Extramedullary Plasmacytomas of the Nasal Cavity: Case-Based Perspectives into Optimizing the Diagnostic Differentiation from Inflammatory Polyps
Abstract
1. Introduction
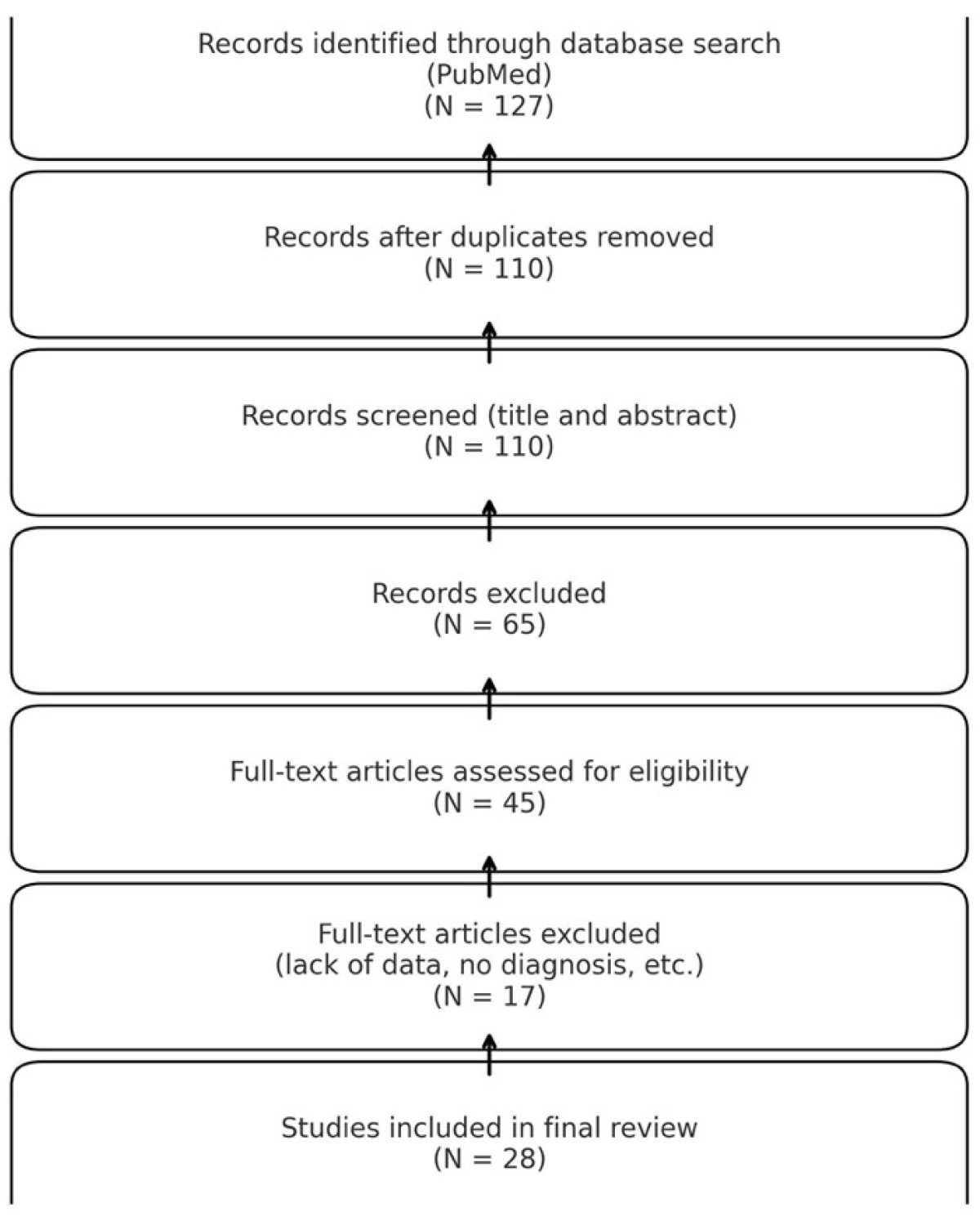
2. Materials and Methods
3. Results
3.1. Literature Data Analysis
3.2. Presentation and Analysis of Our Cohort
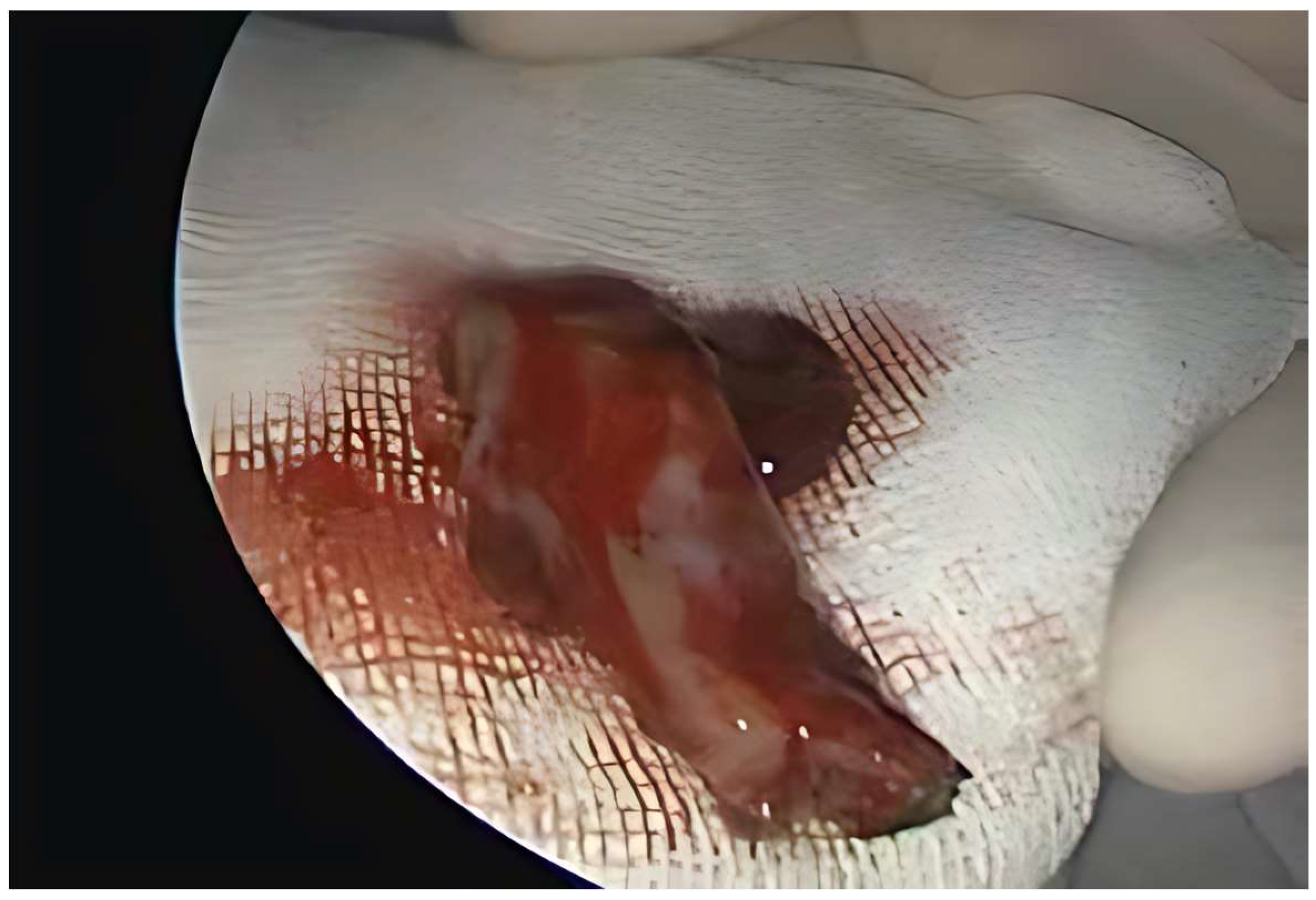
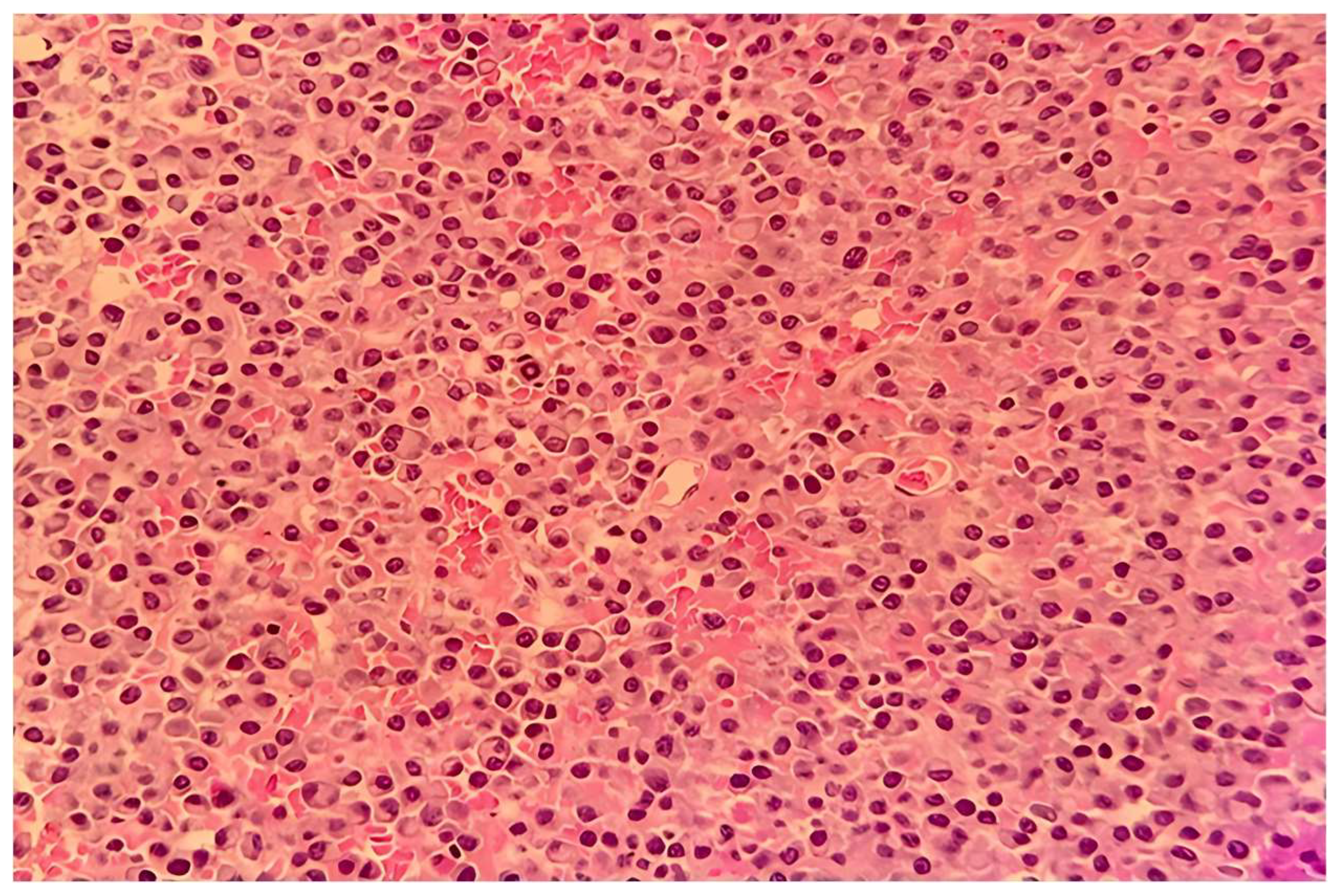
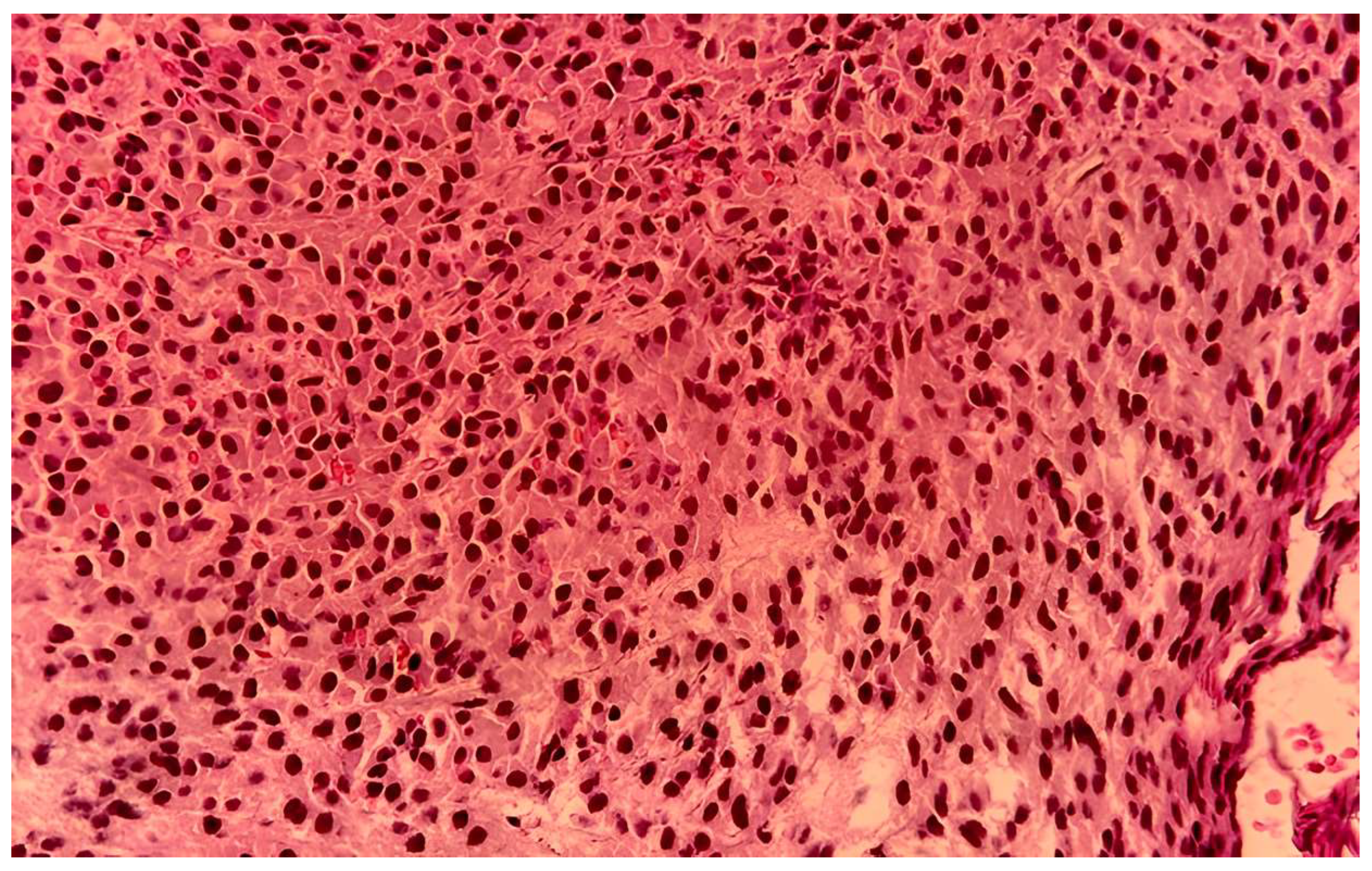
Case Report
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Full Term | Abbreviation |
| Extramedullary plasmacytoma | EMP |
| Multiple myeloma | MM |
| Solitary plasmacytoma of bone | SPB |
| Radiotherapy | RT |
| Chemotherapy | CHT |
| Magnetic resonance imaging | MRI |
| Computed tomography | CT |
| Cluster of differentiation | CD |
| Kappa light chains | KLCs |
| Lambda light chains | LLCs |
| Endoscopic nasal surgery | ENS |
| Complete blood count | CBC |
| Estimated glomerular filtration rate | eGFR |
| Sinonasal extramedullary plasmacytoma | SN-EMP |
References
- Schridde, H. Weitere Untersuchungen uber die kornelungen der Plasmazellen. Cent. Bl F Allg. Path Anat. 1905, 16, 433–435. [Google Scholar]
- Venkatesulu, B.; Mallick, S.; Giridhar, P.; Upadhyay, A.D.; Rath, G.K. Pattern of care and impact of prognostic factors on the outcome of head and neck extramedullary plasmacytoma: A systematic review and individual patient data analysis of 315 cases. Eur. Arch. Otorhinolaryngol. 2018, 275, 595–606. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Hamilos, G. Solitary bone plasmacytoma and extramedullary plasmacytoma. Curr. Treat. Options Oncol. 2002, 3, 255–259. [Google Scholar] [CrossRef]
- Caers, J.; Paiva, B.; Zamagni, E.; Leleu, X.; Bladé, J.; Kristinsson, S.Y.; Touzeau, C.; Abildgaard, N.; Terpos, E.; Heusschen, R.; et al. Diagnosis, treatment, and response assessment in solitary plasmacytoma: Updated recommendations from a European Expert Panel. J. Hematol. Oncol. 2018, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Tanrivermis Sayit, A.; Elmali, M.; Gün, S. Evaluation of Extramedullary Plasmacytoma of the Larynx with Radiologic and Histopathological Findings. Radiologia 2020, 64, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A. Neuroimaging of plasmacytoma. A pictorial review. Neuroradiol. J. 2014, 27, 431–437. [Google Scholar] [CrossRef]
- Gholizadeh, N.; Mehdipour, M.; Rohani, B.; Esmaeili, V. Extramedullary Plasmacytoma of the Oral Cavity in a Young Man: A Case Report. J. Dent. 2016, 17, 155–158. [Google Scholar]
- Strojan, P.; Šoba, E.; Lamovec, J.; Munda, A. Extramedullary plasmacytoma: Clinical and histopathologic study. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 692–701. [Google Scholar] [CrossRef]
