Multidisciplinary Postoperative Ileus Management: A Narrative Review
Abstract
1. Introduction
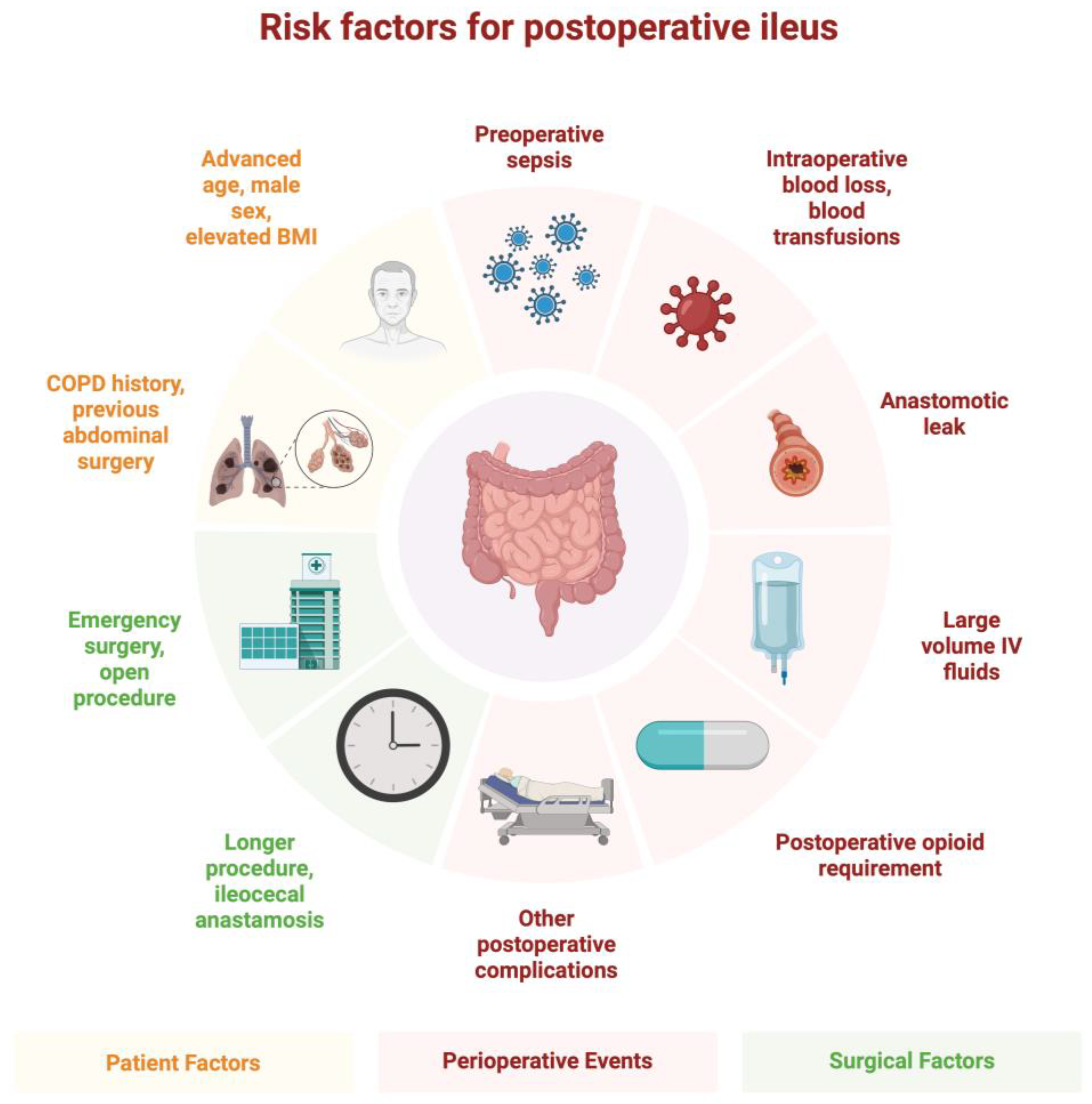
2. Epidemiology
3. Pathophysiology
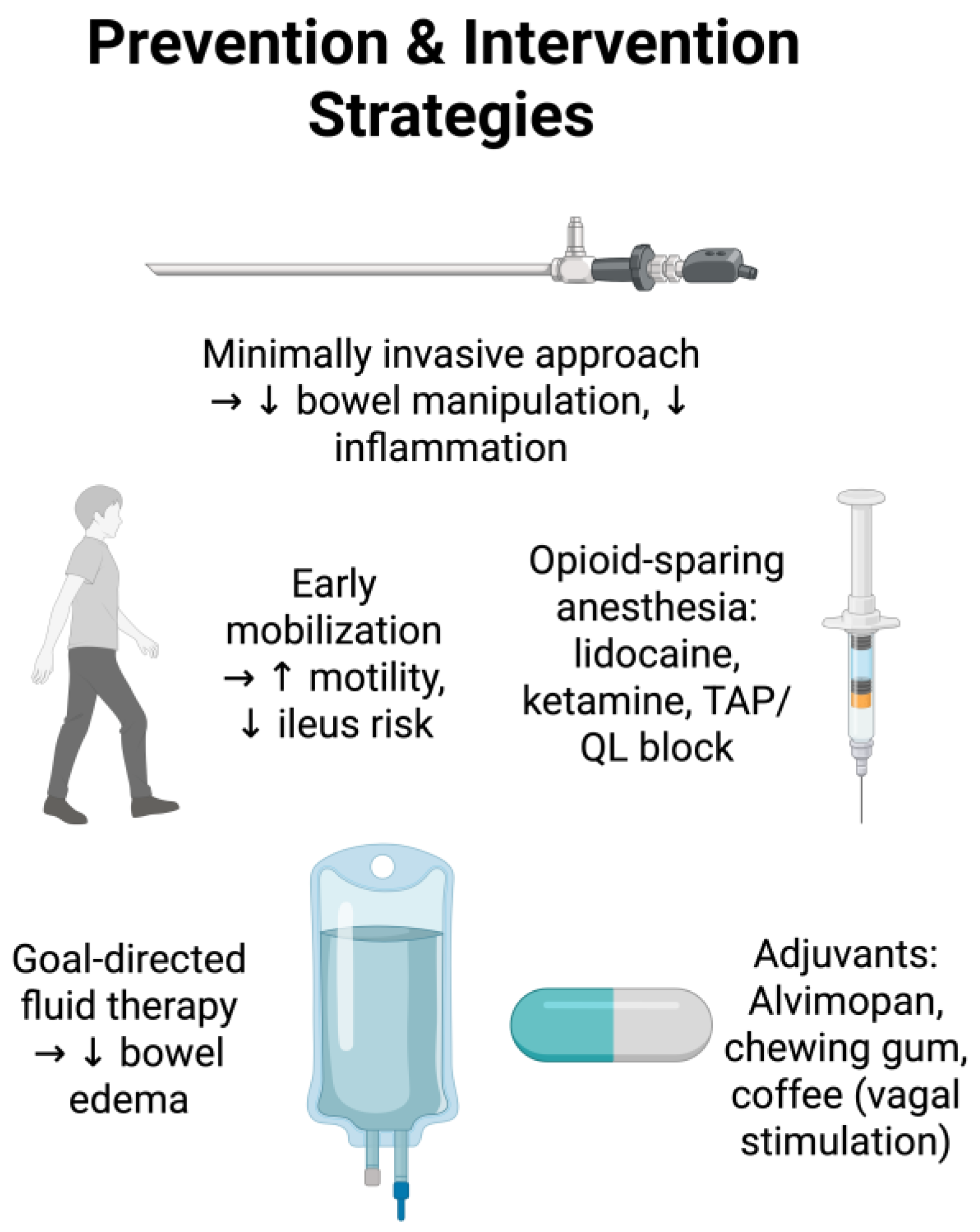
4. Preventative Strategies
4.1. Surgical Planning
4.2. Surgical Approaches
4.3. Intraoperative Opioid-Sparing or Opioid-Free Anesthesia
4.4. Regional Anesthesia and Postoperative Ileus
4.5. Intraoperative Fluid Management
5. Postoperative Considerations
5.1. Postoperative Analgesia
5.2. Alvimopan and Postoperative Ileus
5.3. Acupuncture and Transcutaneous Electrical Nerve Stimulation
5.4. Chewing Gum and Postoperative Ileus
5.5. Coffee and Postoperative Ileus
6. Treatment of Postoperative Ileus
6.1. Prokinetic Agents
6.2. Non-Steroidal Anti-Inflammatory Drugs
6.3. Propranolol and Postoperative Ileus
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CNS | Central nervous system |
| COX | Cyclooxygenase |
| CRF | Corticotropin-releasing factor |
| DAMPs | Damage-associated molecular patterns |
| ENS | Enteric nervous system |
| ERAS | Enhanced recovery after surgery |
| IL | Interleukin |
| IPANs | Intrinsic primary afferent neurons |
| NO | Nitric oxide |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| OR | Odds ratio |
| POI | Postoperative ileus |
| QL | Quadratus lumborum (block) |
| TAP | Transverse abdominis plane (block) |
| TNF | Tumor necrosis factor |
References
- Quiroga-Centeno, A.C.; Jerez-Torra, K.A.; Martin-Mojica, P.A.; Castaneda-Alfonso, S.A.; Castillo-Sanchez, M.E.; Calvo-Corredor, O.F.; Gomez-Ochoa, S.A. Risk Factors for Prolonged Postoperative Ileus in Colorectal Surgery: A Systematic Review and Meta-analysis. World J. Surg. 2020, 44, 1612–1626. [Google Scholar] [CrossRef] [PubMed]
- Wolthuis, A.M.; Bislenghi, G.; Fieuws, S.; de Buck van Overstraeten, A.; Boeckxstaens, G.; D’Hoore, A. Incidence of prolonged postoperative ileus after colorectal surgery: A systematic review and meta-analysis. Color. Dis. 2016, 18, O1–O9. [Google Scholar] [CrossRef] [PubMed]
- Venara, A.; Neunlist, M.; Slim, K.; Barbieux, J.; Colas, P.A.; Hamy, A.; Meurette, G. Postoperative ileus: Pathophysiology, incidence, and prevention. J. Visc. Surg. 2016, 153, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Traeger, L.; Koullouros, M.; Bedrikovetski, S.; Kroon, H.M.; Thomas, M.L.; Moore, J.W.; Sammour, T. Cost of postoperative ileus following colorectal surgery: A cost analysis in the Australian public hospital setting. Color. Dis. 2022, 24, 1416–1426. [Google Scholar] [CrossRef] [PubMed]
- Traeger, L.; Koullouros, M.; Bedrikovetski, S.; Kroon, H.M.; Moore, J.W.; Sammour, T. Global cost of postoperative ileus following abdominal surgery: Meta-analysis. BJS Open 2023, 7, zrad054. [Google Scholar] [CrossRef] [PubMed]
- Harnsberger, C.R.; Maykel, J.A.; Alavi, K. Postoperative Ileus. Clin. Colon Rectal Surg. 2019, 32, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Takahashi, H.; Fujii, M.; Miyoshi, N.; Uemura, M.; Matsuda, C.; Yamamoto, H.; Mizushima, T.; Mori, M.; Doki, Y. Visceral obesity is a preoperative risk factor for postoperative ileus after surgery for colorectal cancer: Single-institution retrospective analysis. Ann. Gastroenterol. Surg. 2019, 3, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Moschen, A.R.; Molnar, C.; Enrich, B.; Geiger, S.; Ebenbichler, C.F.; Tilg, H. Adipose and liver expression of interleukin (IL)-1 family members in morbid obesity and effects of weight loss. Mol. Med. 2011, 17, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Asgeirsson, T.; El-Badawi, K.I.; Mahmood, A.; Barletta, J.; Luchtefeld, M.; Senagore, A.J. Postoperative ileus: It costs more than you expect. J. Am. Coll. Surg. 2010, 210, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Vather, R.; O’Grady, G.; Bissett, I.P.; Dinning, P.G. Postoperative ileus: Mechanisms and future directions for research. Clin. Exp. Pharmacol. Physiol. 