive Heart Failure (MERIT-HF). Lancet 1999, 353, 2001–2007. [Google Scholar] [CrossRef]
- Packer, M.; Fowler, M.B.; Roecker, E.B.; Coats, A.J.S.; Katus, H.A.; Krum, H.; Mohacsi, P.; Rouleau, J.L.; Tendera, M.; Staiger, C.; et al. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: Results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation 2002, 106, 2194–2199. [Google Scholar] [CrossRef] [PubMed]
- Quint, J.K.; Herrett, E.; Bhaskaran, K.; Timmis, A.; Hemingway, H.; Wedzicha, J.A.; Smeeth, L. Effect of β blockers on mortality after myocardial infarction in adults with COPD: Population based cohort study of UK electronic healthcare records. BMJ 2013, 347, f6650. [Google Scholar] [CrossRef] [PubMed]
- Short, P.M.; Lipworth, S.I.W.; Elder, D.H.J.; Schembri, S.; Lipworth, B.J. Effect of beta blockers in treatment of chronic obstructive pulmonary disease: A retrospective cohort study. BMJ 2011, 342, d2549. [Google Scholar] [CrossRef]
- Munsterhjelm, E.; Munsterhjelm, N.M.; Niemi, T.T.; Ylikorkala, O.; Neuvonen, P.J.; Rosenberg, P.H. Dose-dependent inhibition of platelet function by acetaminophen in healthy volunteers. Anesthesiology 2005, 103, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Mahendran, D.; PB, K.K.; Sreeramanan, S.; Venkatachalam, P. Enhanced biosynthesis of colchicine and thiocolchicoside contents in cell suspension cultures of Gloriosa superba L. exposed to ethylene inhibitor and elicitors. Ind. Crops Prod. 2018, 120, 123–130. [Google Scholar] [CrossRef]
- Cimino, M.; Marini, P.; Cattabeni, F. Interaction of thiocolchicoside with [3H]strychnine binding sites in rat spinal cord and brainstem. Eur. J. Pharmacol. 1996, 318, 201–204. [Google Scholar] [CrossRef]
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Inflammation, not Cholesterol, Is a Cause of Chronic Disease. Nutrients 2018, 10, 604. [Google Scholar] [CrossRef]
- Tsoupras, A.; Cholidis, P.; Kranas, D.; Galouni, E.A.; Ofrydopoulou, A.; Efthymiopoulos, P.; Shiels, K.; Saha, S.K.; Kyzas, G.Z.; Anastasiadou, C. Anti-Inflammatory, Antithrombotic, and Antioxidant Properties of Amphiphilic Lipid Bioactives from Shrimp. Pharmaceuticals 2024, 18, 25. [Google Scholar] [CrossRef]
- Papadopoulou, D.; Chrysikopoulou, V.; Rampaouni, A.; Plakidis, C.; Ofrydopoulou, A.; Shiels, K.; Saha, S.K.; Tsoupras, A. Antioxidant, Antithrombotic and Anti-Inflammatory Properties of Amphiphilic Bioactives from Water Kefir Grains and Its Apple Pomace-Based Fermented Beverage. Antioxidants 2025, 14, 164. [Google Scholar] [CrossRef]
- Meade, T.W.; Vickers, M.V.; Thompson, S.G.; Stirling, Y.; Haines, A.P.; Miller, G.J. Epidemiological Characteristics of Platelet Aggregability. Br. Med. J. (Clin. Res. Ed.) 1985, 290, 428–432. [Google Scholar] [CrossRef]
- Tsoupras, A.; Zabetakis, I.; Lordan, R. Platelet aggregometry assay for evaluating the effects of platelet agonists and antiplatelet compounds on platelet function in vitro. MethodsX 2019, 6, 63–70. [Google Scholar] [CrossRef]
- Born, G.V.R. Aggregation of Blood Platelets by Adenosine Diphosphate and its Reversal. Nature 1962, 194, 927–929. [Google Scholar] [CrossRef]
- Thompson, S.G.; Vickers, M.V. Methods in dose response platelet aggregometry. Thromb. Haemost. 1985, 53, 216–218. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A.; Adamantidi, T.; Finos, M.A.; Philippopoulos, A.; Detopoulou, P.; Tsopoki, I.; Kynatidou, M.; Demopoulos, C.A. Re-Assessing the Role of Platelet Activating Factor and Its Inflammatory Signaling and Inhibitors in Cancer and Anti-Cancer Strategies. FBL 2024, 29, 345. [Google Scholar] [CrossRef]
- Sakata, C.; Suzuki, K.; Morita, Y.; Kawasaki, T. Additive antithrombotic effect of ASP6537, a selective cyclooxygenase (COX)-1 inhibitor, in combination with clopidogrel in guinea pigs. Eur. J. Pharmacol. 2017, 798, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Schippinger, G.; Prüller, F.; Divjak, M.; Mahla, E.; Fankhauser, F.; Rackemann, S.; Raggam, R.B. Autologous Platelet-Rich Plasma Preparations: Influence of Nonsteroidal Anti-inflammatory Drugs on Platelet Function. Orthop. J. Sports Med. 2015, 3, 2325967115588896. [Google Scholar] [CrossRef]
- Zarco, P.; Maestre, C.; Herrero-Beaumont, G.; González, E.; Garcia-Hoyo, R.; Navarro, F.J.; Braquet, P.; Egido, J. Involvement of platelet-activating factor and tumour necrosis factor in the pathogenesis of joint inflammation in rabbits. Clin. Exp. Immunol. 1992, 88, 318–323. [Google Scholar] [CrossRef]
- Soussi, R.; Hfaiedh, N.; Sakly, M.; Rhouma, K.B. The aqueous extract of Olea europaea leaves protects from haematotoxicity and kidney damage induced by diclofenac in Swiss albino mice. RSC Adv. 2019, 9, 23352–23361. [Google Scholar] [CrossRef]
- Schmidt, L.; Burmeister, L.S.; Greinacher, A.; König, S.; Garscha, U. Development of SFC-MS Method for Quantification of Eicosanoids Biosynthesized in Primary Human Blood Cells. Metabolites 2022, 12, 1198. [Google Scholar] [CrossRef]
- Osojnik, I.; Kamenik, M. The Effect of Diclofenac on Bleeding, Platelet Function, and Consumption of Opioids Following Cardiac Surgery. Braz. J. Cardiovasc. Surg. 2020, 35, 160–168. [Google Scholar] [CrossRef]
- Niemi, T.T.; Taxell, C.; Rosenberg, P.H. Comparison of the effect of intravenous ketoprofen, ketorolac and diclofenac on platelet function in volunteers. Acta Anaesthesiol. Scand. 1997, 41, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Shtrygol’, S.Y.; Koiro, O.O.; Kudina, O.V.; Yudkevych, T.K.; Gorbach, T.V. Comparative analysis of the effect of diclofenac sodium and etoricoxib on energy metabolism in rat liver in the acute general cooling model. Medicni Perspekt. 2022, 27, 51–57. [Google Scholar] [CrossRef]
- Dubrall, D.; Just, K.S.; Schmid, M.; Stingl, J.C.; Sachs, B. Adverse drug reactions in older adults: A retrospective comparative analysis of spontaneous reports to the German Federal Institute for Drugs and Medical Devices. BMC Pharmacol. Toxicol. 2020, 21, 25. [Google Scholar] [CrossRef] [PubMed]