- Robillard, N.; Wuillème, S.; Moreau, P.; Béné, M.C. Immunophenotype of Normal and Myelomatous Plasma-Cell Subsets. Front. Immunol. 2014, 5, 137. [Google Scholar] [CrossRef]
- Aznab, M. Multifocal Extramedullary and Multiple Solitary Bone Plasmacytoma: A Case Report and Review of the Literature. Int. J. Cancer Manag. 2019, 12, 10–13. [Google Scholar] [CrossRef]
- Report, C. Multiple Solitary Plasmacytoma of Chest Wall and Scalp with Extramedullary Solitary Plasmacytoma of Orbit Occurring Simultaneously: A Rare Case Report. Open Access J. Cancer Oncol. 2019, 3, 1–5. [Google Scholar] [CrossRef]
- Ahnach, M.; Marouan, S.; Rachid, M.; Madani, A.; Quessar, A.; Benchekroun, S.; Quachouh, M. Extramedullary plasmocytoma relapsing at differents sites: An unusual presentation. Pan Afr. Med. J. 2013, 14, 34. [Google Scholar] [CrossRef]
- Bachar, G.; Goldstein, D.; Brown, D.; Tsang, R.; Lockwood, G.; Perez-Ordonez, B.; Irish, J. Solitary extramedullary plasmacytoma of the head and neck--long-term outcome analysis of 68 cases. Head Neck 2008, 30, 1012–1019. [Google Scholar] [CrossRef]
- Tournier-Rangeard, L.; Lapeyre, M.; Graff-Caillaud, P.; Mege, A.; Dolivet, G.; Toussaint, B.; Charra-Brunaud, C.; Hoffstetter, S.; Marchal, C.; Peiffert, D.; et al. Radiotherapy for solitary extramedullary plasmacytoma in the head-and-neck region: A dose greater than 45 Gy to the target volume improves the local control. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 1013–1017. [Google Scholar] [CrossRef]
- Wen, G.; Wang, W.; Zhang, Y.; Niu, S.; Li, Q.; Li, Y. Management of extramedullary plasmacytoma: Role of radiotherapy and prognostic factor analysis in 55 patients. Chin. J. Cancer Res. 2017, 29, 438–446. [Google Scholar] [CrossRef]
- Ashraf, M.J.; Azarpira, N.; Khademi, B.; Abedi, E.; Hakimzadeh, A.; Valibeigi, B. Extramedullary plasmacytoma of the nasal cavity report of three cases with review of the literature. Iran. Red. Crescent Med. J. 2013, 15, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Cantone, E.; Di Lullo, A.M.; Marano, L.; Guadagno, E.; Mansueto, G.; Capriglione, P.; Catalano, L.; Iengo, M. Strategy for the treatment and follow-up of sinonasal solitary extramedullary plasmacytoma: A case series. J. Med. Case Rep. 2017, 11, 219. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Natt, R.S.; O’Sullivan, G. An extraosseous plasmacytoma of the nasopharynx. J. Clin. Med. Res. 2009, 1, 121–122. [Google Scholar] [CrossRef][Green Version]
- Helman, S.N.; Filip, P.; Iacob, C.; Colley, P. Bilateral sinonasal extramedulary plasmacytoma treated with radiotherapy and a medial maxillectomy with a Denker’s procedure. Am. J. Otolaryngol. 2017, 38, 360–362. Available online: https://www.sciencedirect.com/science/article/pii/S0196070917300704 (accessed on 16 March 2025). [CrossRef] [PubMed]