2014, 41, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Buscail, E.; Deraison, C. Postoperative ileus: A pharmacological perspective. Br. J. Pharmacol. 2022, 179, 3283–3305. [Google Scholar] [CrossRef] [PubMed]
- Gershon, M.D.; Margolis, K.G. The gut, its microbiome, and the brain: Connections and communications. J. Clin. Investig. 2021, 131, e143768. [Google Scholar] [CrossRef] [PubMed]
- Boeckxstaens, G.E.; de Jonge, W.J. Neuroimmune mechanisms in postoperative ileus. Gut 2009, 58, 1300–1311. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.J.; Boeckxstaens, G.E. Mechanisms of postoperative ileus. Neurogastroenterol. Motil. 2004, 16 (Suppl. 2), 54–60. [Google Scholar] [CrossRef] [PubMed]
- Browning, K.N.; Travagli, R.A. Central nervous system control of gastrointestinal motility and secretion and modulation of gastrointestinal functions. Compr. Physiol. 2014, 4, 1339–1368. [Google Scholar] [CrossRef] [PubMed]
- Wehner, S.; Vilz, T.O.; Stoffels, B.; Kalff, J.C. Immune mediators of postoperative ileus. Langenbecks Arch. Surg. 2012, 397, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Park, H. Inflammation and Impaired Gut Physiology in Post-operative Ileus: Mechanisms and the Treatment Options. J. Neurogastroenterol. Motil. 2022, 28, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Vilz, T.O.; Roessel, L.; Chang, J.; Pantelis, D.; Schwandt, T.; Koscielny, A.; Wehner, S.; Kalff, J.C. Establishing a biomarker for postoperative ileus in humans—Results of the BiPOI trial. Life Sci. 2015, 143, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, N.T.; Kalff, J.C.; Türler, A.; Engel, B.M.; Watkins, S.C.; Billiar, T.R.; Bauer, A.J. Prostanoid production via COX-2 as a causative mechanism of rodent postoperative ileus. Gastroenterology 2001, 121, 1354–1371. [Google Scholar] [CrossRef] [PubMed]
- van den Heijkant, T.C.; Costes, L.M.M.; van der Lee, D.G.C.; Aerts, B.; Osinga-de Jong, M.; Rutten, H.R.M.; Hulsewé, K.W.E.; de Jonge, W.J.; Buurman, W.A.; Luyer, M.D.P. Randomized clinical trial of the effect of gum chewing on postoperative ileus and inflammation in colorectal surgery. Br. J. Surg. 2015, 102, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Sobczak, M.; Salaga, M.; Storr, M.A.; Fichna, J. Physiology, signaling, and pharmacology of opioid receptors and their ligands in the gastrointestinal tract: Current concepts and future perspectives. J. Gastroenterol. 2014, 49, 24–45. [Google Scholar] [CrossRef] [PubMed]
- Leppert, W. Emerging therapies for patients with symptoms of opioid-induced bowel dysfunction. Drug Des. Dev. Ther. 2015, 9, 2215–2231. [Google Scholar] [CrossRef] [PubMed]
- Oliva, V.; Lippi, M.; Paci, R.; Del Fabro, L.; Delvecchio, G.; Brambilla, P.; De Ronchi, D.; Fanelli, G.; Serretti, A. Gastrointestinal side effects associated with antidepressant treatments in patients with major depressive disorder: A systematic review and meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 109, 110266. [Google Scholar] [CrossRef] [PubMed]