- Dewi, I.M.W.; Aleva, F.E.; Kullaya, V.I.; Garishah, F.M.; de Mast, Q.; van der Ven, A.J.A.M.; van de Veerdonk, F.L. Platelets Modulate IFN-γ Production against Candida albicans in Peripheral Blood Mononuclear Cells via Prostaglandins. J. Immunol. 2020, 204, 122–127. [Google Scholar] [CrossRef]
- Huang, R.; Li, B.; Tamalunas, A.; Waidelich, R.; Stief, C.G.; Hennenberg, M. Inhibition of neurogenic contractions in renal arteries and of cholinergic contractions in coronary arteries by the presumed inhibitor of ADP-ribosylation factor 6, NAV2729. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2022, 395, 471–485. [Google Scholar] [CrossRef]
- Hanumegowda, S.M.; Srinivasa, C.; Shivaiah, A.; Venkatappa, M.M.; Hanumanthappa, R.; Rangappa, R.; Laxmaiah, R.K.; Gonchigar, S.J.; Sannaningaiah, D. Protein extract of kenaf seed exhibits anticoagulant, antiplatelet and antioxidant activities. Asian Pac. J. Trop. Biomed. 2022, 12, 47–58. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, H.; Liu, J.; Wang, K.; Cai, X.; Xiao, W.; Wang, L.; Wang, M.; Zhang, C.; Zhang, J. Metabolomics-based Approach to Analyze the Therapeutic Targets and Metabolites of a Synovitis Ointment for Knee Osteoarthritis. Curr. Pharm. Anal. 2023, 19, 222–234. [Google Scholar] [CrossRef]
- Andrioli, G.; Lussignoli, S.; Gaino, S.; Benoni, G.; Bellavite, P. Study on Paradoxical Effects of NSAIDs on Platelet Activation. Inflammation 1997, 21, 519–530. [Google Scholar] [CrossRef]
- Andrioli, G.; Lussignoli, S.; Ortolani, R.; Minuz, P.; Vella, F.; Bellavite, P. Dual effects of diclofenac on human platelet adhesion in vitro. In Blood Coagulation & Fibrinolysis; LWW: Hosapete, India, 1996; Volume 7, pp. 153–156. [Google Scholar] [CrossRef][Green Version]
- Struthmann, L.; Hellwig, N.; Pircher, J.; Sohn, H.-Y.; Buerkle, M.A.; Klauss, V.; Mannell, H.; Pohl, U.; Krötz, F. Prothrombotic effects of diclofenac on arteriolar platelet activation and thrombosis in vivo. J. Thromb. Haemost. 2009, 7, 1727–1735. [Google Scholar] [CrossRef]
- Falcinelli, E.; Iannone, A.; Mezzasoma, A.M.; Amato, L.; Fierro, T.; Guglielmini, G.; Cagini, C.; Gresele, P. Inhibition of platelet function after ocular administration of non-steroidal anti-inflammatory drugs. Thromb. Res. 2019, 175, 1–5. [Google Scholar] [CrossRef]
- Lara, J.P.; Santana, Y.; Gaddam, M.; Ali, A.; Malik, S.; Khaja, M. Diclofenac-induced thrombotic thrombocytopenic purpura with concomitant complement dysregulation: A case report and review of the literature. J. Med. Case Rep. 2019, 13, 190. [Google Scholar] [CrossRef]
- Özdemir, E.; Karakaya, Z.; Karaca, M.; Topal, F.; Payza, U. Recurrent diclofenac-induced acute myocardial infarction: An interesting case with wandering ST-segment elevation. Hong Kong J. Emerg. Med. 2020, 27, 304–307. [Google Scholar] [CrossRef]
- Korolova, D.; Gryshchenko, V.; Chernyshenko, T.; Platonov, O.; Hornytska, O.; Chernyshenko, V.; Klymenko, P.; Reshetnik, Y.; Platonova, T. Blood coagulation factors and platelet response to drug-induced hepatitis and hepatosis in rats. Anim. Models Exp. Med. 2023, 6, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, O.N.; Abdel-Baky, E.S. Hematological and renoprotective effects of folic acid and lentil extract in diclofenac sodium exposed rats. Braz. J. Biol. 2021, 83, e247360. [Google Scholar] [CrossRef] [PubMed]
- Salim, B.A.; AL-Moziel, M.S.G.; Jaccob, A.A. Subchronic effect of different doses ofDiclofenac Sodium on female reproductive system in rats. Iraqi J. Pharm. Sci. 2023, 32, 227–236. [Google Scholar] [CrossRef]
- Basheeruddin, M.; Lavanya, V.; Ahmed, N.; Jamal, S. Organic osmolyte betaine mitigates the deleterious effects of Diclofenac in vivo in wistar albino rats. Braz. J. Pharm. Sci. 2023, 59, e201178. [Google Scholar] [CrossRef]
- Gut, S.; Rauch, M.; Haschke, M.; Huber, C.A.; Gaertner, J.; Schur, N.; Meier, C.R.; Spoendlin, J. Use of metamizole and other non-opioid analgesics in Switzerland between 2014 and 2019: An observational study using a large health insurance claims database. Swiss Med. Wkly. 2024, 154, 3535. [Google Scholar] [CrossRef]
- Ider, M.; Corum, O.; Ok, M.; Yildiz, R.; Uney, K.; Durna Corum, D.; Atik, O. The effect of tilmicosin and diclofenac sodium combination on cardiac biomarkers in sheep. Pol. J. Vet. Sci. 2023, 26, 5–12. [Google Scholar]
- Rezq, S.; Mahmoud, M.F.; El-Shazly, A.M.; El Raey, M.A.; Sobeh, M. Anti-Inflammatory, Antipyretic, and Analgesic Properties of Potamogeton perfoliatus Extract: In Vitro and In Vivo Study. Molecules 2021, 26, 4826. [Google Scholar] [CrossRef]
- Gurbel, P.A.; Bliden, K.P.; Zhu, J.; Troullos, E.; Centofanti, R.; Jarvis, S.; Venkataraman, P.; Tantry, U.S. Thromboxane inhibition during concurrent therapy with low-dose aspirin and over-the-counter naproxen sodium. J. Thromb. Thrombolysis 2018, 45, 18–26. [Google Scholar] [CrossRef]
- Clarke, A.S.; Rousseau, E.; Wang, K.; Kim, J.-Y.; Murray, B.P.; Bannister, R.; Matzkies, F.; Currie, K.S.; Paolo, J.A.D. Effects of GS-9876, a novel spleen tyrosine kinase inhibitor, on platelet function and systemic hemostasis. Thromb. Res. 2018, 170, 109–118. [Google Scholar] [CrossRef]
- Leach, T.; Huang, B.; Kramer, N.; Challa, S.; Winder, R.P. A Review of Platelet-Rich Plasma Use in Patients Taking Non-steroidal Anti-inflammatory Drugs for Guideline Development. Cureus 2024, 16, e71706. [Google Scholar] [CrossRef] [PubMed]
- Knijff-Dutmer, E.A.J.; Martens, A.; vd Laar, M.A.F.J. Effects of nabumetone compared with naproxen on platelet aggregation in patients with rheumatoid arthritis. Ann. Rheum. Dis. 1999, 58, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Cronberg, S.; Wallmark, E.; Söderberg, I. Effect on platelet aggregation of oral administration of 10 non-steroidal analgesics to humans. Scand. J. Haematol. 1984, 33, 155–159. [Google Scholar] [CrossRef]
- McIntyre, B.A.; Philp, R.B. Effect of three nonsteroidal anti-inflammatory agents on platelet function and prostaglandin synthesis in vitro. Thromb. Res. 1978, 12, 67–77. [Google Scholar] [CrossRef]
