Cerebrospinal Fluid Volume and Other Intracranial Volumes Are Associated with Fazekas Score in Adults: A Single Center Experience
Abstract
1. Introduction
2. Materials and Methods
Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schaefer, A.; Quinque, E.M.; Kipping, J.A.; Arélin, K.; Roggenhofer, E.; Frisch, S.; Villringer, A.; Mueller, K.; Schroeter, M.L. Early small vessel disease affects frontoparietal and cerebellar hubs in close correlation with clinical symptoms—A resting-state fMRI study. J. Cereb. Blood Flow Metab. 2014, 34, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- de Leeuw, F.E.; de Groot, J.C.; Achten, E.; Oudkerk, M.; Ramos, L.M.; Heijboer, R.; Hofman, A.; Jolles, J.; van Gijn, J.; Breteler, M.M. Prevalence of cerebral white matter lesions in elderly people: A population based magnetic resonance imaging study. The Rotterdam Scan Study. J. Neurol. Neurosurg. Psychiatry 2001, 70, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Schmidt, H.; Haybaeck, J.; Loitfelder, M.; Weis, S.; Cavalieri, M.; Seiler, S.; Enzinger, C.; Ropele, S.; Erkinjuntti, T.; et al. Heterogeneity in age-related white matter changes. Acta Neuropathol. 2011, 122, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Fazekas, F.; Chawluk, J.B.; Alavi, A.; Hurtig, H.I.; Zimmerman, R.A. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am. J. Roentgenol. 1987, 149, 351–356. [Google Scholar] [CrossRef]
- Frisoni, G.B.; Galluzzi, S.; Pantoni, L.; Filippi, M. The effect of white matter lesions on cognition in the elderly—Small but detectable. Nat. Clin. Pract. Neurol. 2007, 3, 620–627. [Google Scholar] [CrossRef]
- Breteler, M.M. Vascular involvement in cognitive decline and dementia. Epidemiologic evidence from the Rotterdam Study and the Rotterdam Scan Study. Ann. N. Y. Acad. Sci. 2000, 903, 457–465. [Google Scholar] [CrossRef]
- Longstreth, W.T., Jr.; Arnold, A.M.; Beauchamp, N.J., Jr.; Manolio, T.A.; Lefkowitz, D.; Jungreis, C.; Hirsch, C.H.; O’Leary, D.H.; Furberg, C.D. Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: The Cardiovascular Health Study. Stroke 2005, 36, 56–61. [Google Scholar] [CrossRef]