- Bouras, E.P.; Talley, N.J.; Camilleri, M.; Burton, D.D.; Heckman, M.G.; Crook, J.E.; Richelson, E. Effects of amitriptyline on gastric sensorimotor function and postprandial symptoms in healthy individuals: A randomized, double-blind, placebo-controlled trial. Am. J. Gastroenterol. 2008, 103, 2043–2050. [Google Scholar] [CrossRef] [PubMed]
- Haj Kheder, S.; Heller, J.; Bär, J.K.; Wutzler, A.; Menge, B.A.; Juckel, G. Autonomic dysfunction of gastric motility in major depression. J. Affect. Disord. 2018, 226, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Stakenborg, N.; Gomez-Pinilla, P.J.; Boeckxstaens, G.E. Postoperative Ileus: Pathophysiology, Current Therapeutic Approaches. Handb. Exp. Pharmacol. 2017, 239, 39–57. [Google Scholar] [CrossRef] [PubMed]
- Ljungqvist, O.; Scott, M.; Fearon, K.C. Enhanced Recovery After Surgery: A Review. JAMA Surg. 2017, 152, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Tazreean, R.; Nelson, G.; Twomey, R. Early mobilization in enhanced recovery after surgery pathways: Current evidence and recent advancements. J. Comp. Eff. Res. 2022, 11, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.Y.; Wang, Z.H.; Huang, Z.P.; Zhou, H.; Fu, L.J.; Cai, H.; Huang, X.X.; Yang, Y.; Li, H.F.; Zhou, W.P. Early enforced mobilization after liver resection: A prospective randomized controlled trial. Int. J. Surg. 2018, 54, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Nygren, J.; Thacker, J.; Carli, F.; Fearon, K.C.; Norderval, S.; Lobo, D.N.; Ljungqvist, O.; Soop, M.; Ramirez, J.; Enhanced Recovery After Surgery (ERAS) Society, for Perioperative Care; et al. Guidelines for perioperative care in elective rectal/pelvic surgery: Enhanced Recovery After Surgery (ERAS((R))) Society recommendations. World J. Surg. 2013, 37, 285–305. [Google Scholar] [CrossRef] [PubMed]
- Artinyan, A.; Nunoo-Mensah, J.W.; Balasubramaniam, S.; Gauderman, J.; Essani, R.; Gonzalez-Ruiz, C.; Kaiser, A.M.; Beart, R.W., Jr. Prolonged postoperative ileus-definition, risk factors, and predictors after surgery. World J. Surg. 2008, 32, 1495–1500. [Google Scholar] [CrossRef] [PubMed]
- Sui, C.; Wang, B.; Zhao, Y.; Guo, Y.; Zhu, J.; Yu, F.; Zhou, X.; Bu, X.; Zhang, J. Establishment of an inflammatory cytokine-based predictive model for the onset of prolonged postoperative ileus after radical gastrectomy: A prospective cohort study. Front. Immunol. 2025, 16, 1552944. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.Q.; Chen, Y.; Fu, X.A.; Yin, H.T.; Li, J.S.; Wang, W.S.; Yuan, J.Q.; Guo, S.G. Nomogram for predicting prolonged postoperative ileus in colorectal cancer based on age and inflammatory markers. Biomark. Med. 2023, 17, 921–933. [Google Scholar] [CrossRef] [PubMed]
- Penfold, J.A.; Wells, C.I.; Du, P.; Qian, A.; Vather, R.; Bissett, I.P.; O’Grady, G. Relationships between serum electrolyte concentrations and ileus: A joint clinical and mathematical modeling study. Physiol. Rep. 2021, 9, e14735. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.Q.; Zhang, K.C.; Li, H.; Cui, J.X.; Xi, H.Q.; Li, J.Y.; Cai, A.Z.; Liu, Y.H.; Zhang, W.; Zhang, L.; et al. Preoperative albumin levels predict prolonged postoperative ileus in gastrointestinal surgery. World J. Gastroenterol. 2020, 26, 1185–1196. [Google Scholar] [CrossRef] [PubMed]
