Advancing Diagnostics with Semi-Automatic Tear Meniscus Central Area Measurement for Aqueous Deficient Dry Eye Discrimination
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Diagnostic and Classification Procedures and Criteria
- Participants completed the OSDI questionnaire via a self-administered online form to assess the presence of symptomatology [26]. Positive symptomatology is associated with an OSDI value ≥ 13 points.
- Tear film osmolarity was measured using a TearLab osmometer (TearLab, San Diego, CA, USA) [27]. The diagnostic cut-off values for DED diagnosis were a tear osmolarity ≥ 308 mOsm/L.
- BUT and corneal staining were recorded using a Topcon® SL-D4 slit-lamp equipped with a DV-3 video camera (Topcon Corporation, Tokyo, Japan) and non-preserved fluorescein [12]. The diagnostic cut-off values for DED diagnosis were a BUT ≤ 10 s and/or corneal staining (Oxford grade) ≥ 2.
2.3. TMCA Acquisition and Evaluation
2.3.1. Image Acquisition and Selection
2.3.2. Semi-Automatic Evaluation of the TMCA Parameter
2.4. Statistical Analysis
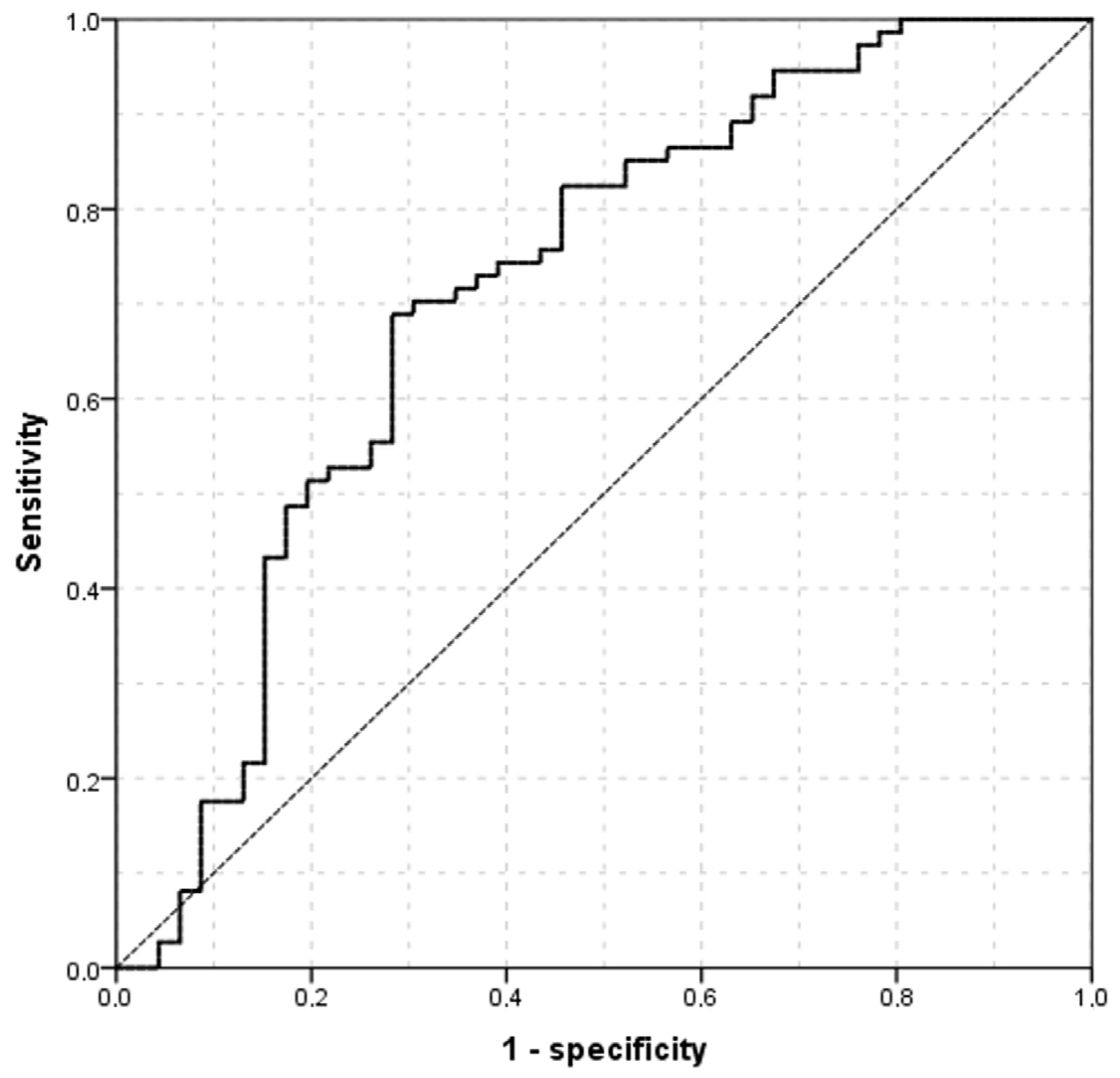
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Braun, R.J.; King-Smith, P.E.; Begley, C.G.; Li, L.; Gewecke, N.R. Dynamics and function of the tear film in relation to the blink cycle. Progress Retin. Eye Res. 2015, 45, 132–164. [Google Scholar] [CrossRef]
- Li, M.; Du, C.; Zhu, D.; Shen, M.; Cui, L.; Wang, J. Daytime variations of tear osmolarity and tear meniscus volume. Eye Contact Lens 2012, 38, 282–287. [Google Scholar] [CrossRef]
- Bron, A.J.; Tomlinson, A.; Foulks, G.N.; Pepose, J.S.; Baudouin, C.; Geerling, G.; Nichols, K.K.; Lemp, M.A. Rethinking dry eye disease: A perspective on clinical implications. Ocul. Surf. 2014, 12, S1–S31. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Wolffsohn, J.S.; Benitez-Del-Castillo, J.; Loya-Garcia, D.; Inomata, T.; Iyar, G.; Liang, L.; Pult, H.; Sabater, A.L.; Starr, C.E.; Vehof, J.; et al. TFOS DEWS III Diagnostic Methodology. Am. J. Ophthalmol. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Wolffsohn, J.S.; Arita, R.; Chalmers, R.; Djalilian, A.; Dogru, M.; Dumbleton, K.; Gupta, P.K.; Karpecki, P.; Lazreg, S.; Pult, H.; et al. TFOS DEWS II Diagnostic Methodology report. Ocul. Surf. 2017, 15, 539–574. [Google Scholar] [CrossRef]
- Wolffsohn, J.S.; Wang, M.T.M.; Vidal-Rohr, M.; Menduni, F.; Dhallu, S.; Ipek, T.; Acar, D.; Recchioni, A.; France, A.; Kingsnorth, A.; et al. Demographic and lifestyle risk factors of dry eye disease subtypes: A cross-sectional study. Ocul. Surf. 2021, 21, 58–63. [Google Scholar] [CrossRef]
- Tukenmez-Dikmen, N.; Yildiz, E.H.; Imamoglu, S.; Turan-Vural, E.; Sevim, M.S. Correlation of Dry Eye Workshop Dry Eye Severity Grading System With Tear Meniscus Measurement by Optical Coherence Tomography and Tear Osmolarity. Eye Contact Lens 2015, 42, 153–157. [Google Scholar] [CrossRef]