- Olsson, E.; Klasson, N.; Berge, J.; Eckerström, C.; Edman, A.; Malmgren, H.; Wallin, A. White Matter Lesion Assessment in Patients with Cognitive Impairment and Healthy Controls: Reliability Comparisons between Visual Rating, a Manual, and an Automatic Volumetrical MRI Method-The Gothenburg MCI Study. J. Aging Res. 2013, 2013, 198471. [Google Scholar] [CrossRef]
- Hilal, S.; Mok, V.; Youn, Y.C.; Wong, A.; Ikram, M.K.; Chen, C.L. Prevalence, risk factors and consequences of cerebral small vessel diseases: Data from three Asian countries. J. Neurol. Neurosurg. Psychiatry 2017, 88, 669–674. [Google Scholar] [CrossRef]
- Ferreira, D.; Shams, S.; Cavallin, L.; Viitanen, M.; Martola, J.; Granberg, T.; Shams, M.; Aspelin, P.; Kristoffersen-Wiberg, M.; Nordberg, A.; et al. The contribution of small vessel disease to subtypes of Alzheimer’s disease: A study on cerebrospinal fluid and imaging biomarkers. Neurobiol. Aging 2018, 70, 18–29. [Google Scholar] [CrossRef]
- Enzinger, C.; Fazekas, F.; Ropele, S.; Schmidt, R. Progression of cerebral white matter lesions—Clinical and radiological considerations. J. Neurol. Sci. 2007, 257, 5–10. [Google Scholar] [CrossRef]
- Cox, R.W. AFNI: What a long strange trip it’s been. Neuroimage 2012, 62, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Acer, N.; Bastepe-Gray, S.; Sagiroglu, A.; Gumus, K.Z.; Degirmencioglu, L.; Zararsiz, G.; Ozic, M.U. Diffusion tensor and volumetric magnetic resonance imaging findings in the brains of professional musicians. J. Chem. Neuroanat. 2018, 88, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Manjón, J.V.; Coupé, P. volBrain: An Online MRI Brain Volumetry System. Front. Neuroinform. 2016, 10, 30. [Google Scholar] [CrossRef]
- Meier-Ruge, W.; Ulrich, J.; Brühlmann, M.; Meier, E. Age-related white matter atrophy in the human brain. Ann. N. Y. Acad. Sci. 1992, 673, 260–269. [Google Scholar] [CrossRef]
- Cole, J.H.; Ritchie, S.J.; Bastin, M.E.; Valdés Hernández, M.C.; Muñoz Maniega, S.; Royle, N.; Corley, J.; Pattie, A.; Harris, S.E.; Zhang, Q.; et al. Brain age predicts mortality. Mol. Psychiatry 2018, 23, 1385–1392. [Google Scholar] [CrossRef]