- The, F.O.; Bennink, R.J.; Ankum, W.M.; Buist, M.R.; Busch, O.R.; Gouma, D.J.; van der Heide, S.; van den Wijngaard, R.M.; de Jonge, W.J.; Boeckxstaens, G.E. Intestinal handling-induced mast cell activation and inflammation in human postoperative ileus. Gut 2008, 57, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Gervaz, P.; Inan, I.; Perneger, T.; Schiffer, E.; Morel, P. A prospective, randomized, single-blind comparison of laparoscopic versus open sigmoid colectomy for diverticulitis. Ann. Surg. 2010, 252, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Tamagawa, H.; Aoyama, T.; Numata, M.; Kazama, K.; Maezawa, Y.; Atsumi, Y.; Hara, K.; Kawahara, S.; Kano, K.; Yukawa, N.; et al. A Comparison of Open and Laparoscopic-assisted Colectomy for Obstructive Colon Cancer. In Vivo 2020, 34, 2797–2801. [Google Scholar] [CrossRef] [PubMed]
- Pitiakoudis, M.; Fotakis, S.N.; Zezos, P.; Kouklakis, G.; Michailidis, L.; Romanidis, K.; Vafiadis, K.; Simopoulos, K. Alterations in colonic transit time after laparoscopic versus open cholecystectomy: A clinical study. Tech. Coloproctol. 2011, 15 (Suppl. 1), S37–S41. [Google Scholar] [CrossRef] [PubMed]
- Twine, C.P.; Humphreys, A.K.; Williams, I.M. Systematic review and meta-analysis of the retroperitoneal versus the transperitoneal approach to the abdominal aorta. Eur. J. Vasc. Endovasc. Surg. 2013, 46, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, P.; Bordeianou, L. Implementation of an ERAS Pathway in Colorectal Surgery. Clin. Colon Rectal Surg. 2019, 32, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, N.J.; Stulc, J.P.; Rodriguez-Bigas, M.; Blumenson, L. Nasogastric decompression following elective colorectal surgery: A prospective randomized study. Am. Surg. 1993, 59, 632–635. [Google Scholar] [PubMed]
- Denost, Q.; Rouanet, P.; Faucheron, J.L.; Panis, Y.; Meunier, B.; Cotte, E.; Meurette, G.; Kirzin, S.; Sabbagh, C.; Loriau, J.; et al. To Drain or Not to Drain Infraperitoneal Anastomosis After Rectal Excision for Cancer: The GRECCAR 5 Randomized Trial. Ann. Surg. 2017, 265, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Kehlet, H.; Mogensen, T. Hospital stay of 2 days after open sigmoidectomy with a multimodal rehabilitation programme. Br. J. Surg. 1999, 86, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Cooke, C.; Kennedy, E.D.; Foo, I.; Nimmo, S.; Speake, D.; Paterson, H.M.; Ventham, N.T. Meta-analysis of the effect of perioperative intravenous lidocaine on return of gastrointestinal function after colorectal surgery. Tech. Coloproctol. 2019, 23, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Wu, Y.; Zhou, C. Effect of intravenous ketamine for postoperative analgesia in patients undergoing laparoscopic cholecystectomy: A meta-analysis. Medicine 2017, 96, e9147. [Google Scholar] [CrossRef] [PubMed]
- Shariat Moharari, R.; Motalebi, M.; Najafi, A.; Zamani, M.M.; Imani, F.; Etezadi, F.; Pourfakhr, P.; Khajavi, M.R. Magnesium Can Decrease Postoperative Physiological Ileus and Postoperative Pain in Major non Laparoscopic Gastrointestinal Surgeries: A Randomized Controlled Trial. Anesthesiol. Pain Med. 2014, 4, e12750. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.L.; King, A.B.; Geiger, T.M.; Grant, M.C.; Grocott, M.P.W.; Gupta, R.; Hah, J.M.; Miller, T.E.; Shaw, A.D.; Gan, T.J.; et al. American Society for Enhanced Recovery and Perioperative Quality Initiative Joint Consensus Statement on Perioperative Opioid Minimization in Opioid-Naive Patients. Anesthesiol. Analg. 