- Georgin, J.; Franco, D.S.P.; da Boit Martinello, K.; Lima, E.C.; Silva, L.F.O. A review of the toxicology presence and removal of ketoprofen through adsorption technology. J. Environ. Chem. Eng. 2022, 10, 107798. [Google Scholar] [CrossRef]
- Van Solingen, R.M.; Rosenstein, E.D.; Mihailescu, G.; Drejka, M.L.; Kalia, A.; Cohen, A.J.; Kramer, N. Comparison of the effects of ketoprofen on platelet function in the presence and absence of aspirin. Am. J. Med. 2001, 111, 285–289. [Google Scholar] [CrossRef]
- Stichtenoth, D.O.; Tsikas, D.; Gutzki, F.M.; Frölich, J.C. Effects of ketoprofen and ibuprofen on platelet aggregation and prostanoid formation in man. Eur. J. Clin. Pharmacol. 1996, 51, 231–234. [Google Scholar] [CrossRef]
- da Silva, M.B.; Gustin, P.; Herion, F.; David, J.L.; Van de Weerdt, M.L.; Lekeux, P. Effect of ketoprofen on PAF-induced bovine platelet aggregation. Vet. J. 1998, 155, 201–203. [Google Scholar] [CrossRef]
- Dordoni, P.L.; Ventura, M.D.; Stefanelli, A.; Iannace, E.; Paparella, P.; Rocca, B.; Accorra, F. Effect of ketorolac, ketoprofen and nefopam on platelet function. Anaesthesia 1994, 49, 1046–1049. [Google Scholar] [CrossRef]
- Gandini, R.; Cunietti, E.; Pappalepore, V.; Ferrari, M.; Deleo, B.; Locatelli, E.; Fasoli, A.; Liverta, C. Effects of Intravenous High Doses of Ketoprofen on Blood Clotting, Bleeding Time and Platelet Aggregation in Man. J. Int. Med. Res. 1983, 11, 243–246. [Google Scholar] [CrossRef]
- Halmay, D.; Gaál, T.; Kocsis, R. Influencing factors of ADP-induced, epinephrine-induced and ristomycin-induced platelet aggregation in dogs. Blood Coagul. Fibrinolysis 2008, 19, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Gaál, T.; Halmay, D.; Kocsis, R.; Abonyi-Tóth, Z. Evaluation of the effect of ketoprofen and carprofen on platelet function in dogs studied by PFA-100 point-of-care analyser. Acta Vet. Hung. 2007, 55, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.; Silvestre, S.; Duarte, A.P.; Alves, G. Safety of Non-Steroidal Anti-Inflammatory Drugs in the Elderly: An Analysis of Published Literature and Reports Sent to the Portuguese Pharmacovigilance System. Int. J. Environ. Res. Public Health 2022, 19, 3541. [Google Scholar] [CrossRef] [PubMed]
- Lemke, K.A.; Runyon, C.L.; Horney, B.S. Effects of preoperative administration of ketoprofen on whole blood platelet aggregation, buccal mucosal bleeding time, and hematologic indices in dogs undergoing elective ovariohysterectomy. J. Am. Vet. Med. Assoc. 2002, 220, 1818–1822. [Google Scholar] [CrossRef]
- Lees, P.; Taylor, P.M.; Landoni, F.M.; Arifah, A.K.; Waters, C. Ketoprofen in the Cat: Pharmacodynamics and Chiral Pharmacokinetics. Vet. J. 2003, 165, 21–35. [Google Scholar] [CrossRef]
- Grossman, C.J.; Wiseman, J.; Lucas, F.S.; Trevethick, M.A.; Birch, P.J. Inhibition of constitutive and inducible cyclooxygenase activity in human platelets and mononuclear cells by NSAIDS and Cox 2 inhibitors. Inflamm. Res. 1995, 44, 253–257. [Google Scholar] [CrossRef]
- Razi, M.T.; Javed, I.; Choudry, M.Z.; Khan, M.T.; Mukhtar, N. Effect of Ketoprofen on Lactic Dehydrogenase from Human Platelets. Adv. Clin. Exp. Med. 2014, 23, 377–380. [Google Scholar] [CrossRef]
- Lucarini, L.; Durante, M.; Sgambellone, S.; Lanzi, C.; Bigagli, E.; Akgul, O.; Masini, E.; Supuran, C.T.; Carta, F. Effects of New NSAID-CAI Hybrid Compounds in Inflammation and Lung Fibrosis. Biomolecules 2020, 10, 1307. [Google Scholar] [CrossRef]
- Blaicher, A.M.; Landsteiner, H.T.; Al-Falaki, O.; Zwerina, J.; Volf, I.; Gruber, D.; Zimpfer, M.; Hoerauf, K. Acetylsalicylic Acid, Diclofenac, and Lornoxicam, but Not Rofecoxib, Affect Platelet CD 62 Expression. Anesth. Analg. 2004, 98, 1082–1085. [Google Scholar] [CrossRef]
- Blaicher, A.M.; Landsteiner, H.T.; Zwerina, J.; Leitgeb, U.; Volf, I.; Hoerauf, K. Effect of non-selective, non-steroidal anti-inflammatory drugs and cyclo-oxygenase-2 selective inhibitors on the PFA-100 closure time. Anaesthesia 2004, 59, 1100–1103. [Google Scholar] [CrossRef]
- Tsakiridis, K.; Mpakas, A.; Kesisis, G.; Arikas, S.; Argyriou, M.; Siminelakis, S.; Zarogoulidis, P.; Katsikogiannis, N.; Kougioumtzi, I.; Tsiouda, T.; et al. Lung inflammatory response syndrome after cardiac-operations and treatment of lornoxicam. J. Thorac. Dis. 2014, 6 (Suppl. S1), S78–S98. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.; Fellier, H.; Christoph, T.; Grarup, J.; Stimmeder, D. The analgesic NSAID lornoxicam inhibits cyclooxygenase (COX)-1/-2, inducible nitric oxide synthase (iNOS), and the formation of interleukin (IL)-6 in vitro. Inflamm. Res. 1999, 48, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Felfernig, M.; Salat, A.; Kimberger, O.; Gradisek, P.; Müller, M.R.; Felfernig, D. Preemptive analgesia by lornoxicam--an NSAID--significantly inhibits perioperative platelet aggregation. Eur. J. Anaesthesiol. 2008, 25, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Ghaffar, H.S.; Sayed, J.A.; Fathy, M.A.; Abdel-Azeem, H.G.; Salem, M.A.M. Preincisional peritonsillar vs. intravenous lornoxicam for posttonsillectomy analgesia: A clinical and platelet aggregometry comparative study. Egypt. J. Anaesth. 2012, 28, 107–115. [Google Scholar] [CrossRef]
- Dallob, A.; Hawkey, C.J.; Greenberg, H.; Wight, N.; De Schepper, P.; Waldman, S.; Wong, P.; DeTora, L.; Gertz, B.; Agrawal, N.; et al. Characterization of etoricoxib, a novel, selective COX-2 inhibitor. J. Clin. Pharmacol. 2003, 43, 573–585. [Google Scholar] [CrossRef]
- Escudero-Contreras, A.; Cervantes, J.V.-M.; Collantes-Estevez, E. Update on the clinical pharmacology of etoricoxib, a potent cyclooxygenase-2 inhibitor. Future Rheumatol. 2007, 2, 545–565. [Google Scholar] [CrossRef]
- Patrignani, P.; Capone, M.L.; Tacconelli, S. Clinical pharmacology of etoricoxib: A novel selectiveCOX2 inhibitor. Expert Opin. Pharmacother. 2003, 4, 265–284. [Google Scholar] [CrossRef]
- Mohd, R.; Yee, L.K. Etoricoxib Induced Thrombotic Thrombocytopenic Purpura. Int. J. Fam. Med. Prim. Care 2020, 1, 1028. [Google Scholar]
- PubChem Niflumic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/4488 (accessed on 7 May 2025).