- Bonilha, L.; Eckert, M.A.; Fridriksson, J.; Hirth, V.A.; Moser, D.; Morgan, P.S.; Rorden, C. Age-related relative volume preservation of the dominant hand cortical region. Brain Res. 2009, 1305, 14–19. [Google Scholar] [CrossRef][Green Version]
- Giorgio, A.; Santelli, L.; Tomassini, V.; Bosnell, R.; Smith, S.; De Stefano, N.; Johansen-Berg, H. Age-related changes in grey and white matter structure throughout adulthood. Neuroimage 2010, 51, 943–951. [Google Scholar] [CrossRef]
- Lambert, C.; Benjamin, P.; Zeestraten, E.; Lawrence, A.J.; Barrick, T.R.; Markus, H.S. Longitudinal patterns of leukoaraiosis and brain atrophy in symptomatic small vessel disease. Brain 2016, 139, 1136–1151. [Google Scholar] [CrossRef]
- Prins, N.D.; van Dijk, E.J.; den Heijer, T.; Vermeer, S.E.; Jolles, J.; Koudstaal, P.J.; Hofman, A.; Breteler, M.M. Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain 2005, 128, 2034–2041. [Google Scholar] [CrossRef]
- Prins, N.D.; van Dijk, E.J.; den Heijer, T.; Vermeer, S.E.; Koudstaal, P.J.; Oudkerk, M.; Hofman, A.; Breteler, M.M. Cerebral white matter lesions and the risk of dementia. Arch. Neurol. 2004, 61, 1531–1534. [Google Scholar] [CrossRef] [PubMed]
- Garnier-Crussard, A.; Bougacha, S.; Wirth, M.; André, C.; Delarue, M.; Landeau, B.; Mézenge, F.; Kuhn, E.; Gonneaud, J.; Chocat, A.; et al. White matter hyperintensities across the adult lifespan: Relation to age, Aβ load, and cognition. Alzheimers Res. Ther. 2020, 12, 127. [Google Scholar] [CrossRef]
- Huang, C.C.; Chou, K.H.; Lee, W.J.; Yang, A.C.; Tsai, S.J.; Chen, L.K.; Chung, C.P.; Lin, C.P. Brain white matter hyperintensities-predicted age reflects neurovascular health in middle-to-old aged subjects. Age Ageing 2022, 51, afac106. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, J.M.; Smith, C.; Dichgans, M. Mechanisms of sporadic cerebral small vessel disease: Insights from neuroimaging. Lancet Neurol. 2013, 12, 483–497. [Google Scholar] [CrossRef]