- Park, S.H.; Kim, Y.Z.; Lee, E.H.; Kim, K.H. Endoscopic endonasal transsphenoidal resection of solitary extramedullary plasmacytoma in the sphenoid sinus with destruction of skull base. J. Korean Neurosurg. Soc. 2009, 46, 156–160. [Google Scholar] [CrossRef]
- Report, C. Extramedullary plasmacytoma in the maxillary sinus. Singapore Med. J. 2008, 49, 310–311. [Google Scholar]
- Attanasio, G.; Viccaro, M.; Barbaro, M.; De Seta, E.; Filipo, R. Extramedullary plasmacytoma of paranasal sinuses. A combined therapeutic strategy. Acta Otorhinolaryngol. Ital. 2006, 26, 118–120. [Google Scholar]
- Shreif, J.A.; Goumas, P.D.; Mastronikolis, N.; Naxakis, S.S. Extramedullary plasmacytoma of the nasal cavity. Otolaryngol. Head Neck Surg. 2001, 124, 119–120. Available online: https://www.sciencedirect.com/science/article/pii/S0194599801701714 (accessed on 16 March 2025). [CrossRef]
- Lomeo, P.E.; McDonald, J.E.; Finneman, J.; Shoreline. Extramedullary plasmacytoma of the nasal sinus cavities. Am. J. Otolaryngol. 2007, 28, 50–51. Available online: https://www.sciencedirect.com/science/article/pii/S0196070905001833 (accessed on 16 March 2025). [CrossRef]
- Erkal, H.; Han, Ü.; Ulubay, H. Extramedullary plasmacytoma presenting as a large lesion protruding from the nasal cavity with massive hemorrhage. J. Neuroradiol. 2006, 33, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Kalayoglu-Besisik, S.; Yonal, I.; Hindilerden, F.; Agan, M.; Sargin, D. Plasmacytoma of the nasolacrimal duct simulating dacryocystitis: An uncommon presentation for extramedullary relapse of multiple myeloma. Case Rep. Oncol. 2012, 5, 119–124. [Google Scholar] [CrossRef]
- Ghazizadeh, M.; Amlashi, H.A.; Mehrparvar, G. Radioresistant Extramedullary Plasmacytoma of the Maxillary Sinus: A Case Report and review article. Iran. J. Otorhinolaryngol. 2015, 27, 313–318. [Google Scholar] [PubMed]
- Araújo, R.d.P.; Gomes, E.F.; Menezes, D.B.d.; Ferreira, L.M.d.B.M.; Rios, A.S.d.N. Rare nasosinusal tumors: Case series and literature review. Braz. J. Otorhinolaryngol. 2008, 74, 307–314. [Google Scholar] [CrossRef]
- Pantazidou, G.; Papaioannou, I.; Karagkouni, E.; Fragkakis, I.; Korovessis, P. Sinonasal Extramedullary Plasmacytoma with Rare Osteolytic Lesions. Cureus 2021, 13, e14220. [Google Scholar] [CrossRef]
- Hazarika, P.; Balakrishnan, R.; Singh, R.; Pujary, K.; Aziz, B. Solitary extramedullary plasmacytoma of the sinonasal region. Indian J. Otolaryngol. Head Neck Surg. 2011, 63 (Suppl. S1), 33–35. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Qi, X.Q.; Wu, X.J.; Luo, C.; Lu, Y.C. Solitary Intracranial Plasmacytoma Located in the Spheno-Clival Region Mimicking Chordoma: A Case Report. J. Int. Med. Res. 2010, 38, 1868–1875. [Google Scholar] [CrossRef]