- Nie, D.; Yin, S.; Xie, S.; Wang, X.; Li, Y.; Ma, L.; Wang, X.; Wu, Y.; Feng, J. Effects of Niflumic Acid on Thrombocytic Cytoplasmic Free Calcium and Platelet Aggregation in Patients with Diabetes Mellitus. Blood 2006, 108, 3909. [Google Scholar] [CrossRef]
- Перлович, Г.Л.; Манин, А.Н.; Манин, Н.Г.; Суров, А.О.; Воронин, А.П. Cocrystalline Form of Niflumic Acid with Isonicotinamide ot Caffeine. Patent No. RU2536484C1, 27 December 2014. [Google Scholar]
- Cruickshank, S.F.; Baxter, L.M.; Drummond, R.M. The Cl− channel blocker niflumic acid releases Ca2+ from an intracellular store in rat pulmonary artery smooth muscle cells. Br. J. Pharmacol. 2003, 140, 1442–1450. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.A.; Afzal, M.N.; Shah, B.H. Dual effects of nimesulide, a COX-2 inhibitor, in human platelets. Life Sci. 1998, 63, 1835–1841. [Google Scholar] [CrossRef] [PubMed]
- Uemura, T.; Kawasaki, T.; Taniguchi, M.; Moritani, Y.; Hayashi, K.; Saito, T.; Takasaki, J.; Uchida, W.; Miyata, K. Biological properties of a specific Gαq/11 inhibitor, YM-254890, on platelet functions and thrombus formation under high-shear stress. Br. J. Pharmacol. 2006, 148, 61–69. [Google Scholar] [CrossRef]
- Saeed, S.A.; Rasheed, H.; Hoodbhoy, Z.A.; Pasha, S.R.; Mapara, Z.; Kumar, H.; Shah, B.H. Involvement of cyclooxygenase, phospholipase C and MAP kinase pathways in human platelet aggregation mediated by the synergistic interaction of adrenaline and PAF. Inflammopharmacology 2001, 9, 147–155. [Google Scholar] [CrossRef]
- Tool, A.T.; Verhoeven, A.J. Inhibition of the production of platelet activating factor and of leukotriene B4 in activated neutrophils by nimesulide due to an elevation of intracellular cyclic adenosine monophosphate. Arzneimittelforschung 1995, 45, 1110–1114. [Google Scholar]
- Verhoeven, A.J.; Tool, A.T.; Kuijpers, T.W.; Roos, D. Nimesulide inhibits platelet-activating factor synthesis in activated human neutrophils. Drugs 1993, 46 (Suppl. S1), 52–58. [Google Scholar] [CrossRef]
- Drug Interaction Report: Allopurinol, Warfarin. Available online: https://www.drugs.com/drug-interactions/allopurinol-with-warfarin-127-0-2311-0.html (accessed on 7 May 2025).
- Macfarlane, D.G.; Slade, R.; Hopes, P.A.; Hartog, M.H. A study of platelet aggregation and adhesion in gout. Clin. Exp. Rheumatol. 1983, 1, 63–66. [Google Scholar]
- Ciompi, M.L.; De Caterina, R.; Bertolucci, D.; Bernini, W.; Michelassi, C.; L’Abbate, A. Uric acid levels and platelet function in humans. An in-vivo ex-vivo study. Clin. Exp. Rheumatol. 1983, 1, 143–147. [Google Scholar]
- Qu, X.W.; Rozenfeld, R.A.; Huang, W.; Bulkley, G.B.; Hsueh, W. The role of xanthine oxidase in platelet activating factor induced intestinal injury in the rat. Gut 1999, 44, 203–211. [Google Scholar] [CrossRef]
- Kitazono, T.; Kamouchi, M.; Matsumaru, Y.; Shirai, T.; Takita, A.; Kuroda, T.; Kimura, K. Comparison of Prasugrel and Clopidogrel in Thrombotic Stroke Patients with Risk Factors for Ischemic Stroke Recurrence: An Integrated Analysis of PRASTRO-I, PRASTRO-II, and PRASTRO-III. Cerebrovasc. Dis. 2023, 52, 720–729. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, Y.; Guo, L.; Shen, D.; Dong, Z.; Lin, Y.; Liu, H.; Wei, Y.; Zhang, B. Effect of ticagrelor versus clopidogrel after implantation of drug-eluting stents guided by either intravascular ultrasound or angiography in patients with acute coronary syndrome—Propensity score matching analysis. BMC Cardiovasc. Disord. 2024, 24, 58. [Google Scholar] [CrossRef]
- Chi, G.; AlKhalfan, F.; Lee, J.J.; Montazerin, S.M.; Fitzgerald, C.; Korjian, S.; Omar, W.; Barnathan, E.; Plotnikov, A.; Gibson, C.M. Factors associated with early, late, and very late stent thrombosis among patients with acute coronary syndrome undergoing coronary stent placement: Analysis from the ATLAS ACS 2-TIMI 51 trial. Front. Cardiovasc. Med. 2024, 10, 1269011. [Google Scholar] [CrossRef]
- Özer, A.; Demirtaş, H.; Tak, S.; Koçak, B.; Yiğiter, E.N.; Oktar, G.L.; Kaya, Z. Assessment of Aspirin and Clopidogrel Resistance in Patients Undergoing Cardiovascular Surgery: A Single-Center Cross- Sectional Study. Turk. J. Hematol. 2024, 41, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.R.; Lim, S.T.; Murphy, S.J.X.; Hickey, F.B.; Offiah, C.; Murphy, S.M.; Collins, D.R.; Coughlan, T.; O’Neill, D.; Egan, B.; et al. von Willebrand factor antigen, von Willebrand factor propeptide and ADAMTS13 activity in TIA or ischaemic stroke patients changing antiplatelet therapy. J. Neurol. Sci. 2024, 463, 123118. [Google Scholar] [CrossRef] [PubMed]
- Olivier, C.B.; Mulder, H.; Hiatt, W.R.; Jones, W.S.; Fowkes, F.G.R.; Rockhold, F.W.; Berger, J.S.; Baumgartner, I.; Held, P.; Katona, B.G.; et al. Incidence, Characteristics, and Outcomes of Myocardial Infarction in Patients with Peripheral Artery Disease: Insights from the EUCLID Trial. JAMA Cardiol. 2019, 4, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, M.; Hall, R.P.; Feldman, Z.; Goudot, G.; Sumetsky, N.; Jessula, S.; Kirshkaln, A.; Bellomo, T.; Chang, D.; Cardenas, J.; et al. Predicting Arterial Thrombotic Events Following Peripheral Revascularization Using Objective Viscoelastic Data. J. Am. Heart Assoc. 2023, 12, e027790. [Google Scholar] [CrossRef]