2019, 129, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Beloeil, H.; Garot, M.; Lebuffe, G.; Gerbaud, A.; Bila, J.; Cuvillon, P.; Dubout, E.; Oger, S.; Nadaud, J.; Becret, A.; et al. Balanced Opioid-free Anesthesia with Dexmedetomidine versus Balanced Anesthesia with Remifentanil for Major or Intermediate Noncardiac Surgery. Anesthesiology 2021, 134, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Rekatsina, M.; Theodosopoulou, P.; Staikou, C. Effects of Intravenous Dexmedetomidine Versus Lidocaine on Postoperative Pain, Analgesic Consumption and Functional Recovery After Abdominal Gynecological Surgery: A Randomized Placebo-controlled Double Blind Study. Pain Physician 2021, 24, e997–e1006. [Google Scholar] [PubMed]
- Chen, J.; Luo, Q.; Huang, S.; Jiao, J. Effect of opioid-free anesthesia on postoperative analgesia after laparoscopic gynecologic surgery. Minerva Anestesiol. 2022, 88, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Tochie, J.N.; Bengono Bengono, R.S.; Metogo, J.M.; Ndikontar, R.; Ngouatna, S.; Ntock, F.N.; Minkande, J.Z. The efficacy and safety of an adapted opioid-free anesthesia regimen versus conventional general anesthesia in gynecological surgery for low-resource settings: A randomized pilot study. BMC Anesthesiol. 2022, 22, 325. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, H.Y.; Yuan, Z.Y.; Han, Y.; Chen, Y.R.; Liu, Q.L.; Zhu, T. Analgesic efficacy of postoperative bilateral, ultrasound-guided, posterior transversus abdominis plane block for laparoscopic colorectal cancer surgery: A randomized, prospective, controlled study. BMC Anesthesiol. 2021, 21, 107. [Google Scholar] [CrossRef] [PubMed]
- Ris, F.; Findlay, J.M.; Hompes, R.; Rashid, A.; Warwick, J.; Cunningham, C.; Jones, O.; Crabtree, N.; Lindsey, I. Addition of transversus abdominis plane block to patient controlled analgesia for laparoscopic high anterior resection improves analgesia, reduces opioid requirement and expedites recovery of bowel function. Ann. R. Coll. Surg. Engl. 2014, 96, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.J.; Sun, X.; Jiang, H.; Yin, Y.H.; Weng, M.L.; Sun, Z.R.; Chen, W.K.; Miao, C.H. Randomized clinical trial of continuous transversus abdominis plane block, epidural or patient-controlled analgesia for patients undergoing laparoscopic colorectal cancer surgery. Br. J. Surg. 2020, 107, e133–e141. [Google Scholar] [CrossRef] [PubMed]
- Emile, S.H.; Elfeki, H.; Elbahrawy, K.; Sakr, A.; Shalaby, M. Ultrasound-guided versus laparoscopic-guided subcostal transversus abdominis plane (TAP) block versus No TAP block in laparoscopic cholecystectomy; a randomized double-blind controlled trial. Int. J. Surg. 2022, 101, 106639. [Google Scholar] [CrossRef] [PubMed]
- Peltrini, R.; Cantoni, V.; Green, R.; Greco, P.A.; Calabria, M.; Bucci, L.; Corcione, F. Efficacy of transversus abdominis plane (TAP) block in colorectal surgery: A systematic review and meta-analysis. Tech. Coloproctol. 2020, 24, 787–802. [Google Scholar] [CrossRef] [PubMed]
- Bernard, L.; Lavecchia, M.; Trepanier, G.; Mah, S.; Pokoradi, A.; McGinnis, J.M.; Alyafi, M.; Glezerson, B.; Nguyen, J.; Carlson, V.; et al. A double-blinded, randomized trial comparing surgeon-administered transversus abdominis plane block with placebo after midline laparotomy in gynecologic oncology surgery. Am. J. Obstet. Gynecol. 2023, 228, 553.e1–553.e8. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Lu, S.; Cui, X.; Zhang, Y.; Xie, Y.; Zhang, Y.; Yan, W.; Ji, Z.; Huang, Y. Transmuscular quadratus lumborum block for postoperative pain and recovery after laparoscopic adrenalectomy: A randomized controlled trial. BMC Anesthesiol. 2021, 21, 274. [Google Scholar] [CrossRef] [PubMed]