- Meziane, M.; Boulaadas, M.; Essakalli, L.; Kzadri, M.; Harmouch, A. Solitary plasmocytoma: Ghost tumour? Int. J. Oral Maxillofac. Surg. 2012, 41, 17–19. [Google Scholar] [CrossRef]
- Çakir, E.; Karaarslan, G.; Usul, H.; Baykal, S.; Arslan, E. Solitary plasmacytoma with intracranial intraorbital and, paranasal sinus extension. J. Clin. Neurosci. 2003, 10, 266–268. [Google Scholar] [CrossRef]
- Alexiou, C.; Kau, R.J.; Dietzfelbinger, H.; Kremer, M.; Spiess, J.C.; Schratzenstaller, B.; Arnold, W. Extramedullary plasmacytoma: Tumor occurrence and therapeutic concepts. Cancer 1999, 85, 2305–2314. [Google Scholar] [CrossRef]
- Mcquiston, R.J.; Jones, D.E. Extramedullary Plasmacytoma of the Nasal Cavity and Lateral Wall of the Nose. AMA Arch. Otolaryngol. 1959, 69, 457–458. [Google Scholar] [CrossRef]
- Sodhi, K.S.; Khandelwal, N.; Virmani, V.; Das, A.; Panda, N. Solitary extramedullary plasmacytoma of the nasal tract: An unusual cause of epistaxis. Ear Nose Throat J. 2013, 92, E51. [Google Scholar] [CrossRef]
- Kapadia, S.B.; Desai, U.; Cheng, V.S. Extramedullary plasmacytoma of the head and neck. A clinicopathologic study of 20 cases. Medicine 1982, 61, 317–329. [Google Scholar] [CrossRef]
- Ching, A.S.-C.; Khoo, J.B.-K.; Chong, V.F.-H. CT and MR imaging of solitary extramedullary plasmacytoma of the nasal tract. Am. J. Neuroradiol. 2002, 23, 1632–1636. [Google Scholar] [PubMed]
- Hu, H.; Hu, X.; Hu, G.; Li, D.; Cai, J. Diagnosis and management of extramedullary plasmacytoma in nasal cavity: Clinical experience and literature review. Medicine 2023, 102, e32647. Available online: https://journals.lww.com/md-journal/fulltext/2023/01130/diagnosis_and_management_of_extramedullary.17.aspx (accessed on 16 March 2025). [CrossRef] [PubMed]
- Ooi, G.C.; Chim, J.C.-S.; Au, W.-Y.; Khong, P.-L. Radiologic manifestations of primary solitary extramedullary and multiple solitary plasmacytomas. AJR Am. J. Roentgenol. 2006, 186, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Soutar, R.; Lucraft, H.; Jackson, G.; Reece, A.; Bird, J.; Low, E.; Samson, D. Guidelines on the diagnosis and management of solitary plasmacytoma of bone and solitary extramedullary plasmacytoma. Br. J. Haematol. 2004, 124, 717–726. [Google Scholar] [CrossRef]