- Andere, A.; Jindal, G.; Molino, J.; Collins, S.; Merck, D.; Burton, T.; Stretz, C.; Yaghi, S.; Sacchetti, D.C.; Jamal, S.E.; et al. Volumetric White Matter Hyperintensity Ranges Correspond to Fazekas Scores on Brain MRI. J. Stroke Cerebrovasc. Dis. 2022, 31, 106333. [Google Scholar] [CrossRef]
- Cedres, N.; Ferreira, D.; Machado, A.; Shams, S.; Sacuiu, S.; Waern, M.; Wahlund, L.O.; Zettergren, A.; Kern, S.; Skoog, I.; et al. Predicting Fazekas scores from automatic segmentations of white matter signal abnormalities. Aging 2020, 12, 894–901. [Google Scholar] [CrossRef]
- Chappell, F.M.; Del Carmen Valdés Hernández, M.; Makin, S.D.; Shuler, K.; Sakka, E.; Dennis, M.S.; Armitage, P.A.; Muñoz Maniega, S.; Wardlaw, J.M. Sample size considerations for trials using cerebral white matter hyperintensity progression as an intermediate outcome at 1 year after mild stroke: Results of a prospective cohort study. Trials 2017, 18, 78. [Google Scholar] [CrossRef]
- Valdés Hernández Mdel, C.; Morris, Z.; Dickie, D.A.; Royle, N.A.; Muñoz Maniega, S.; Aribisala, B.S.; Bastin, M.E.; Deary, I.J.; Wardlaw, J.M. Close correlation between quantitative and qualitative assessments of white matter lesions. Neuroepidemiology 2013, 40, 13–22. [Google Scholar] [CrossRef]
- Tran, P.; Thoprakarn, U.; Gourieux, E.; Dos Santos, C.L.; Cavedo, E.; Guizard, N.; Cotton, F.; Krolak-Salmon, P.; Delmaire, C.; Heidelberg, D.; et al. Automatic segmentation of white matter hyperintensities: Validation and comparison with state-of-the-art methods on both Multiple Sclerosis and elderly subjects. Neuroimage Clin. 2022, 33, 102940. [Google Scholar] [CrossRef]
- Soysal, H.; Acer, N.; Özdemir, M.; Eraslan, Ö. Volumetric measurements of the subcortical structures of healthy adult brains in the Turkish population. Folia Morphol. 2022, 81, 294–306. [Google Scholar] [CrossRef]
- Öztürk, İ.; Pak, A.T.; Şengül, H.S.; Doğan, S.N.; Şengül, Y. The effect on cognitive functions of vascular lesion localizations and vascular load in the brain. Turk. J. Cerebrovasc. Dis. 2021, 27, 133–138. [Google Scholar]
- Kloppenborg, R.P.; Nederkoorn, P.J.; Grool, A.M.; Vincken, K.L.; Mali, W.P.; Vermeulen, M.; van der Graaf, Y.; Geerlings, M.I. Cerebral small-vessel disease and progression of brain atrophy: The SMART-MR study. Neurology 2012, 79, 2029–2036. [Google Scholar] [CrossRef] [PubMed]