- Franczyk-Skóra, B.; Gluba, A.; Banach, M.; Rysz, J. State of the art paper Treatment of non-ST-elevation myocardial infarction and ST-elevation myocardial infarction in patients with chronic kidney disease. Arch. Med. Sci. 2013, 9, 1019–1027. [Google Scholar] [CrossRef]
- Liu, R.; Li, T.; Yuan, D.; Chen, Y.; Tang, X.; Gao, L.; Zhang, C.; Jia, S.; Zhu, P.; Xu, O.; et al. Long-term effects of baseline on-treatment platelet reactivity in patients with acute coronary syndrome and thrombocytopenia undergoing percutaneous coronary intervention. J. Int. Med. Res. 2022, 50, 03000605221081725. [Google Scholar] [CrossRef]
- Cebo, M.; Dittrich, K.; Fu, X.; Manke, M.C.; Emschermann, F.; Rheinlaender, J.; von Eysmondt, H.; Ferreirós, N.; Sudman, J.; Witte, A.; et al. Platelet ACKR3/CXCR7 favors antiplatelet lipids over an atherothrombotic lipidome and regulates thromboinflammation. Blood 2022, 139, 1722–1742. [Google Scholar] [CrossRef]
- Sikora, J.; Pstrągowski, K.; Karczmarska-Wódzka, A.; Wszelaki, P.; Buszko, K.; Włodarczyk, Z. Impact of Levosimendan and Its Metabolites on Platelet Activation Mechanisms in Patients during Antiplatelet Therapy—Pilot Study. Int. J. Mol. Sci. 2024, 25, 1824. [Google Scholar] [CrossRef]
- Mazereeuw, G.; Herrmann, N.; Xu, H.; Blanchard, A.P.; Figeys, D.; Oh, P.I.; Bennett, S.A.; Lanctôt, K.L. Platelet activating factors are associated with depressive symptoms in coronary artery disease patients: A hypothesis-generating study. NDT 2015, 11, 2309–2314. [Google Scholar] [CrossRef]
- Lim, K.K.; Koleva-Kolarova, R.; Kamaruzaman, H.F.; Kamil, A.A.; Chowienczyk, P.; Wolfe, C.D.A.; Fox-Rushby, J. Genetic-Guided Pharmacotherapy for Coronary Artery Disease: A Systematic and Critical Review of Economic Evaluations. J. Am. Heart Assoc. 2024, 13, e030058. [Google Scholar] [CrossRef]
- Zwart, B.; Bor, W.L.; de Veer, A.J.W.M.; Mahmoodi, B.K.; Kelder, J.C.; Lip, G.Y.; Bhatt, D.L.; Cannon, C.P.; Ten Berg, J.M. A novel risk score to identify the need for triple antithrombotic therapy in patients with atrial fibrillation undergoing percutaneous coronary intervention: A post hoc analysis of the RE-DUAL PCI trial. EuroIntervention 2022, 18, e292. [Google Scholar] [CrossRef]
- Hamid, N.; Sarkar, J.; Redfors, B.; Balani, A.; Ramaswamy, R.; Ghosh, A.; Alu, M.; Crowley, A.; Zhang, Y.; Leon, M.B.; et al. A neural system dynamics modeling platform and its applications in randomized controlled trial data analysis. Inform. Med. Unlocked 2021, 24, 100612. [Google Scholar] [CrossRef]
- Nagrani, T.; Zaher, M.; Gaddam, S.; Jabbour, G.; Baldari, D.; Baglini, R.; Duvvuri, S. In-stent thrombosis after 68 months of implantation inspite of continuous dual antiplatelet therapy: A case report. Cases J. 2010, 3, 68. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Russell, L.; Weihe, S.; Madsen, E.K.; Hvas, C.L.; Leistner, J.W.; Michelsen, J.; Brøchner, A.C.; Bastiansen, A.; Nielsen, F.M.; Meier, N.; et al. Thromboembolic and bleeding events in ICU patients with COVID-19: A nationwide, observational study. Acta Anaesthesiol. Scand. 2023, 67, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.T.; Li, R.H.L.; Georges, C.J.; Nguyen, N.; Chen, C.K.; Stuhlmann, C.; Oldach, M.S.; Rivas, V.N.; Fousse, S.; Harris, S.P.; et al. Synergistic inhibitory effects of clopidogrel and rivaroxaban on platelet function and platelet-dependent thrombin generation in cats. J. Vet. Intern. Med. 2023, 37, 1390–1400. [Google Scholar] [CrossRef] [PubMed]
- Kuszynski, D.S.; Christian, B.D.; Bernard, M.P.; Lauver, D.A. Evaluation of the Efficacy and Safety of Antiplatelet Therapeutics in Rabbits. Curr. Protoc. 2023, 3, e711. [Google Scholar] [CrossRef]
- Jiang, L.-P.; Ji, J.-Z.; Ge, P.-X.; Zhu, T.; Mi, Q.-Y.; Tai, T.; Li, Y.-F.; Xie, H.-G. Is platelet responsiveness to clopidogrel attenuated in overweight or obese patients and why? A reverse translational study in mice. Br. J. Pharmacol. 2022, 179, 46–64. [Google Scholar] [CrossRef]
- Iba, T.; Levy, J.H. Inflammation and thrombosis: Roles of neutrophils, platelets and endothelial cells and their interactions in thrombus formation during sepsis. J. Thromb. Haemost. 2018, 16, 231–241. [Google Scholar] [CrossRef]
- Palur Ramakrishnan, A.V.K.; Varghese, T.P.; Vanapalli, S.; Nair, N.K.; Mingate, M.D. Platelet activating factor: A potential biomarker in acute coronary syndrome? Cardiovasc. Ther. 2017, 35, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Tunehag, K.R.; Thomas, C.D.; Franchi, F.; Rossi, J.S.; Keeley, E.C.; Anderson, R.D.; Beitelshees, A.L.; Duarte, J.D.; Gong, Y.; Kerensky, R.A.; et al. CYP2C19 Genotype Is Associated with Adverse Cardiovascular Outcomes in Black Patients Treated With Clopidogrel Undergoing Percutaneous Coronary Intervention. J. Am. Heart Assoc. 2024, 13, e033791. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.; Persad-Ramdeensingh, S.; Abrahim, S.C.; Seecheran, N.; Haraksingh, R.R. Prevalence of CYP2C19*2 and CYP2C19*3 Allelic Variants and Clopidogrel Use in Patients with Cardiovascular Disease in Trinidad & Tobago. Cardiol. Ther. 2024, 13, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Shahim, B.; Redfors, B.; Stuckey, T.D.; Liu, M.; Zhou, Z.; Witzenbichler, B.; Weisz, G.; Rinaldi, M.J.; Neumann, F.; Metzger, D.C.; et al. On-Treatment Platelet Reactivity and Ischemic Outcomes in Patients with Diabetes Mellitus: Two-Year Results From ADAPT-DES. J. Am. Heart Assoc. 2023, 12, e026482. [Google Scholar] [CrossRef]