- Lam, A.K.; Tai, S.K.; Chan, J.K.; Ng, R.W. Lower Tear Meniscus Height Measurements Using Keratography and Swept-Source Optical Coherence Tomography and Effect of Fluorescein Instillation Methods. Curr. Eye Res. 2019, 44, 1203–1208. [Google Scholar] [CrossRef]
- Niedernolte, B.; Trunk, L.; Wolffsohn, J.S.; Pult, H.; Bandlitz, S. Evaluation of tear meniscus height using different clinical methods. Clin. Exp. Optom. J. Aust. Optom. Assoc. 2021, 104, 583–588. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Z.; Gong, Q.; Ren, W.; Chen, L.; Lu, F.; Hu, L. The Role of Different Tear Volume Detection Methods in the Evaluation and Diagnosis of Mild Dry Eye Disease. Transl. Vis. Sci. Technol. 2022, 11, 15. [Google Scholar] [CrossRef]
- Sabucedo-Villamarin, B.; Pena-Verdeal, H.; Garcia-Queiruga, J.; Giraldez, M.J.; Garcia-Resua, C.; Yebra-Pimentel, E. Categorization of the Aqueous Deficient Dry Eye by a Cut-Off Criterion of TMH Measured with Tearscope. Life 2022, 12, 2007. [Google Scholar] [CrossRef]
- Patel, S.; Wallace, I. Tear meniscus height, lower punctum lacrimale, and the tear lipid layer in normal aging. Optom. Vis. Sci. 2006, 83, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Bandlitz, S.; Purslow, C.; Murphy, P.J.; Pult, H. The relationship between tear meniscus regularity and conjunctival folds. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 2014, 91, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Soares, I.; Ramalho, E.; Brardo, F.M.; Nunes, A.F. Tear meniscus height agreement and reproducibility between two corneal topographers and spectral-domain optical coherence tomography. Clin. Exp. Optom. 2025, 108, 430–436. [Google Scholar] [CrossRef]
- Chen, M.; Wei, A.; Xu, J.; Zhou, X.; Hong, J. Application of Keratograph and Fourier-Domain Optical Coherence Tomography in Measurements of Tear Meniscus Height. J. Clin. Med. 2022, 11, 1343. [Google Scholar] [CrossRef]
- Pult, H.; Riede-Pult, B. Comparison of subjective grading and objective assessment in meibography. Contact Lens Anterior Eye J. Br. Contact Lens Assoc. 2013, 36, 22–27. [Google Scholar] [CrossRef]
- Srinivasan, S.; Menzies, K.; Sorbara, L.; Jones, L. Infrared imaging of meibomian gland structure using a novel keratograph. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 2012, 89, 788–794. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Sanchez Brea, M.L.; Barreira Rodriguez, N.; Mosquera Gonzalez, A.; Evans, K.; Pena-Verdeal, H. Defining the Optimal Region of Interest for Hyperemia Grading in the Bulbar Conjunctiva. Comput. Math. Methods Med. 2016, 2016, 3695014. [Google Scholar] [CrossRef]
- Pena-Verdeal, H.; Garcia-Resua, C.; Barreira, N.; Giraldez, M.J.; Yebra-Pimentel, E. Interobserver variability of an open-source software for tear meniscus height measurement. Contact Lens Anterior Eye J. Br. Contact Lens Assoc. 2016, 39, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Stanislaw, P.A.; Stawicki, M.D. RESEARCH GUIDE: Guide to free statistical software: General applications. OPUS 12 Sci. 2010, 4, 12–13. [Google Scholar]