- Nie, B.Q.; Niu, L.X.; Yang, E.; Yao, S.L.; Yang, L. Effect of Subcostal Anterior Quadratus Lumborum Block vs. Oblique Subcostal Transversus Abdominis Plane Block after Laparoscopic Radical Gastrectomy. Curr. Med. Sci. 2021, 41, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Jiao, R.; Peng, S.; Wang, L.; Feng, M.; Li, Y.; Sun, J.; Liu, D.; Fu, J.; Feng, C. Ultrasound-Guided Quadratus Lumborum Block Combined with General Anaesthesia or General Anaesthesia Alone for Laparoscopic Radical Gastrectomy for Gastric Adenocarcinoma: A Monocentric Retrospective Study. Int. J. Gen. Med. 2022, 15, 7739–7750. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Li, L.; Yang, Z.; Shen, J.; Zhu, R.; Wen, Y.; Cai, W.; Liu, L. Ultrasound guided continuous Quadratus Lumborum block hastened recovery in patients undergoing open liver resection: A randomized controlled, open-label trial. BMC Anesthesiol. 2019, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- Hamid, H.K.S.; Marc-Hernández, A.; Saber, A.A. Transversus abdominis plane block versus thoracic epidural analgesia in colorectal surgery: A systematic review and meta-analysis. Langenbecks Arch. Surg. 2021, 406, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Lee, S.; Kim, G.S.; Jeong, J.S.; Gwak, M.S.; Kim, J.M.; Choi, G.S.; Cho, Y.J.; Ko, J.S. Comparison of Analgesic Efficacy of Erector Spinae Plane Block and Posterior Quadratus Lumborum Block in Laparoscopic Liver Resection: A Randomized Controlled Trial. J. Pain Res. 2021, 14, 3791–3800. [Google Scholar] [CrossRef] [PubMed]
- She, H.; Jiang, P.; Zhu, J.; Zhou, Y.; Wang, Y.; Kan, M.; Wu, J. Comparison of the analgesic effect of quadratus lumborum block and epidural block in open uterine surgery: A randomized controlled trial. Minerva Anestesiol. 2021, 87, 414–422. [Google Scholar] [CrossRef] [PubMed]
- VandeHei, M.S.; Papageorge, C.M.; Murphy, M.M.; Kennedy, G.D. The effect of perioperative fluid management on postoperative ileus in rectal cancer patients. Surgery 2017, 161, 1628–1632. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.S.; Noh, T.I.; Ku, J.H.; Lee, S.; Kwon, T.G.; Kim, T.H.; Jeon, S.H.; Lee, S.H.; Nam, J.K.; Kim, W.S.; et al. Effect of intraoperative fluid volume on postoperative ileus after robot-assisted radical cystectomy. Sci. Rep. 2021, 11, 10522. [Google Scholar] [CrossRef] [PubMed]
- Koch, K.E.; Hahn, A.; Hart, A.; Kahl, A.; Charlton, M.; Kapadia, M.R.; Hrabe, J.E.; Cromwell, J.W.; Hassan, I.; Gribovskaja-Rupp, I. Male sex, ostomy, infection, and intravenous fluids are associated with increased risk of postoperative ileus in elective colorectal surgery. Surgery 2021, 170, 1325–1330. [Google Scholar] [CrossRef] [PubMed]
- Thacker, J.K.; Mountford, W.K.; Ernst, F.R.; Krukas, M.R.; Mythen, M.M. Perioperative Fluid Utilization Variability and Association with Outcomes: Considerations for Enhanced Recovery Efforts in Sample US Surgical Populations. Ann. Surg. 2016, 263, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Ghodraty, M.R.; Rokhtabnak, F.; Dehghan, H.R.; Pournajafian, A.; Baghaee Vaji, M.; Koleini, Z.S.; Porhomayon, J.; Nader, N.D. Crystalloid versus colloid fluids for reduction of postoperative ileus after abdominal operation under combined general and epidural anesthesia. Surgery 2017, 162, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Cvetkovic, A.; Kalezic, N.; Milicic, B.; Nikolic, S.; Zegarac, M.; Gavrilovic, D.; Stojiljkovic, D. The impact of different infusion solutions on postoperative recovery following colorectal surgery. J. BUON 2018, 23, 1369–1379. [Google Scholar]