- Knoops, A.J.; van der Graaf, Y.; Appelman, A.P.; Mali, W.P.; Geerlings, M.I. Total cerebral blood flow and hippocampal volume in patients with arterial disease. The SMART-MR study. J. Cereb. Blood Flow Metab. 2009, 29, 1727–1733. [Google Scholar] [CrossRef] [PubMed]
- Habes, M.; Erus, G.; Toledo, J.B.; Zhang, T.; Bryan, N.; Launer, L.J.; Rosseel, Y.; Janowitz, D.; Doshi, J.; Van der Auwera, S.; et al. White matter hyperintensities and imaging patterns of brain ageing in the general population. Brain 2016, 139, 1164–1179. [Google Scholar] [CrossRef]
- Aribisala, B.S.; Valdés Hernández, M.C.; Royle, N.A.; Morris, Z.; Muñoz Maniega, S.; Bastin, M.E.; Deary, I.J.; Wardlaw, J.M. Brain atrophy associations with white matter lesions in the ageing brain: The Lothian Birth Cohort 1936. Eur. Radiol. 2013, 23, 1084–1092. [Google Scholar] [CrossRef]
- Wang, R.; Fratiglioni, L.; Laveskog, A.; Kalpouzos, G.; Ehrenkrona, C.H.; Zhang, Y.; Bronge, L.; Wahlund, L.O.; Bäckman, L.; Qiu, C. Do cardiovascular risk factors explain the link between white matter hyperintensities and brain volumes in old age? A population-based study. Eur. J. Neurol. 2014, 21, 1076–1082. [Google Scholar] [CrossRef]
- Tuladhar, A.M.; Reid, A.T.; Shumskaya, E.; de Laat, K.F.; van Norden, A.G.; van Dijk, E.J.; Norris, D.G.; de Leeuw, F.E. Relationship between white matter hyperintensities, cortical thickness, and cognition. Stroke 2015, 46, 425–432. [Google Scholar] [CrossRef]
- Wen, W.; Sachdev, P.S.; Chen, X.; Anstey, K. Gray matter reduction is correlated with white matter hyperintensity volume: A voxel-based morphometric study in a large epidemiological sample. Neuroimage 2006, 29, 1031–1039. [Google Scholar] [CrossRef]
- Debette, S.; Markus, H.S. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: Systematic review and meta-analysis. Br. Med. J. 2010, 341, c3666. [Google Scholar] [CrossRef]