- Miller, K.L.; Walt, J.G.; Mink, D.R.; Satram-Hoang, S.; Wilson, S.E.; Perry, H.D.; Asbell, P.A.; Pflugfelder, S.C. Minimal clinically important difference for the ocular surface disease index. Arch. Ophthalmol. 2010, 128, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.D.; Watanabe, J.H.; Strauss, J.A.; Dubey, A.T. Work productivity loss in patients with dry eye disease: An online survey. Curr. Med. Res. Opin. 2011, 27, 1041–1048. [Google Scholar] [CrossRef]
- Arita, R.; Minoura, I.; Morishige, N.; Shirakawa, R.; Fukuoka, S.; Asai, K.; Goto, T.; Imanaka, T.; Nakamura, M. Development of Definitive and Reliable Grading Scales for Meibomian Gland Dysfunction. Am. J. Ophthalmol. 2016, 169, 125–137. [Google Scholar] [CrossRef]
- Facchin, A.; Boccardo, L. Psychometric properties and diagnostic performance of three dry eye questionnaires in Italian: OSDI, OSDI-6, and SPEED. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 2024, 101, 579–588. [Google Scholar] [CrossRef]
- Tavakoli, A.; Markoulli, M.; Flanagan, J.; Papas, E. The validity of point of care tear film osmometers in the diagnosis of dry eye. Ophthalmic Physiol. Opt. 2022, 42, 140–148. [Google Scholar] [CrossRef]
- Johnson, M.E.; Murphy, P.J. The Effect of instilled fluorescein solution volume on the values and repeatability of TBUT measurements. Cornea 2005, 24, 811–817. [Google Scholar] [CrossRef]
- Bandlitz, S.; Purslow, C.; Murphy, P.J.; Pult, H. Comparison of a new portable digital meniscometer and optical coherence tomography in tear meniscus radius measurement. Acta Ophthalmol. 2014, 92, e112–e118. [Google Scholar] [CrossRef]
- Dunn, G. Design and Analysis of Reliability Studies: The Statistical Evaluation of Measurement Errors; Edward Arnold Publishers: New York, NY, USA; London, UK; Oxford University Press: Oxford, UK, 1989. [Google Scholar]
- Nahm, F.S. Receiver operating characteristic curve: Overview and practical use for clinicians. Korean J. Anesthesiol. 2022, 75, 25–36. [Google Scholar] [CrossRef]
- Johnson, M.E.; Murphy, P.J. The agreement and repeatability of tear meniscus height measurement methods. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 2005, 82, 1030–1037. [Google Scholar] [CrossRef]
- Pult, H.; Purslow, C.; Murphy, P.J. The relationship between clinical signs and dry eye symptoms. Eye 2011, 25, 502–510. [Google Scholar] [CrossRef]
- Bandlitz, S.; Purslow, C.; Murphy, P.J.; Pult, H.; Bron, A.J. A new portable digital meniscometer. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 2014, 91, e1–e8. [Google Scholar] [CrossRef]
- Uchida, A.; Uchino, M.; Goto, E.; Hosaka, E.; Kasuya, Y.; Fukagawa, K.; Dogru, M.; Ogawa, Y.; Tsubota, K. Noninvasive interference tear meniscometry in dry eye patients with Sjogren syndrome. Am. J. Ophthalmol. 2007, 144, 232–237. [Google Scholar] [CrossRef]