- Galieni, P.; Cavo, M.; Pulsoni, A.; Avvisati, G.; Bigazzi, C.; Neri, S.; Caliceti, U.; Benni, M.; Ronconi, S.; Lauria, F. Clinical outcome of extramedullary plasmacytoma. Haematologica 2000, 85, 47–51. [Google Scholar] [PubMed]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.-V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.; Jawad, S.; Kowa, X.-Y. Head and neck manifestations of extramedullary plasmacytomas and their differential diagnoses: A pictorial review. Br. J. Radiol. 2025, 98, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Raghuram, S.; Faizal, B.; Sanjeevan, K.; Eapen, M.; Nair, I.R.; Philip, A.; Pavithran, K. Recurrent extramedullary plasmacytomas without multiple myeloma: A case report with review of the literature. Cancer Treat. Res. Commun. 2022, 31, 100550. [Google Scholar] [CrossRef]
- Hughes, M.; Doig, A.; Soutar, R. Solitary plasmacytoma and multiple myeloma: Adhesion molecule and chemokine receptor expression patterns. Br. J. Haematol. 2007, 137, 486–487. [Google Scholar] [CrossRef]
- Tojima, I.; Ogawa, T.; Kouzaki, H.; Seno, S.; Shibayama, M.; Shimizu, T. Endoscopic resection of malignant sinonasal tumours with or without chemotherapy and radiotherapy. J. Laryngol. Otol. 2012, 126, 1027–1032. [Google Scholar] [CrossRef]
- Rawal, R.B.; Farzal, Z.; Federspiel, J.J.; Sreenath, S.B.; Thorp, B.D.; Zanation, A.M. Endoscopic Resection of Sinonasal Malignancy: A Systematic Review and Meta-analysis. Otolaryngol. Head Neck Surg. 2016, 155, 376–386. [Google Scholar] [CrossRef]
- D’AGuillo, C.; Soni, R.S.; Gordhan, C.; Liu, J.K.; Baredes, S.; Eloy, J.A. Sinonasal extramedullary plasmacytoma: A systematic review of 175 patients. Int. Forum Allergy Rhinol. 2014, 4, 156–163. [Google Scholar] [CrossRef]
- Mishra, U.P.; Verma, A.K.; Chaurasia, J.K. Solitary Extramedullary Plasmacytoma of Nasal Cavity. Indian J. Otolaryngol. Head Neck Surg. 2023, 75, 4060–4065. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, L.; Zhu, Y.; Diao, W.; Li, W.; Gao, Z.; Chen, X. Extramedullary Plasmacytoma: Long-Term Clinical Outcomes in a Single-Center in China and Literature Review. Ear Nose Throat J. 2021, 100, 227–232. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network (NCCN). Clinical Practice Guidelines in Oncology: Multiple Myeloma, 4th ed.; National Comprehensive Cancer Network: Plymouth, PA, USA, 2024. [Google Scholar]
- Tsang, R.W.; Gospodarowicz, M.K.; Pintilie, M.; Bezjak, A.; Wells, W.; Hodgson, D.C.; Stewart, A. Solitary Plasmacytoma Treated with Radiotherapy: Impact of Tumor Size on Outcome. Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Kilciksiz, S.; Karakoyun-Celik, O.; Agaoglu, F.Y.; Haydaroglu, A. A Review for Solitary Plasmacytoma of Bone and Extramedullary Plasmacytoma. Sci. World J. 2012, 2012, 895765. [Google Scholar] [CrossRef] [PubMed]