- Savi, P.; Pereillo, J.M.; Uzabiaga, M.F.; Combalbert, J.; Picard, C.; Maffrand, J.P.; Pascal, M.; Herbert, J.M. Identification and biological activity of the active metabolite of clopidogrel. Thromb. Haemost. 2000, 84, 891–896. [Google Scholar] [CrossRef]
- Gurbel, P.A.; Bliden, K.P.; Hiatt, B.L.; O’Connor, C.M. Clopidogrel for Coronary Stenting. Circulation 2003, 107, 2908–2913. [Google Scholar] [CrossRef]
- Weber, A.-A.; Reimann, S.; Schrör, K. Specific inhibition of ADP-induced platelet aggregation by clopidogrel in vitro. Br. J. Pharmacol. 1999, 126, 415–420. [Google Scholar] [CrossRef]
- Hinz, B.; Cheremina, O.; Brune, K. Acetaminophen (paracetamol) is a selective cyclooxygenase-2 inhibitor in man. FASEB J. 2008, 22, 383–390. [Google Scholar] [CrossRef]
- Graham, G.G.; Robins, S.-A.; Bryant, K.J.; Scott, K.F. Inhibition of prostaglandin synthesis in intact cells by paracetamol (acetaminophen). Inflammopharmacology 2001, 9, 131–142. [Google Scholar] [CrossRef]
- Kao, D.S.; Zhang, S.W.; Vap, A.R. A Systematic Review on the Effect of Common Medications on Platelet Count and Function: Which Medications Should Be Stopped Before Getting a Platelet-Rich Plasma Injection? Orthop. J. Sports Med. 2022, 10, 23259671221088820. [Google Scholar] [CrossRef]
- Gonzalez-Valcarcel, J.; Sissani, L.; Labreuche, J.; Bousser, M.-G.; Chamorro, A.; Fisher, M.; Ford, I.; Fox, K.M.; Hennerici, M.G.; Mattle, H.P.; et al. Paracetamol, Ibuprofen, and Recurrent Major Cardiovascular and Major Bleeding Events in 19 120 Patients with Recent Ischemic Stroke. Stroke 2016, 47, 1045–1052. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Catella-Lawson, F.; Reilly, M.P.; Kapoor, S.C.; Cucchiara, A.J.; DeMarco, S.; Tournier, B.; Vyas, S.N.; FitzGerald, G.A. Cyclooxygenase Inhibitors and the Antiplatelet Effects of Aspirin. N. Engl. J. Med. 2001, 345, 1809–1817. [Google Scholar] [CrossRef] [PubMed]
- Brossi, A.; Dumont, R.; Yun-Choi, H.S.; Lee, J.R. Inhibition of platelet aggregation by colchicine, thiocolchicine, and their phenolic and glucosidic congeners. Arch. Pharm. Res. 1987, 10, 100–102. [Google Scholar] [CrossRef]
- Reddel, C.J.; Pennings, G.J.; Chen, V.M.; Gnanenthiran, S.; Kritharides, L. Colchicine as a Modulator of Platelet Function: A Systematic Review. Semin. Thromb. Hemost. 2022, 48, 552–567. [Google Scholar] [CrossRef]
- Punda, A.; Polić, S.; Rumboldt, Z.; Bagatin, J.; Marković, V.; Lukin, A. Effects of atenolol and propranolol on platelet aggregation in moderate essential hypertension: Randomized crossover trial. Croat. Med. J. 2005, 46, 219–224. [Google Scholar]
- Bonten, T.N.; Plaizier, C.E.I.; Snoep, J.-J.D.; Stijnen, T.; Dekkers, O.M.; van der Bom, J.G. Effect of β-blockers on platelet aggregation: A systematic review and meta-analysis. Br. J. Clin. Pharmacol. 2014, 78, 940–949. [Google Scholar] [CrossRef]
- Kerry, R.; Scrutton, M.C. Platelet β-adrenoceptors. Br. J. Pharmacol. 1983, 79, 681–691. [Google Scholar] [CrossRef]
- Sharma, K.K.; Mathur, M.; Gupta, R.; Guptha, S.; Roy, S.; Khedar, R.S.; Gupta, N.; Gupta, R. Epidemiology of cardioprotective pharmacological agent use in stable coronary heart disease. Indian Heart J. 2013, 65, 250–255. [Google Scholar] [CrossRef][Green Version]
- Michiels, I.; Bodem, F. The deltoid muscle: An electromyographical analysis of its activity in arm abduction in various body postures. Int. Orthop. 1992, 16, 268–271. [Google Scholar] [CrossRef]
- Iwamura, M.; Ishimori, T.; Making, M.; Yasuda, K.; Izumi, A.; Himori, N. Drug-induced inhibition of guinea pig platelet aggregation unrelated to their β-adrenolytic actions. Jpn. J. Pharmacol. 1983, 33, 219–226. [Google Scholar] [CrossRef]
- Nosál, R.; Jancinová, V.; Petríková, M. The role of prostaglandins in the effect of beta-blockers on stimulated blood platelets. Vnitr. Lek. 1991, 37, 261–267. [Google Scholar]
- Anfossi, G.; Trovati, M.; Mularoni, E.; Massucco, P.; Calcamuggi, G.; Emanuelli, G. Influence of propranolol on platelet aggregation and thromboxane B2 production from platelet-rich plasma and whole blood. Prostaglandins Leukot. Essent. Fat. Acids 1989, 36, 1–7. [Google Scholar] [CrossRef]
- Weksler, B.; Gillick, M.; Pink, J. Effect of propranolol on platelet function. Blood 1977, 49, 185–196. [Google Scholar] [CrossRef]
- Winther, K.; Knudsen, J.B.; Gormsen, J.; Jensen, J. Effect of metoprolol and propranolol on platelet aggregation and cAMP level in hypertensive patients. Eur. J. Clin. Pharmacol. 1986, 29, 561–564. [Google Scholar] [CrossRef]
- Ignjatovic, V.; Pavlovic, S.; Miloradovic, V.; Andjelkovic, N.; Davidovic, G.; Djurdjevic, P.; Stolic, R.; Iric-Cupic, V.; Simic, I.; Ignjatovic, V.D.; et al. Influence of Different β-Blockers on Platelet Aggregation in Patients with Coronary Artery Disease on Dual Antiplatelet Therapy. J. Cardiovasc. Pharmacol. Ther. 2016, 21, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Larsson, P.T.; Olsson, G.; Angelin, B.; Granström, E.; Hansson, G.; Hjemdahl, P. Metoprolol does not reduce platelet aggregability during sympatho-adrenal stimulation. Eur. J. Clin. Pharmacol. 1992, 42, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Korbut, R.; Swies, J.; Marcinkiewicz, E.; Gryglewski, R.J. Thrombolytic activity of beta-adrenolytic drug, sotalol. J. Physiol. Pharmacol. 1998, 49, 51–60. [Google Scholar] [PubMed]