- Smith, E.E.; Egorova, S.; Blacker, D.; Killiany, R.J.; Muzikansky, A.; Dickerson, B.C.; Tanzi, R.E.; Albert, M.S.; Greenberg, S.M.; Guttmann, C.R. Magnetic resonance imaging white matter hyperintensities and brain volume in the prediction of mild cognitive impairment and dementia. Arch. Neurol. 2008, 65, 94–100. [Google Scholar] [CrossRef]
- Rhodius-Meester, H.F.M.; Benedictus, M.R.; Wattjes, M.P.; Barkhof, F.; Scheltens, P.; Muller, M.; van der Flier, W.M. MRI Visual Ratings of Brain Atrophy and White Matter Hyperintensities across the Spectrum of Cognitive Decline Are Differently Affected by Age and Diagnosis. Front. Aging Neurosci. 2017, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, J.M.; Smith, E.E.; Biessels, G.J.; Cordonnier, C.; Fazekas, F.; Frayne, R.; Lindley, R.I.; O’Brien, J.T.; Barkhof, F.; Benavente, O.R.; et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013, 12, 822–838. [Google Scholar] [CrossRef] [PubMed]
- Jessen, N.A.; Munk, A.S.; Lundgaard, I.; Nedergaard, M. The Glymphatic System: A Beginner’s Guide. Neurochem. Res. 2015, 40, 2583–2599. [Google Scholar] [CrossRef] [PubMed]
- Nedergaard, M.; Goldman, S.A. Glymphatic failure as a final common pathway to dementia. Science 2020, 370, 50–56. [Google Scholar] [CrossRef]
- Taoka, T.; Naganawa, S. Glymphatic imaging using MRI. J. Magn. Reson. Imaging 2020, 51, 11–24. [Google Scholar] [CrossRef]
- Waymont, J.M.J.; Valdés Hernández, M.D.C.; Bernal, J.; Duarte Coello, R.; Brown, R.; Chappell, F.M.; Ballerini, L.; Wardlaw, J.M. Systematic review and meta-analysis of automated methods for quantifying enlarged perivascular spaces in the brain. Neuroimage 2024, 297, 120685. [Google Scholar] [CrossRef]
- Chung, S.J.; Yoo, H.S.; Shin, N.Y.; Park, Y.W.; Lee, H.S.; Hong, J.M.; Kim, Y.J.; Lee, S.K.; Lee, P.H.; Sohn, Y.H. Perivascular Spaces in the Basal Ganglia and Long-term Motor Prognosis in Newly Diagnosed Parkinson Disease. Neurology 2021, 96, e2121–e2131. [Google Scholar] [CrossRef]
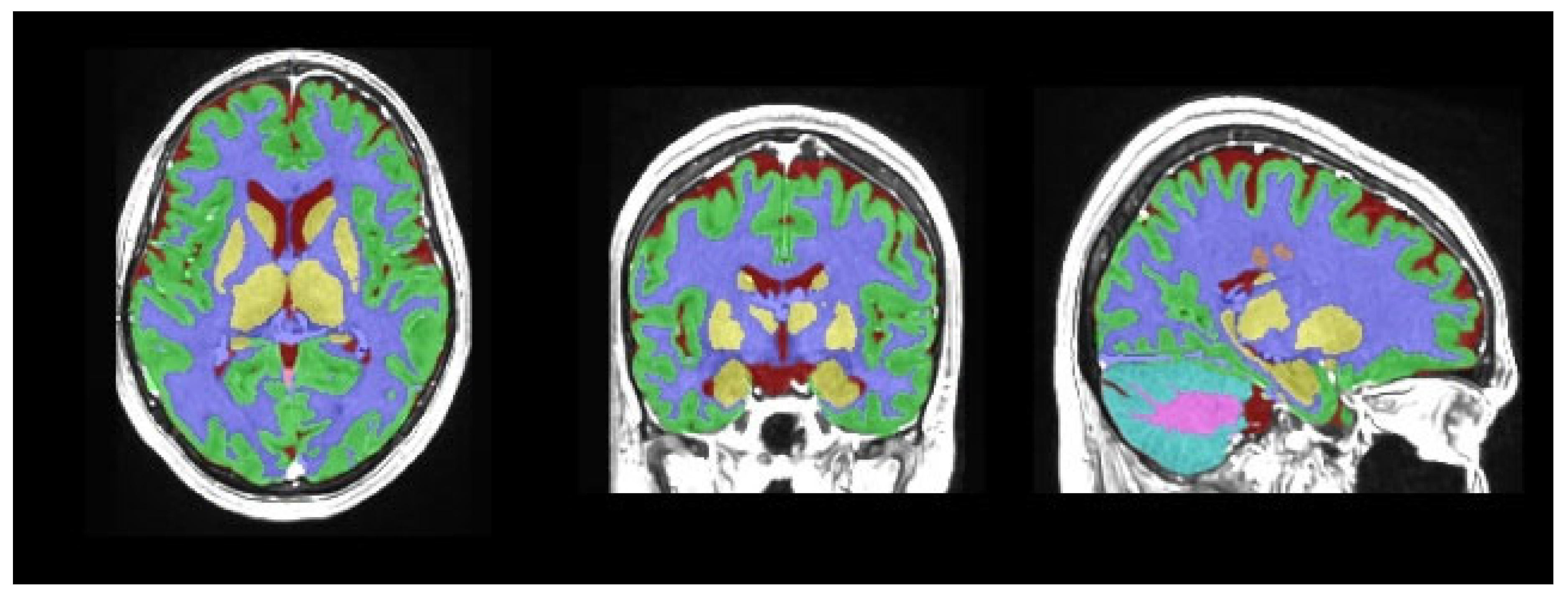
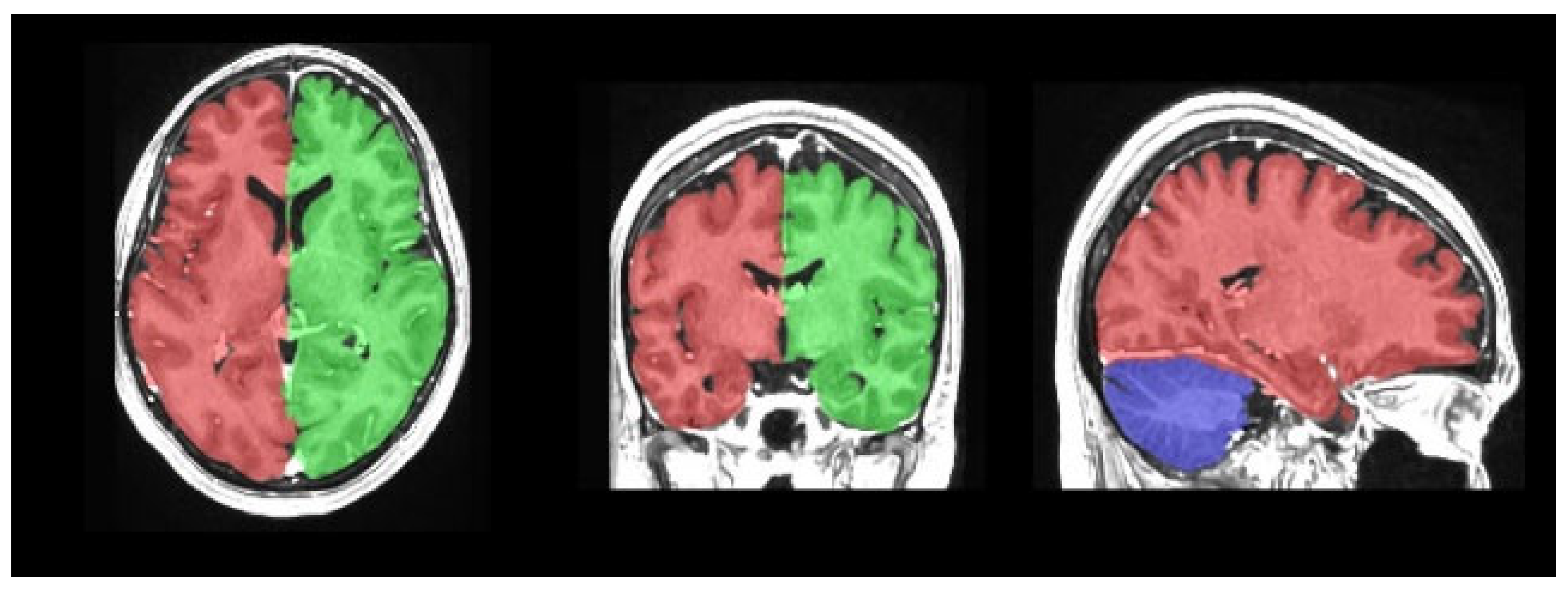
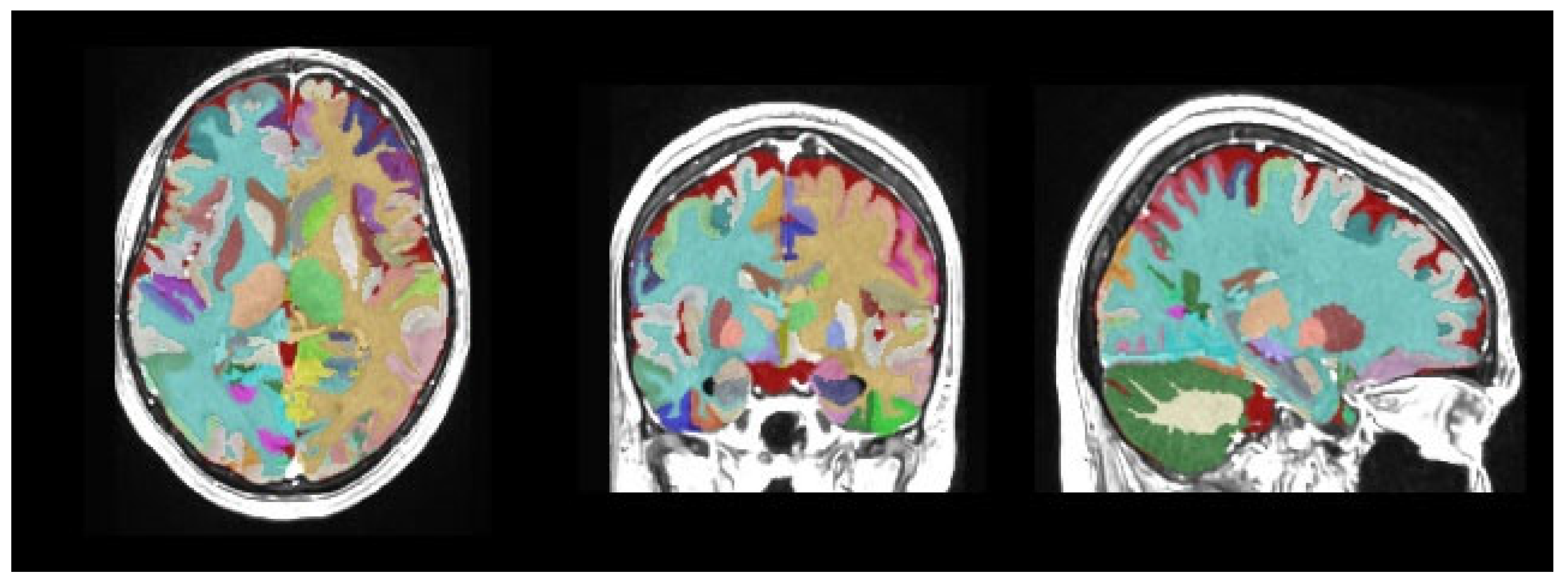
| Group 1 (n = 114) | Group 2 (n = 122) | p | ||
|---|---|---|---|---|
| Gender (n, %) | Women | 72 (63%) | 60 (49%) | <0.001 |
| Men | 42 (37%) | 62 (51%) | <0.001 | |