- Holler, A.; Cicha, I.; Eckstein, M.; Haderlein, M.; Pöttler, M.; Rappl, A.; Iro, H.; Alexiou, C. Extramedullary plasmacytoma: Tumor occurrence and therapeutic concepts–a follow-up. Cancer Med. 2022, 11, 4743–4755. [Google Scholar] [CrossRef]
- Ozsahin, M.; Tsang, R.W.; Poortmans, P.; Belkacemi, Y.; Bolla, M.; Djonov, M.; Dinçbas, F.Ö.; Landmann, C.; Castelain, B.; Buijsen, J.; et al. Outcomes and patterns of failure in solitary plasmacytoma: A multicenter Rare Cancer Network study of 258 patients. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 210–217. [Google Scholar] [CrossRef] [PubMed]


| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Original articles published in English (January 2000 and December 2023) | Articles not published in English |
| Histopathologically confirmed EMP | Non-human or animal studies |
| Human subjects only | Review articles, editorials, or conference abstracts without case-level data |
| Clinical details on diagnosis, treatment, follow-up, and outcomes | No histopathological confirmation of EMP |
| Reports missing clinical information or follow-up information | |
| Studies that grouped sinonasal EMP cases with other head and neck locations |
| Case No./Ref. | Gender/Age | Symptoms | Localization | Treatment | Outcome on Follow-Up | Multiple Myeloma |
|---|---|---|---|---|---|---|
| 1/[1] | M/20 | No symptoms | Nasal cavity | Surgery | No recurrence | No |
| 2/[1] | M/48 | Epistaxis | Nasal cavity | Surgery + RT | No recurrence | No |
| 3/[1] | M/60 | Epistaxis | Nasal cavity | RT | Recurrence | No |
| 4/[2] | M/61 | Epistaxis Facial pain Rhinorrhea | Nasal cavity Maxillary sinus | RT + CHT | Recurrence | No |
| 5/[2] | M/60 | Nasal obstruction Epistaxis Rhinorrhea | Nasal cavity Ethmoid sinus | RT | Recurrence | No |
| 6/[2] | F/37 | Nasal obstruction | Nasal cavity Maxillary sinus | CHT | No recurrence | No |
| 7/[3] | M/75 | Epistaxis | Nasopharynx | RT | No recurrence | No |
| 8/[4] | F/44 | Epistaxis | Nasal cavity Maxillary sinus | Surgery | No recurrence | No |
| 9/[5] | M/32 | Change/loss of vision | Sphenoid sinus | Surgery + RT | No recurrence | No |
| 10/[6] | M/56 | Nasal obstruction Epistaxis Facial pain and swelling | Nasal cavity Maxillary sinus Ethmoid sinus Sphenoid sinus nasopharynx | CHT | Died of the disease | No |
| 11/[7] | F/67 | Nasal obstruction Epistaxis Rhinorrhea | Nasal cavity Ethmoid sinus | Surgery + RT | No recurrence | No |
| 12/[8] | F/75 | Nasal obstruction | Nasal cavity Maxillary sinus | Surgery + RT | No recurrence | No |
| 13/[9] | F/32 | Nasal obstruction Epistaxis Facial pain and swelling Rhinorrhea Chance/loss of vision | Nasal cavity Maxillary sinus Ethmoid sinus Frontal sinus | Surgery + RT | No recurrence | No |