- Premuzic Mestrovic, I.; Smoday, I.M.; Kalogjera, L.; Krezic, I.; Zizek, H.; Vranes, H.; Vukovic, V.; Oroz, K.; Skorak, I.; Brizic, I.; et al. Antiarrhythmic Sotalol, Occlusion/Occlusion-like Syndrome in Rats, and Stable Gastric Pentadecapeptide BPC 157 Therapy. Pharmaceuticals 2023, 16, 977. [Google Scholar] [CrossRef]
- Sato, Y.; Fujii, S.; Imagawa, S.; Ohmura, K.; Ohmura, Y.; Andoh, Y.; Dong, J.; Ishimori, N.; Furumoto, T.; Tsutsui, H. Platelet aggregability in patients with hypertension treated with angiotensin II type 1 receptor blockers. J. Atheroscler. Thromb. 2007, 14, 31–35. [Google Scholar] [CrossRef][Green Version]
- Fogari, R.; Derosa, G.; Mugellini, A.; Preti, P.; Rinaldi, A.; Pasotti, C.; Malacco, E.; Corradi, L. P-86: Effect of losartan irbesartan and candesartan on platelet aggregability and fibrinolysis in type 2 diabetic hypertensive patients. Am. J. Hypertens. 2003, 14, 70A. [Google Scholar] [CrossRef]
- Núñez, A.; Gómez, J.; Zalba, L.R.; Montón, M.; Jiménez, A.; Velasco, S.; López-Blaya, A.; Celdrán Uriarte, A.; Casado, S.; López-Farré, A. Losartan inhibits in vitro platelet activation: Comparison with candesartan and valsartan. J. Renin Angiotensin Aldosterone Syst. 2000, 1, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, A.M.; Montón, M.; García, R.; Núñez, A.; Gómez, J.; Rico, L.; García-Colis, E.; de Miguel, L.S.; Arriero, M.M.; Cabestrero, F.; et al. Inhibition of Platelet Activation in Stroke-Prone Spontaneously Hypertensive Rats: Comparison of Losartan, Candesartan, and Valsartan. J. Cardiovasc. Pharmacol. 2001, 37, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Nossaman, B.D.; Baber, S.R.; Nazim, M.M.; Detrolio, J.D.; Kadowitz, P.J. Differential effects of losartan and candesartan on vasoconstrictor responses in the ratThis paper is one of a selection of papers published in this Special Issue, entitled The Cellular and Molecular Basis of Cardiovascular Dysfunction, Dhalla 70th Birthday Tribute. Can. J. Physiol. Pharmacol. 2007, 85, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Sekizuka, E.; Yamaguchi, N.; Nakadate, H.; Terao, S.; Granger, D.N.; Minamitani, H. Angiotensin II type 1 receptor signaling contributes to platelet-leukocyte-endothelial cell interactions in the cerebral microvasculature. Am. J. Physiol. -Heart Circ. Physiol. 2007, 292, H2306–H2315. [Google Scholar] [CrossRef]
- Hallevi, H.; Hazan-Halevy, I.; Paran, E. Modification of neutrophil adhesion to human endothelial cell line in acute ischemic stroke by dipyridamole and candesartan. Eur. J. Neurol. 2007, 14, 1002–1007. [Google Scholar] [CrossRef]
- Buda, V.; Andor, M.; Cristescu, C.; Voicu, M.; Cochera, F.; Tuduce, P.; Petrescu, L.; Tomescu, M.C. The effect of candesartan on pentraxin-3 plasma levels as marker of endothelial dysfunction in patients with essential arterial hypertension. Ir. J. Med. Sci. 2017, 186, 621–629. [Google Scholar] [CrossRef]
- Al-Azzam, S.I.; Alzoubi, K.H.; Khabour, O.F.; Quttina, M.; Zayadeen, R. Evaluation of the effect of angiotensin converting enzyme inhibitors and angiotensin receptors blockers on aspirin antiplatelet effect. Int. J. Clin. Pharmacol. Ther. 2016, 54, 96–101. [Google Scholar] [CrossRef]
- McClellan, K.J.; Goa, K.L. Candesartan cilexetil. A review of its use in essential hypertension. Drugs 1998, 56, 847–869. [Google Scholar] [CrossRef]
- Kratochwil, N.A.; Huber, W.; Müller, F.; Kansy, M.; Gerber, P.R. Predicting plasma protein binding of drugs: A new approach. Biochem. Pharmacol. 2002, 64, 1355–1374. [Google Scholar] [CrossRef]
- Colmenarejo, G. In silico prediction of drug-binding strengths to human serum albumin. Med. Res. Rev. 2003, 23, 275–301. [Google Scholar] [CrossRef]
- Malinin, A.; Serebruany, V.L.; Webb, R.L. Use of Valsartan ot Its Metabolite to Inhibit Platelet Aggregation. Patent No. WO2003094915A1, 20 November 2003. [Google Scholar]
- Serebruany, V.L.; Malinin, A.I.; Lowry, D.R.; Sane, D.C.; Webb, R.L.; Gottlieb, S.O.; O’Connor, C.M.; Hennekens, C.H. Effects of Valsartan and Valeryl 4-Hydroxy Valsartan on Human Platelets: A Possible Additional Mechanism for Clinical Benefits. J. Cardiovasc. Pharmacol. 2004, 43, 677–684. [Google Scholar] [CrossRef]
- Serebruany, V.L.; Pokov, A.N.; Malinin, A.I.; O’Connor, C.; Bhatt, D.L.; Tanguay, J.-F.; Sane, D.C.; Hennekens, C.H. Valsartan inhibits platelet activity at different doses in mild to moderate hypertensives: Valsartan Inhibits Platelets (VIP) trial. Am. Heart J. 2006, 151, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wang, H.-Y.; Cai, F.; Wang, L.-J.; Zhang, F.-R.; Chen, X.-N.; Yang, Q.; Jiang, M.-H.; Wang, X.-F.; Shen, W.-F. Valsartan Decreases Platelet Activity and Arterial Thrombotic Events in Elderly Patients with Hypertension. Chin. Med. J. 2015, 128, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Serebruany, V.L.; Pokov, A.N.; Aradi, D.; Can, M.; DiNicolantonio, J.; Kipshidze, N.; Atar, D. Effect of Aliskiren and Valsartan Combination Versus Aliskiren Monotherapy on Hemostatic Biomarkers in Hypertensive Diabetics: Aliskiren and Valsartan Impact in Diabetics Pilot Trial. Am. J. Ther. 2014, 21, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Kalinowski, L.; Matys, T.; Chabielska, E.; Buczko, W.; Malinski, T. Angiotensin II AT1 receptor antagonists inhibit platelet adhesion and aggregation by nitric oxide release. Hypertension 2002, 40, 521–527. [Google Scholar] [CrossRef]