- Chong, M.A.; Wang, Y.; Berbenetz, N.M.; McConachie, I. Does goal-directed haemodynamic and fluid therapy improve peri-operative outcomes?: A systematic review and meta-analysis. Eur. J. Anaesthesiol. 2018, 35, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chai, F.; Pan, C.; Romeiser, J.L.; Gan, T.J. Effect of perioperative goal-directed hemodynamic therapy on postoperative recovery following major abdominal surgery-a systematic review and meta-analysis of randomized controlled trials. Crit. Care 2017, 21, 141. [Google Scholar] [CrossRef] [PubMed]
- Jessen, M.K.; Vallentin, M.F.; Holmberg, M.J.; Bolther, M.; Hansen, F.B.; Holst, J.M.; Magnussen, A.; Hansen, N.S.; Johannsen, C.M.; Enevoldsen, J.; et al. Goal-directed haemodynamic therapy during general anaesthesia for noncardiac surgery: A systematic review and meta-analysis. Br. J. Anaesth. 2022, 128, 416–433. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.J.; Robinson, S.B.; Oderda, G.M.; Scranton, R.; Pepin, J.; Ramamoorthy, S. Impact of postsurgical opioid use and ileus on economic outcomes in gastrointestinal surgeries. Curr. Med. Res. Opin. 2015, 31, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Aryaie, A.H.; Lalezari, S.; Sergent, W.K.; Puckett, Y.; Juergens, C.; Ratermann, C.; Ogg, C. Decreased opioid consumption and enhance recovery with the addition of IV Acetaminophen in colorectal patients: A prospective, multi-institutional, randomized, double-blinded, placebo-controlled study (DOCIVA study). Surg. Endosc. 2018, 32, 3432–3438. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Wu, G.J.; Mok, M.S.; Chou, Y.H.; Sun, W.Z.; Chen, P.L.; Chan, W.S.; Yien, H.W.; Wen, Y.R. Effect of adding ketorolac to intravenous morphine patient-controlled analgesia on bowel function in colorectal surgery patients--a prospective, randomized, double-blind study. Acta Anaesthesiol. Scand. 2005, 49, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Bakkum-Gamez, J.N.; Langstraat, C.L.; Martin, J.R.; Lemens, M.A.; Weaver, A.L.; Allensworth, S.; Dowdy, S.C.; Cliby, W.A.; Gostout, B.S.; Podratz, K.C. Incidence of and risk factors for postoperative ileus in women undergoing primary staging and debulking for epithelial ovarian carcinoma. Gynecol. Oncol. 2012, 125, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, J.M.; Chen, R.; Messer, K.; Veerapong, J.; Kelly, K.J.; Ramamoorthy, S.; Lowy, A.M. Alvimopan for Enhanced Gastrointestinal Recovery after Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: A Randomized Controlled Trial. J. Am. Coll. Surg. 2022, 235, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Chen, H.; Qi, L.; Zu, X.; Li, Y. Effect of alvimopan on accelerates gastrointestinal recovery after radical cystectomy: A systematic review and meta-analysis. Int. J. Surg. 2016, 25, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Highlights of Prescribing Information, Entereg (Alvimopan) Capsules, for Oral Use. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021775s010lbl.pdf (accessed on 4 May 2025).