| 14/[10] | F/87 | Epistaxis | Nasal cavity Maxillary sinus, Sphenoid sinus Frontal sinus Nasopharynx | RT | No recurrence | No |
| 15/[11] | F/50 | Epistaxis Rhinorrhea Facial swelling | Nasal cavity Maxillary sinus Ethmoid sinus | Surgery + RT + CHT | - | Yes |
| 16/[12] | M/24 | Nasal obstruction Facial swelling and pain Rhinorrhea proptosis | Maxillary sinus | Surgery + RT | No recurrence | No |
| 17/[13] | F/16 | Nasal obstruction Epistaxis | Nasal cavity Maxillary sinus Ethmoid sinus Sphenoid sinus | Surgery + RT | No recurrence | No |
| 18/[13] | F/26 | Nasal obstruction Facial swelling | Maxillary sinus | Surgery | No recurrence | No |
| 19/[13] | F/52 | Nasal obstruction Epistaxis | Nasal cavity Nasopharynx | Surgery + RT | No recurrence | No |
| 20/[13] | F/15 | Epistaxis Facial swelling | Nasal cavity Maxillary sinus Sphenoid sinus | Surgery + RT + CHT | Died of the disease | No |
| 21/[13] | M/23 | Nasal obstruction epistaxis | Nasal cavity Maxillary sinus Ethmoid sinus | Surgery | No recurrence | No |
| 22/[13] | F/15 | Facial swelling | Maxillary sinus | No treatment | - | - |
| 23/[14] | M/51 | Nasal obstruction Change/loss of vision Headache | Nasal cavity Maxillary sinus | RT + CHT | - | Yes |
| 24/[15] | M/31 | Nasal obstruction Rhinorrhea Facial swelling | Nasal cavity Maxillary sinus, Ethmoid sinus, Sphenoid sinus Nasopharynx | RT | No recurrence | No |
| 25/[15] | M/60 | Nasal obstruction Epistaxis | Nasal cavity Nasopharynx | Surgery + RT | No recurrence | No |
| 26/[16] | F/54 | Change/loss of vision Headache | Sphenoid sinus | Surgery + RT | No recurrence | No |
| 27/[17] | M/42 | Nasal obstruction Headache | Nasal cavity Maxillary sinus | No treatment | - | - |
| 28/[18] | M/49 | Facial swelling and pain proptosis | Maxillary sinus Frontal sinus | Surgery + RT + CHT | No recurrence | No |
| Parameter | Value |
|---|---|
| Total cases | 28 |
| Mean age at diagnosis (±SD) | 45.07 years (±19.70) |
| Sex distribution | 15 males (53.57%), 13 females (46.42%) |
| Site | Cases (%) |
|---|---|
| Nasal cavity and paranasal sinuses | 22 (78.57%) |
| -Localized only in nasal cavity | 3 (13.63%) |
| -With extension beyond nasal cavity | 19 (86.37%) |
| Paranasal sinus involvement | 22 (78.57%) |
| -Localized only in sinuses | 5–3 maxillary, 2 sphenoid (22.72%) |
| Nasopharyngeal involvement | 7 (25%) |
| -Localized only in nasopharynx | 2 (28.57%) |
| -With extension beyond nasopharynx | 5 (71.42%) |
| Treatment | Cases (%) |
|---|---|
| Surgery + Radiotherapy | 10 (35.71%) |
| Radiotherapy alone | 5 (17.86%) |
| Surgery alone | 4 (14.29%) |
| Radiotherapy + Chemotherapy | 3 (10.71%) |
| Surgery + Radiotherapy + Chemotherapy | 3 (10.71%) |
| Chemotherapy alone | 1 (3.57%) |