- Shen, M.; Li, J.; Wang, J.; Ma, H.; Cai, C.; Tao, A.; Yuan, Y.; Lu, F. Upper and lower tear menisci in the diagnosis of dry eye. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2722–2726. [Google Scholar] [CrossRef]
- Tian, L.; Qu, J.H.; Zhang, X.Y.; Sun, X.G. Repeatability and Reproducibility of Noninvasive Keratograph 5M Measurements in Patients with Dry Eye Disease. J. Ophthalmol. 2016, 2016, 8013621. [Google Scholar] [CrossRef]
- Singh, A.; Vanathi, M.; Kishore, A.; Gupta, N.; Tandon, R. Evaluation of strip meniscometry, tear meniscus height and depth in the diagnosis of dry eye disease in asian Indian eyes. Ocul. Surf. 2019, 17, 747–752. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.Y.; Seo, K.Y.; Kim, T.I.; Chin, H.S.; Jung, J.W. Location and pattern of non-invasive keratographic tear film break-up according to dry eye disease subtypes. Acta Ophthalmol. 2019, 97, e1089–e1097. [Google Scholar] [CrossRef]
- Lee, K.W.; Kim, J.Y.; Chin, H.S.; Seo, K.Y.; Kim, T.I.; Jung, J.W. Assessment of the Tear Meniscus by Strip Meniscometry and Keratograph in Patients With Dry Eye Disease According to the Presence of Meibomian Gland Dysfunction. Cornea 2017, 36, 189–195. [Google Scholar] [CrossRef]
- Tung, C.I.; Perin, A.F.; Gumus, K.; Pflugfelder, S.C. Tear meniscus dimensions in tear dysfunction and their correlation with clinical parameters. Am. J. Ophthalmol. 2014, 157, 301–310.e1. [Google Scholar] [CrossRef]
- Palakuru, J.R.; Wang, J.; Aquavella, J.V. Effect of blinking on tear dynamics. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3032–3037. [Google Scholar] [CrossRef]
- Eroglu, F.C.; Karalezli, A.; Dursun, R. Is optical coherence tomography an effective device for evaluation of tear film meniscus in patients with acne rosacea? Eye 2016, 30, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Pena-Verdeal, H.; Garcia-Queiruga, J.; Sabucedo-Villamarin, B.; Garcia-Resua, C.; Giraldez, M.J.; Yebra-Pimentel, E. A Comprehensive Study on Tear Meniscus Height Inter-Eye Differences in Aqueous Deficient Dry Eye Diagnosis. J. Clin. Med. 2024, 13, 659. [Google Scholar] [CrossRef] [PubMed]
- Sabucedo-Villamarin, B.; Pena-Verdeal, H.; Garcia-Queiruga, J.; Giraldez, M.J.; Garcia-Resua, C.; Yebra-Pimentel, E. Longitudinal analysis of variation in status and diagnostic stability of untreated dry eye disease. Ocul. Surf. 2023, 29, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.J.; Lee, J.H. Measurement of the tear meniscus height using 0.25% fluorescein sodium. Korean J. Ophthalmol. KJO 1991, 5, 34–36. [Google Scholar] [CrossRef]
- Oguz, H.; Yokoi, N.; Kinoshita, S. The height and radius of the tear meniscus and methods for examining these parameters. Cornea 2000, 19, 497–500. [Google Scholar] [CrossRef]
- Kawai, M.; Yamada, M.; Kawashima, M.; Inoue, M.; Goto, E.; Mashima, Y.; Tsubota, K. Quantitative evaluation of tear meniscus height from fluorescein photographs. Cornea 2007, 26, 403–406. [Google Scholar] [CrossRef]