- Ru, O.; Jin, X.; Qu, L.; Long, D.; Liu, M.; Cheng, L.; Jiang, Y. Low-intensity transcutaneous auricular vagus nerve stimulation reduces postoperative ileus after laparoscopic radical resection of colorectal cancer: A randomized controlled trial. Minerva Anestesiol. 2023, 89, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Li, W.; Yan, Y.; Yang, R.; Zhang, Y.; Jin, M.; Luo, Z.; Xie, L.; Ma, Y.; Xu, X.; et al. Transcutaneous electrical acupoint stimulation applied in lower limbs decreases the incidence of paralytic ileus after colorectal surgery: A multicenter randomized controlled trial. Surgery 2021, 170, 1618–1626. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Gao, C.; An, L.X.; Ji, Y.W.; Xue, F.S.; Du, Y. Perioperative transcutaneous electrical acupoint stimulation for improving postoperative gastrointestinal function: A randomized controlled trial. J. Integr. Med. 2021, 19, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Venara, A.; Bougard, M., Jr.; Mucci, S.; Lemoult, A., Jr.; Le Naoures, P.; Darsonval, A.; Barbieux, J.; Neunlist, M.; Hamy, A.P. Perioperative Transcutaneous Tibial Nerve Stimulation to Reduce Postoperative Ileus After Colorectal Resection: A Pilot Study. Dis. Colon Rectum 2018, 61, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- Short, V.; Herbert, G.; Perry, R.; Atkinson, C.; Ness, A.R.; Penfold, C.; Thomas, S.; Andersen, H.K.; Lewis, S.J. Chewing gum for postoperative recovery of gastrointestinal function. Cochrane Database Syst. Rev. 2015, 2015, CD006506. [Google Scholar] [CrossRef] [PubMed]
- Su’a, B.U.; Pollock, T.T.; Lemanu, D.P.; MacCormick, A.D.; Connolly, A.B.; Hill, A.G. Chewing gum and postoperative ileus in adults: A systematic literature review and meta-analysis. Int. J. Surg. 2015, 14, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Su’a, B.U.; Hill, A.G. Perioperative use of chewing gum affects the inflammatory response and reduces postoperative ileus following major colorectal surgery. Evid. Based Med. 2015, 20, 185–186. [Google Scholar] [CrossRef] [PubMed]
- Hasler-Gehrer, S.; Linecker, M.; Keerl, A.; Slieker, J.; Descloux, A.; Rosenberg, R.; Seifert, B.; Nocito, A. Does Coffee Intake Reduce Postoperative Ileus After Laparoscopic Elective Colorectal Surgery? A Prospective, Randomized Controlled Study: The Coffee Study. Dis. Colon Rectum 2019, 62, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, J.; Miki, A.; Koizumi, M.; Kotani, K.; Sata, N. Effect of Postoperative Coffee Consumption on Postoperative Ileus after Abdominal Surgery: An Updated Systematic Review and Meta-Analysis. Nutrients 2021, 13, 4394. [Google Scholar] [CrossRef] [PubMed]
- Gkegkes, I.D.; Minis, E.E.; Iavazzo, C. Effect of Caffeine Intake on Postoperative Ileus: A Systematic Review and Meta-Analysis. Dig. Surg. 2020, 37, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, A.V.; Nasi, M.; Farinetti, A.; Gelmini, R. Effects of Caffeine on Colon: A Potential Clinical Use of Coffee in Surgical Patients. Dig. Surg. 2020, 37, 265–266. [Google Scholar] [CrossRef] [PubMed]
- Isola, S.; Hussain, A.; Dua, A.; Singh, K.; Adams, N. Metoclopramide. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Stewart, D.; Waxman, K. Management of postoperative ileus. Dis. Mon. 2010, 56, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Traut, U.; Brugger, L.; Kunz, R.; Pauli-Magnus, C.; Haug, K.; Bucher, H.C.; Koller, M.T. Systemic prokinetic pharmacologic treatment for postoperative adynamic ileus following abdominal surgery in adults. Cochrane Database Syst. Rev. 2008, CD004930. [Google Scholar] [CrossRef] [PubMed]
- Safety and efficacy of non-steroidal anti-inflammatory drugs to reduce ileus after colorectal surgery. Br. J. Surg. 2020, 107, e161–e169. [CrossRef] [PubMed]
- Holte, K.; Kehlet, H. Postoperative ileus: Progress towards effective management. Drugs 2002, 62, 2603–2615. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, S.; Kerolus, K.; Jin, Z.; Bajrami, S.; Denoya, P.; Bergese, S.D. Multidisciplinary Postoperative Ileus Management: A Narrative Review. Medicina 2025, 61, 1344. https://doi.org/10.3390/medicina61081344
Yu S, Kerolus K, Jin Z, Bajrami S, Denoya P, Bergese SD. Multidisciplinary Postoperative Ileus Management: A Narrative Review. Medicina. 2025; 61(8):1344. https://doi.org/10.3390/medicina61081344
Chicago/Turabian StyleYu, Sun, Katrina Kerolus, Zhaosheng Jin, Sandi Bajrami, Paula Denoya, and Sergio D. Bergese. 2025. "Multidisciplinary Postoperative Ileus Management: A Narrative Review" Medicina 61, no. 8: 1344. https://doi.org/10.3390/medicina61081344
APA StyleYu, S., Kerolus, K., Jin, Z., Bajrami, S., Denoya, P., & Bergese, S. D. (2025). Multidisciplinary Postoperative Ileus Management: A Narrative Review. Medicina, 61(8), 1344. https://doi.org/10.3390/medicina61081344