| Age (years) | 59 (37–81) | 69 (34–95) | <0.001 | |
| Volume (mm3) | Group 1 (n = 114) | Group 2 (n = 122) | p |
|---|---|---|---|
| Mean ± Std | |||
| Total intracranial cavity | 1305 ± 122 | 1312 ± 137 | 0.37 |
| Cortical gray matter | 521 ± 59 | 510 ± 62 | 0.68 |
| Globus pallidus | 2.6 ± 0.4 | 2.6 ± 0.4 | 0.51 |
| Temporal lobe | 110 ± 12 | 107 ± 13 | 0.78 |
| Median (Min–Max) | |||
| Subcortical gray matter | 6.2 (4.6–9.8) | 6.1 (1.6–9.1) | 0.48 |
| Cerebrospinal fluid | 119 (53–292) | 193 (53–981) | <0.001 |
| Total brain white + gray matter | 1158 (825–1207) | 1113 (611–1491) | 0.009 |
| Total cerebrum | 1046 (734–1257) | 992 (492–1326) | <0.001 |
| Cerebrum white matter | 461 (232–688) | 441 (134–889) | 0.049 |
| Cerebrum gray matter | 558 (414–1314) | 542 (239–989) | 0.11 |
| Accumbens | 0.6 (0.3–0.9) | 0.5 (0.2–0.9) | <0.001 |
| Amygdala | 1.9 (0.7–4.7) | 1.8 (1–9.1) | 0.52 |
| Caudate | 38 (29–86) | 38 (22–49) | 0.20 |
| Putamen | 7.3 (5.2–9.6) | 7.1 (1–9.8) | 0.12 |
| Thalamus | 11.8 (8.1–16.2) | 10.8 (3.3–16.4) | <0.001 |
| Hippocampus | 7.7 (2.8–9.9) | 7.8 (3.4–10.1) | 0.53 |
| Frontal lobe | 180 (124–255) | 167 (53–250) | <0.001 |
| Occipital lobe | 72 (46–95) | 72 (28–88) | 0.88 |
| Parietal lobe | 85 (69–132) | 96 (43–143) | <0.001 |
| Lateral ventricle | 22 (7–87) | 33 (8–95) | <0.001 |
| CSF Volume | Total Brain White + Gray Matter | Total Cerebrum | Cerebrum White Matter Volume | Accumbens Volume | Thalamus Volume | Frontal Lobe Volume | Parietal Lobe Volume | Lateral Ventricle Volume | |
|---|---|---|---|---|---|---|---|---|---|
| CSF volume | - | NS | NS | NS | r = −015 p = 0.02 | r = −0.33 p < 0.001 | r = −0.20 p = 0.02 | r = 0.13 p = 0.04 | r = 0.22 p < 0.001 |
| Total brain white + gray matter | NS | - | r = 0.20 p = 0.003 | r = 0.15 p = 0.025 | NS | NS | r = 0.14 p = 0.033 | NS | NS |
| Total cerebrum | NS | r = 0.20 p = 0.003 | - | r = 0.68 p < 0.001 | r = 0.61 p < 0.001 | r = 0.66 p < 0.001 | r = 0.78 p < 0.001 | r = 0.48 p < 0.001 | r = −0.14 p = 0.029 |
| Cerebrum white matter volume | NS | r = 0.15 p = 0.025 | r = 0.68 p < 0.001 | - | r = 0.32 p < 0.001 | r = 0.34 p < 0.001 | r = 0.34 p < 0.001 | r = 0.40 p < 0.001 | r = −0.20 p = 0.002 |