- López-Farré, A.; Sánchez De Miguel, L.; Montón, M.; Jiménez, A.; Lopez-Bloya, A.; Gómez, J.; Núñez, A.; Rico, L.; Casado, S. Angiotensin II AT1 receptor antagonists and platelet activation. Nephrol. Dial. Transplant. 2001, 16, 45–49. [Google Scholar] [CrossRef]
- Sironi, L.; Calvio, A.M.; Arnaboldi, L.; Corsini, A.; Parolari, A.; de Gasparo, M.; Tremoli, E.; Mussoni, L. Effect of Valsartan on Angiotensin II–Induced Plasminogen Activator Inhibitor-1 Biosynthesis in Arterial Smooth Muscle Cells. Hypertension 2001, 37, 961–966. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, S.-P.; Zhou, H.-N.; Li, Q.-Z.; Li, J.-X. Effect of Fluvastatin and Valsartan, Alone and in Combination, on Postprandial Vascular Inflammation and Fibrinolytic Activity in Patients with Essential Hypertension. J. Cardiovasc. Pharmacol. 2007, 50, 50. [Google Scholar] [CrossRef]
- Malinin, A.I.; Ong, S.; Makarov, L.M.; Petukhova, E.Y.; Serebruany, V.L. Platelet inhibition beyond conventional antiplatelet agents: Expanding role of angiotensin receptor blockers, statins and selective serotonin reuptake inhibitors. Int. J. Clin. Pract. 2006, 60, 993–1002. [Google Scholar] [CrossRef]
- Colussi, D.M.; Parisot, C.; Rossolino, M.L.; Brunner, L.A.; Lefèvre, G.Y. Protein binding in plasma of valsartan, a new angiotensin II receptor antagonist. J. Clin. Pharmacol. 1997, 37, 214–221. [Google Scholar] [CrossRef]
- Mil, K.M.; Gryciuk, M.E.; Pawlukianiec, C.; Żendzian-Piotrowska, M.; Ładny, J.R.; Zalewska, A.; Maciejczyk, M. Pleiotropic Properties of Valsartan: Do They Result from the Antiglycooxidant Activity? Literature Review and In Vitro Study. Oxid. Med. Cell. Longev. 2021, 2021, 5575545. [Google Scholar] [CrossRef]
- Tomczyk, M.D.; Matczak, K.; Denel-Bobrowska, M.; Dzido, G.; Kubicka, A.; Gendosz de Carrillo, D.; Cichoń, T.; Golec, M.; Powieczko, B.; Rzetelny, W.; et al. Combining Sulfonylureas with Anticancer Drugs: Evidence of Synergistic Efficacy with Doxorubicin In Vitro and In Vivo. Int. J. Mol. Sci. 2025, 26, 1429. [Google Scholar] [CrossRef]
- Tsoupras, A.; Gkika, D.A.; Siadimas, I.; Christodoulopoulos, I.; Efthymiopoulos, P.; Kyzas, G.Z. The Multifaceted Effects of Non-Steroidal and Non-Opioid Anti-Inflammatory and Analgesic Drugs on Platelets: Current Knowledge, Limitations, and Future Perspectives. Pharmaceuticals 2024, 17, 627. [Google Scholar] [CrossRef]
- Grosser, T.; Fries, S.; FitzGerald, G.A. Biological basis for the cardiovascular consequences of COX-2 inhibition: Therapeutic challenges and opportunities. J. Clin. Investig. 2006, 116, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Fox, K.A.A.; Hacke, W.; Berger, P.B.; Black, H.R.; Boden, W.E.; Cacoub, P.; Cohen, E.A.; Creager, M.A.; Easton, J.D.; et al. Clopidogrel and Aspirin versus Aspirin Alone for the Prevention of Atherothrombotic Events. N. Engl. J. Med. 2006, 354, 1706–1717. [Google Scholar] [CrossRef] [PubMed]
- Burnier, M.; Brunner, H.R. Angiotensin II receptor antagonists. Lancet 2000, 355, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Weiss, H.J.; Rogers, J. Thrombocytopathia Due to Abnormalities in Platelet Release Reaction—Studies on Six Unrelated Patients. Blood 1972, 39, 187–196. [Google Scholar] [CrossRef]
- Trovati, M.; Anfossi, G.; Cavalot, F.; Vitali, S.; Massucco, P.; Mularoni, E.; Schinco, P.; Tamponi, G.; Emanuelli, G. Studies on Mechanisms Involved in Hypoglycemia-Induced Platelet Activation. Diabetes 1986, 35, 818–825. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katsanopoulou, M.; Zannas, Z.; Ofrydopoulou, A.; Maria, C.; Krokidis, X.; Lambropoulou, D.A.; Tsoupras, A. Anti-Inflammatory and Antiplatelet Interactions on PAF and ADP Pathways of NSAIDs, Analgesic and Antihypertensive Drugs for Cardioprotection—In Vitro Assessment in Human Platelets. Medicina 2025, 61, 1413. https://doi.org/10.3390/medicina61081413
Katsanopoulou M, Zannas Z, Ofrydopoulou A, Maria C, Krokidis X, Lambropoulou DA, Tsoupras A. Anti-Inflammatory and Antiplatelet Interactions on PAF and ADP Pathways of NSAIDs, Analgesic and Antihypertensive Drugs for Cardioprotection—In Vitro Assessment in Human Platelets. Medicina. 2025; 61(8):1413. https://doi.org/10.3390/medicina61081413
Chicago/Turabian StyleKatsanopoulou, Makrina, Zisis Zannas, Anna Ofrydopoulou, Chatzikamari Maria, Xenophon Krokidis, Dimitra A. Lambropoulou, and Alexandros Tsoupras. 2025. "Anti-Inflammatory and Antiplatelet Interactions on PAF and ADP Pathways of NSAIDs, Analgesic and Antihypertensive Drugs for Cardioprotection—In Vitro Assessment in Human Platelets" Medicina 61, no. 8: 1413. https://doi.org/10.3390/medicina61081413
APA StyleKatsanopoulou, M., Zannas, Z., Ofrydopoulou, A., Maria, C., Krokidis, X., Lambropoulou, D. A., & Tsoupras, A. (2025). Anti-Inflammatory and Antiplatelet Interactions on PAF and ADP Pathways of NSAIDs, Analgesic and Antihypertensive Drugs for Cardioprotection—In Vitro Assessment in Human Platelets. Medicina, 61(8), 1413. https://doi.org/10.3390/medicina61081413