| Interpretation | OSDI | Tear Film Osmolarity (mOsm/L) | BUT (s) | Corneal Staining (Oxford Grade) | TMH (mm) | |
|---|---|---|---|---|---|---|
| Diagnosis | Categorization | |||||
| Non-DED | - | <13 | <308 | >10 | <2 | - |
| DED | Non-ADDE | ≥13 | ≥308 (or) | ≤10 (or) | ≥2 (or) | ≥0.20 |
| DED | ADDE | ≥13 | ≥308 (or) | ≤10 (or) | ≥2 (or) | <0.20 |
| Characteristic | Value | |
|---|---|---|
| Non-ADDE n = 74 | ADDE n = 46 | |
| Female gender (%) | 48 (64.9%) | 36 (78.3%) |
| Age (years), mean (SD) | 21.37 (4.88) | 22.76 (7.98) |
| OSDI, median (IQR) | 16.67 (14.58–17.19) | 16.67 (14.58–22.76) |
| BUT (s), median (IQR) | 8.10 (5.21–9.77) | 6.12 (4.29–8.74) |
| Corneal staining (Oxford grade), median (IQR) | 0.00 (0.00–0.25) | 0.00 (0.00–1.00) |
| Osmolarity (mOsm/L), mean (SD) | 308.53 (14.58) | 312.30 (12.63) |
| TMH (mm), mean (SD) | 0.237 (0.03) | 0.154 (0.03) |
| TMCA (mm2), mean (SD) | 62.23 (15.60) | 54.94 (29.46) |
| OSDI | Tear Film Osmolarity (mOsm/L) | BUT (s) | Corneal Staining (Oxford Grade) | TMH (mm) | ||
|---|---|---|---|---|---|---|
| TMCA (mm2) | r | −0.091 | 0.157 | −0.070 | −0.094 | 0.417 |
| p | 0.320 | 0.087 | 0.445 | 0.308 | <0.001 |
| TFOS DEWS II Diagnostic Methodology Subcommittee | ||||
|---|---|---|---|---|
| Non-ADDE | ADDE | Total | ||
| TMCA cut-off value | Non-ADDE | 26 (27.1%) | 16 (16.7%) | 42 (43.8%) |
| ADDE | 12 (12.5%) | 42 (43.8%) | 54 (56.3%) | |
| Total | 38 (39.6%) | 58 (60.4%) | 96 (100%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pena-Verdeal, H.; Garcia-Queiruga, J.; Sabucedo-Villamarin, B.; Garcia-Resua, C.; Giraldez, M.J.; Yebra-Pimentel, E. Advancing Diagnostics with Semi-Automatic Tear Meniscus Central Area Measurement for Aqueous Deficient Dry Eye Discrimination. Medicina 2025, 61, 1322. https://doi.org/10.3390/medicina61081322
Pena-Verdeal H, Garcia-Queiruga J, Sabucedo-Villamarin B, Garcia-Resua C, Giraldez MJ, Yebra-Pimentel E. Advancing Diagnostics with Semi-Automatic Tear Meniscus Central Area Measurement for Aqueous Deficient Dry Eye Discrimination. Medicina. 2025; 61(8):1322. https://doi.org/10.3390/medicina61081322
Chicago/Turabian StylePena-Verdeal, Hugo, Jacobo Garcia-Queiruga, Belen Sabucedo-Villamarin, Carlos Garcia-Resua, Maria J. Giraldez, and Eva Yebra-Pimentel. 2025. "Advancing Diagnostics with Semi-Automatic Tear Meniscus Central Area Measurement for Aqueous Deficient Dry Eye Discrimination" Medicina 61, no. 8: 1322. https://doi.org/10.3390/medicina61081322
APA StylePena-Verdeal, H., Garcia-Queiruga, J., Sabucedo-Villamarin, B., Garcia-Resua, C., Giraldez, M. J., & Yebra-Pimentel, E. (2025). Advancing Diagnostics with Semi-Automatic Tear Meniscus Central Area Measurement for Aqueous Deficient Dry Eye Discrimination. Medicina, 61(8), 1322. https://doi.org/10.3390/medicina61081322