| No treatment | 2 (7.14%) |
| Outcome | Cases (%) |
|---|---|
| Disease-free, no recurrence | 19 (67.89%) |
| Relapse | 3 (10.71%) |
| Deaths | 2 (7.14%) |
| No available data | 4–2 MM, 2 refused treatment (14.28%) |
| Parameter | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| Age/Sex | 43 M | 79 F | 47 M |
| Symptoms | Bilateral nasal obstruction, epistaxis (3 months) | Left nasal obstruction, epistaxis (2 years) | Right nasal obstruction (1 + year) |
| Clinical and Endoscopic Findings | Infiltrating, vegetative mass in the left nasal fossa and nasopharynx | Sessile, reddish mass, in the left nasal fossa | Sessile mass in the right nasal cavity |
| Imaging | No bone damage | No bone invasion | No bone lesion |
| Surgery | En bloc resection, endoscopic | En bloc resection, endoscopic | En bloc resection, endoscopic |
| Histopathology | Plasmacytoid cells, hemorrhage | Plasmacytoid cells, necrosis | Compact eosinophilic cells |
| Immunohistochemistry | CD38+, CD138+ | CD79a+, CD138+, CD56+ | CD138+ |
| Radiation | - | 40 Gy/3 weeks | 44 Gy/1 month |
| Follow-up | 1 year, no recurrence | 2 years, no recurrence | 18 months, no recurrence |
| Multiple Myeloma | Negative | Negative | Negative |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mogoantă, C.A.; Sarafoleanu, C.; Osman, A.; Enache, I.; Tarabichi, S.; Busuioc, C.-I.; Liliac, I.M.; Iovanescu, D.; Tănase, I. Extramedullary Plasmacytomas of the Nasal Cavity: Case-Based Perspectives into Optimizing the Diagnostic Differentiation from Inflammatory Polyps. Medicina 2025, 61, 1406. https://doi.org/10.3390/medicina61081406
Mogoantă CA, Sarafoleanu C, Osman A, Enache I, Tarabichi S, Busuioc C-I, Liliac IM, Iovanescu D, Tănase I. Extramedullary Plasmacytomas of the Nasal Cavity: Case-Based Perspectives into Optimizing the Diagnostic Differentiation from Inflammatory Polyps. Medicina. 2025; 61(8):1406. https://doi.org/10.3390/medicina61081406
Chicago/Turabian StyleMogoantă, Carmen Aurelia, Codruț Sarafoleanu, Andrei Osman, Irina Enache, Shirley Tarabichi, Constantin-Ioan Busuioc, Ilona Mihaela Liliac, Dan Iovanescu, and Ionuţ Tănase. 2025. "Extramedullary Plasmacytomas of the Nasal Cavity: Case-Based Perspectives into Optimizing the Diagnostic Differentiation from Inflammatory Polyps" Medicina 61, no. 8: 1406. https://doi.org/10.3390/medicina61081406
APA StyleMogoantă, C. A., Sarafoleanu, C., Osman, A., Enache, I., Tarabichi, S., Busuioc, C.-I., Liliac, I. M., Iovanescu, D., & Tănase, I. (2025). Extramedullary Plasmacytomas of the Nasal Cavity: Case-Based Perspectives into Optimizing the Diagnostic Differentiation from Inflammatory Polyps. Medicina, 61(8), 1406. https://doi.org/10.3390/medicina61081406