| Accumbens volume | NS | r = −015 p = 0.02 | r = 0.61 p < 0.001 | r = 0.32 p < 0.001 | - | r = 0.61 p < 0.001 | r = 0.60 p < 0.001 | r = 0.32 p < 0.001 | r = −0.31 p < 0.001 |
| Thalamus volume | r = −0.33 p < 0.001 | NS | r = 0.66 p < 0.001 | r = 0.34 p < 0.001 | r = 0.61 p < 0.001 | - | r = 0.65 p < 0.001 | r = 0.25 p < 0.001 | r = −0.24 p < 0.001 |
| Frontal lobe volume | r = −0.20 p = 0.02 | r = 0.14 p = 0.033 | r = 0.78 p < 0.001 | r = 0.34 p < 0.001 | r = 0.60 p < 0.001 | r = 0.65 p < 0.001 | - | r = 0.37 p < 0.001 | NS |
| Parietal lobe volume | r = 0.13 p = 0.04 | NS | r = 0.48 p < 0.001 | r = 0.40 p < 0.001 | r = 0.32 p < 0.001 | r = 0.25 p < 0.001 | r = 0.37 p < 0.001 | - | NS |
| Lateral ventricle volume | r = 0.22 p < 0.001 | NS | r = −0.14 p = 0.029 | r = −0.20 p = 0.002 | r = −0.31 p < 0.001 | r = −0.24 p < 0.001 | NS | NS | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalfaoglu, M.E.; Cosgun, Z.; Buz Yasar, A.; Sarioglu, A.E.; Aktas, G. Cerebrospinal Fluid Volume and Other Intracranial Volumes Are Associated with Fazekas Score in Adults: A Single Center Experience. Medicina 2025, 61, 1411. https://doi.org/10.3390/medicina61081411
Kalfaoglu ME, Cosgun Z, Buz Yasar A, Sarioglu AE, Aktas G. Cerebrospinal Fluid Volume and Other Intracranial Volumes Are Associated with Fazekas Score in Adults: A Single Center Experience. Medicina. 2025; 61(8):1411. https://doi.org/10.3390/medicina61081411
Chicago/Turabian StyleKalfaoglu, Melike Elif, Zeliha Cosgun, Aysenur Buz Yasar, Abdullah Emre Sarioglu, and Gulali Aktas. 2025. "Cerebrospinal Fluid Volume and Other Intracranial Volumes Are Associated with Fazekas Score in Adults: A Single Center Experience" Medicina 61, no. 8: 1411. https://doi.org/10.3390/medicina61081411
APA StyleKalfaoglu, M. E., Cosgun, Z., Buz Yasar, A., Sarioglu, A. E., & Aktas, G. (2025). Cerebrospinal Fluid Volume and Other Intracranial Volumes Are Associated with Fazekas Score in Adults: A Single Center Experience. Medicina, 61(8), 1411. https://doi.org/10.3390/medicina61081411







