Long-Term Impact of COVID-19 on Osteoporosis Risk Among Patients Aged ≥50 Years with New-Onset Overweight, Obesity, or Type 2 Diabetes: A Multi-Institutional Retrospective Cohort Study
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Data Source
Data Source and Study Design
2.3. Inclusion and Exclusion Criteria
2.3.1. Inclusion Criteria
2.3.2. Exclusion Criteria
2.4. Non-COVID-19 Cohort Definition
2.5. Outcomes and Follow-Up
2.6. Study Covariates
2.7. Statistical Analysis
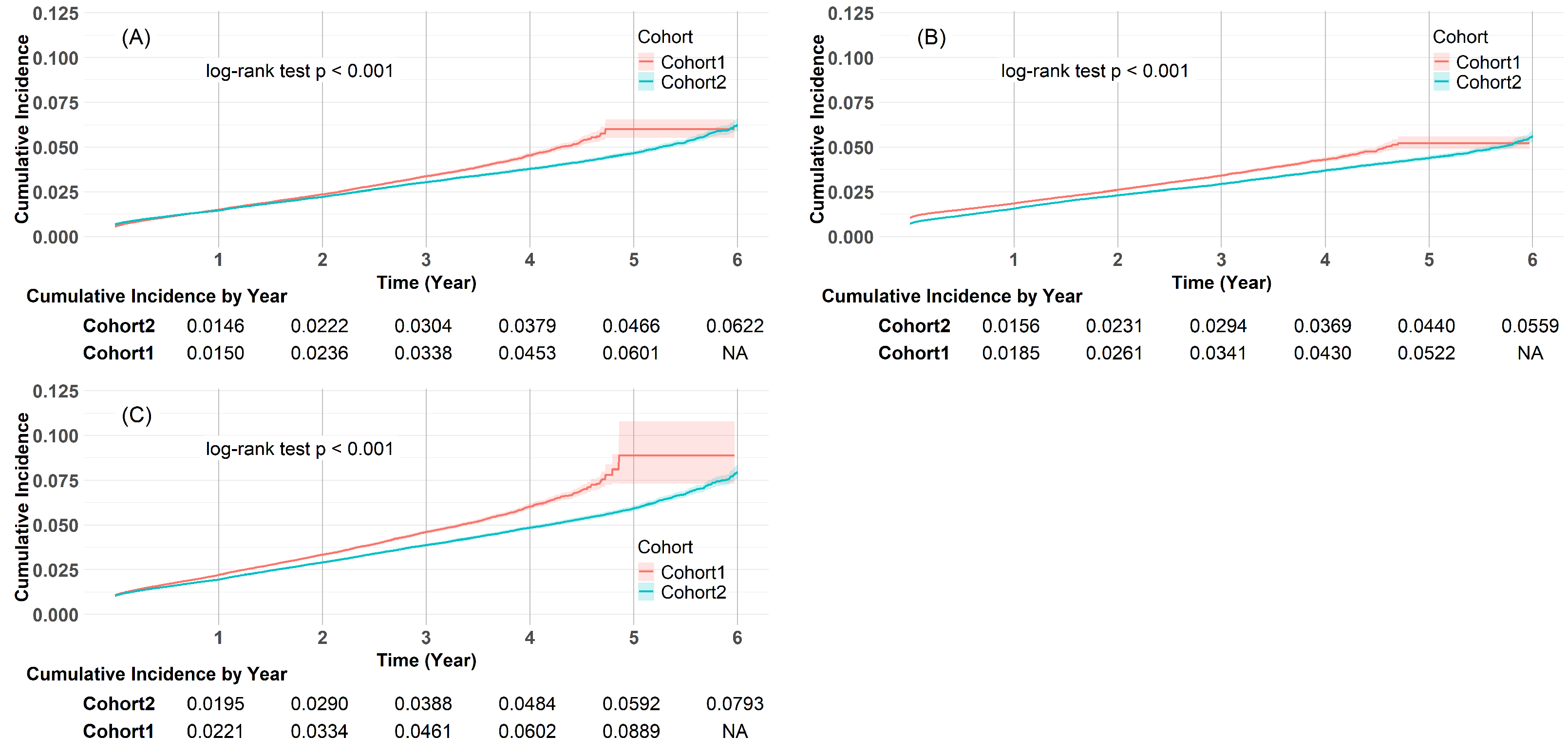
2.8. Prespecified Outcomes
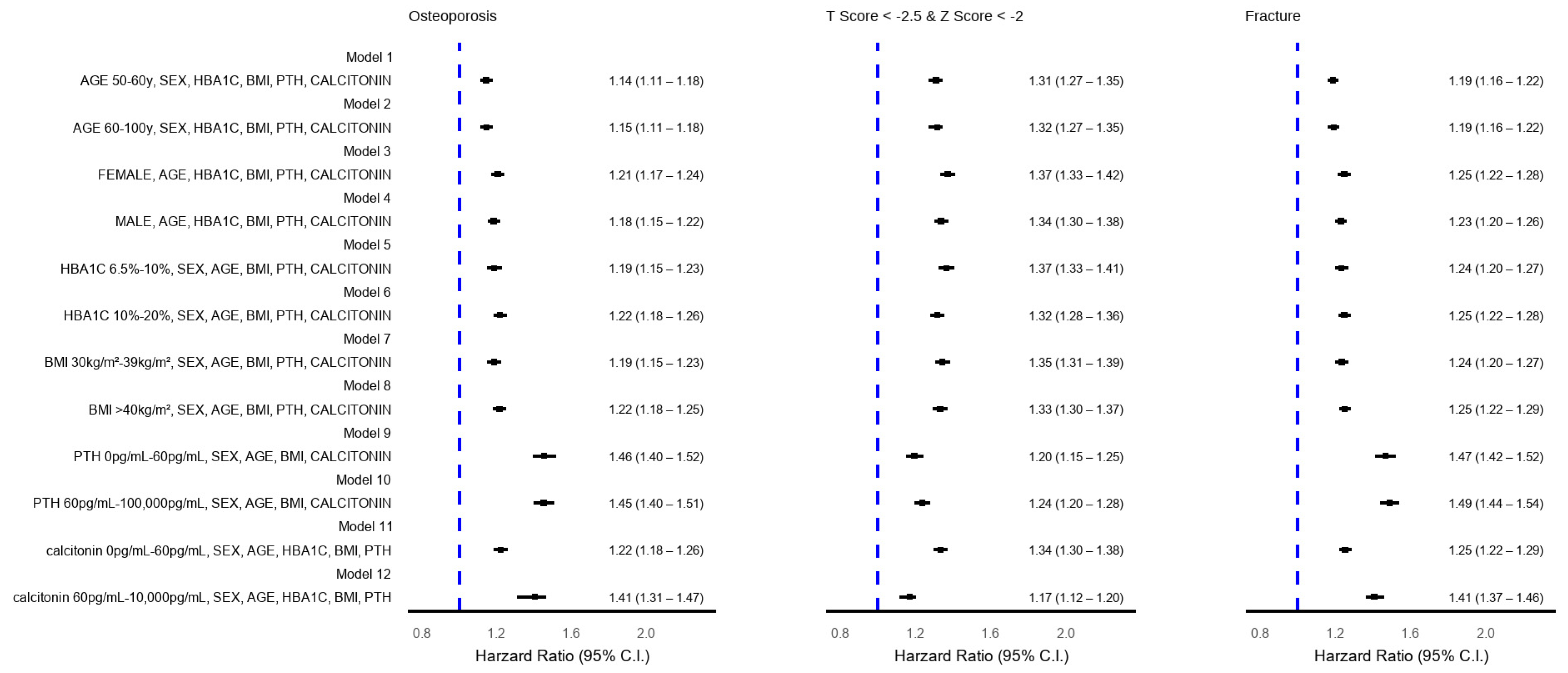
2.9. Sensitivity Analysis
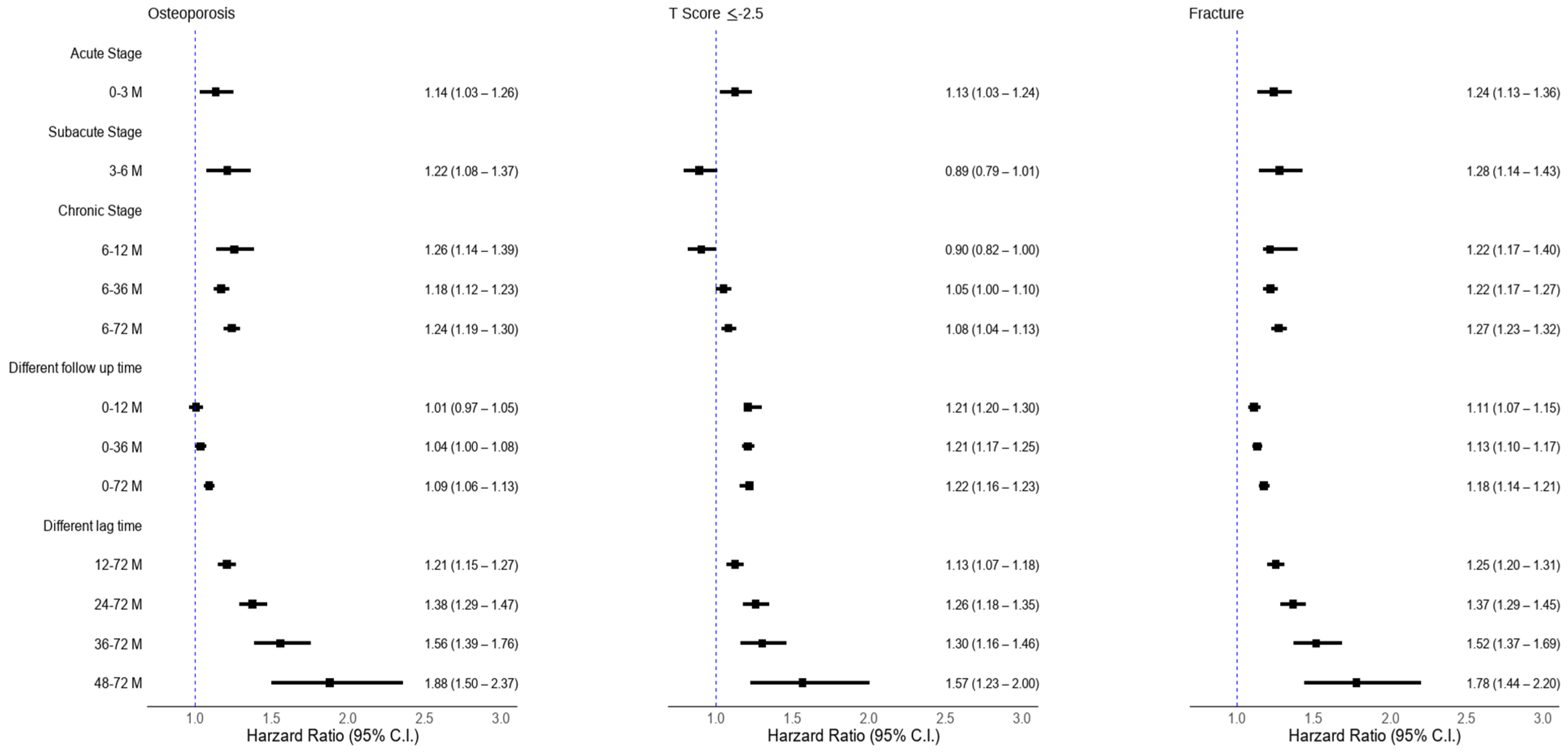
2.10. Stratified Analyses
3. Results
4. Discussion
4.1. Key Findings
4.2. Comparison with the Existing Literature
4.3. Clinical Implications
4.4. Strengths
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guimarães, G.C.; Coelho, J.B.C.; Silva, J.G.O.; de Sant’Ana, A.C.C.; de Sá, C.A.C.; Moreno, J.M.; Reis, L.M.; de Oliveira Guimarães, C.S. Obesity, diabetes and risk of bone fragility: How BMAT behavior is affected by metabolic disturbances and its influence on bone health. Osteoporos. Int. 2024, 35, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Martiniakova, M.; Biro, R.; Penzes, N.; Sarocka, A.; Kovacova, V.; Mondockova, V.; Omelka, R. Links among Obesity, Type 2 Diabetes Mellitus, and Osteoporosis: Bone as a Target. Int. J. Mol. Sci. 2024, 25, 4827. [Google Scholar] [CrossRef] [PubMed]
- Anghel, L.; Manole, C.; Nechita, A.; Tatu, A.L.; Ștefănescu, B.I.; Nechita, L.; Bușilă, C.; Zainea, P.; Baroiu, L.; Mușat, C.L. Calcium, Phosphorus and Magnesium Abnormalities Associated with COVID-19 Infection, and Beyond. Biomedicines 2023, 11, 2362. [Google Scholar] [CrossRef] [PubMed]
- Lopez, N.; Cohen, S.M.; Emanuele, M. Type 2 Diabetes and Bone Disease. Clin. Rev. Bone Miner. Metab. 2023, 21, 21–31. [Google Scholar] [CrossRef]
- Haudenschild, A.K.; Christiansen, B.A.; Orr, S.; Ball, E.E.; Weiss, C.M.; Liu, H.; Fyhrie, D.P.; Yik, J.H.N.; Coffey, L.L.; Haudenschild, D.R. Acute bone loss following SARS-CoV-2 infection in mice. J. Orthop. Res. 2023, 41, 1945–1952. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, F.; Mokbel, K.; Meertens, R.; Obotiba, A.D.; Alharbi, M.; Knapp, K.M.; Strain, W.D. Bone Mineral Density, Bone Biomarkers, and Joints in Acute, Post, and Long COVID-19: A Systematic Review. Viruses 2024, 16, 1694. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Tahtabasi, M.; Kilicaslan, N.; Akin, Y.; Karaman, E.; Gezer, M.; Icen, Y.K.; Sahiner, F. The Prognostic Value of Vertebral Bone Density on Chest CT in Hospitalized COVID-19 Patients. J. Clin. Densitom. 2021, 24, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Elmedany, S.H.; Badr, O.I.; Abu-Zaid, M.H.; Tabra, S.A.A. Bone mineral density changes in osteoporotic and osteopenic patients after COVID-19 infection. Egypt. Rheumatol. Rehabil. 2022, 49, 64. [Google Scholar] [CrossRef]
- Soeroto, A.Y.; Soetedjo, N.N.; Purwiga, A.; Santoso, P.; Kulsum, I.D.; Suryadinata, H.; Ferdian, F. Effect of increased BMI and obesity on the outcome of COVID-19 adult patients: A systematic review and meta-analysis. Diabetes Metab. Syndr. 2020, 14, 1897–1904. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.; Creecy, A.; Awosanya, O.D.; McCune, T.; Ozanne, M.V.; Toepp, A.J.; Kacena, M.A.; Qiao, X. SARS-CoV-2 and its Multifaceted Impact on Bone Health: Mechanisms and Clinical Evidence. Curr. Osteoporos. Rep. 2024, 22, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Amin, H.; Khan, M.A.; Bukhari, M. Investigating the impact of COVID-19 lockdowns on fragility fracture risk and bone mineral density in a large observational cohort: A cross-sectional study. Rheumatol. Adv. Pract. 2024, 8, rkae115. [Google Scholar] [CrossRef] [PubMed]
- Tang, J. COVID-19 Pandemic and Osteoporosis in Elderly Patients. Aging Dis 2022, 13, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Lui, D.T.W.; Xiong, X.; Cheung, C.-L.; Lai, F.T.T.; Li, X.; Wan, E.Y.F.; Chui, C.S.L.; Chan, E.W.Y.; Cheng, F.W.T.; Chung, M.S.H.; et al. Risks of incident major osteoporotic fractures following SARS-CoV-2 infection among older individuals: A population-based cohort study in Hong Kong. J. Bone Miner. Res. 2024, 39, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Galliera, E.; Massaccesi, L.; Mangiavini, L.; De Vecchi, E.; Villa, F.; Corsi Romanelli, M.M.; Peretti, G. Effects of COVID-19 on bone fragility: A new perspective from osteoimmunological biomarkers. Front. Immunol. 2024, 15, 1493643. [Google Scholar] [CrossRef] [PubMed]
- Sapra, L.; Saini, C.; Garg, B.; Gupta, R.; Verma, B.; Mishra, P.K.; Srivastava, R.K. Long-term implications of COVID-19 on bone health: Pathophysiology and therapeutics. Inflamm. Res. 2022, 71, 1025–1040. [Google Scholar] [CrossRef] [PubMed]
- Gittoes, N.J.; Criseno, S.; Appelman-Dijkstra, N.M.; Bollerslev, J.; Canalis, E.; Rejnmark, L.; Hassan-Smith, Z. ENDOCRINOLOGY IN THE TIME OF COVID-19: Management of calcium metabolic disorders and osteoporosis. Eur. J. Endocrinol. 2020, 183, G57–G65. [Google Scholar] [CrossRef] [PubMed]
- Berktaş, B.M.; Gökçek, A.; Hoca, N.T.; Koyuncu, A. COVID-19 illness and treatment decrease bone mineral density of surviving hospitalized patients. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 3046–3056. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Z.; Shen, Y.; Qian, S.; Tang, M.; He, J.; Lu, H.; Zhang, N. Long-term effects of COVID-19 infection on bone mineral density. J. Glob. Health 2024, 14, 05029. [Google Scholar] [CrossRef] [PubMed]
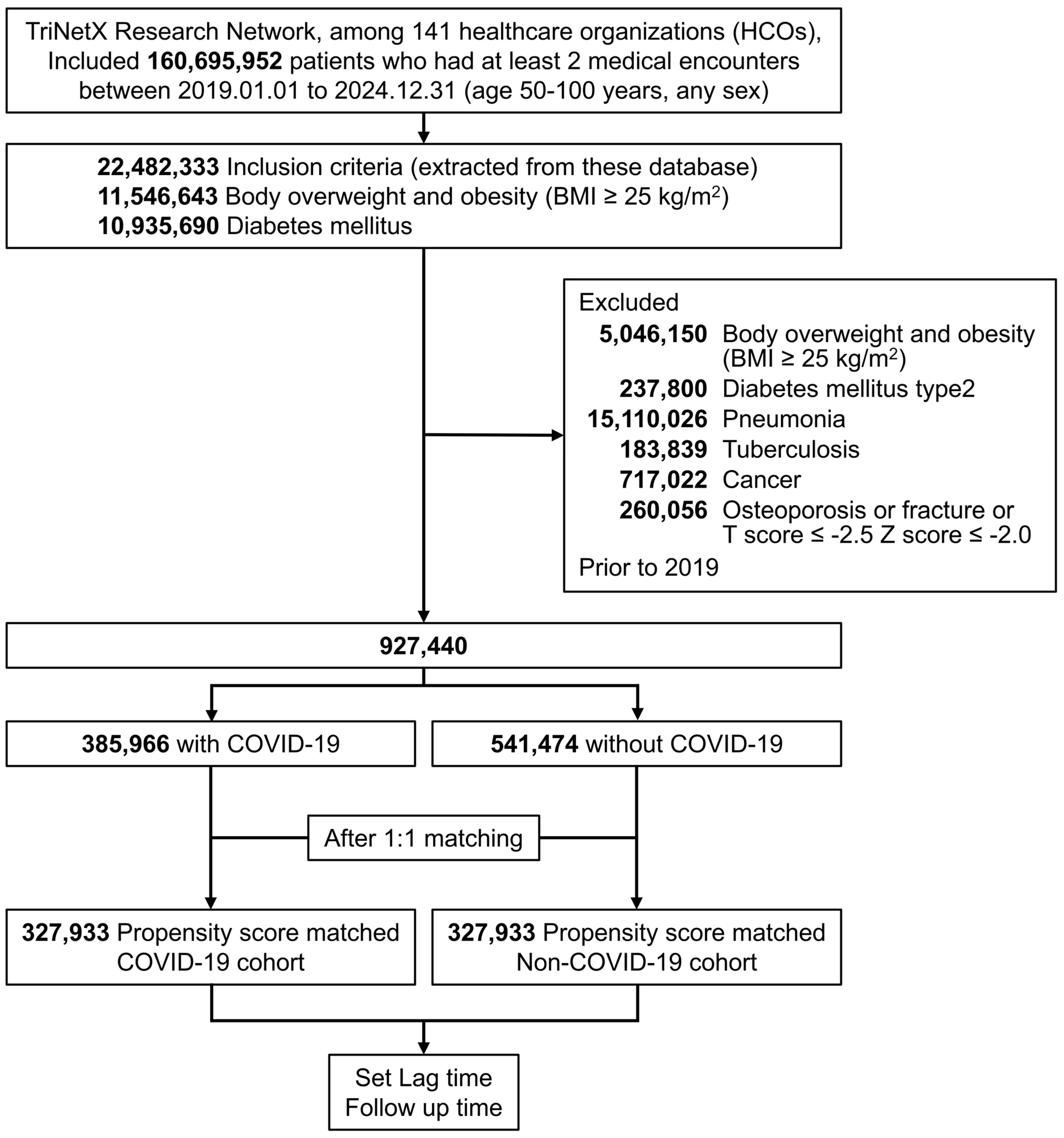
| Cohort Before Matching, No (%) | Cohort After Matching, No (%) | |||||
|---|---|---|---|---|---|---|
| Characteristics | COVID-19 (n = 385, 966) | Non-COVID (n = 541, 474) | SMD | COVID-19 (n = 327, 933) | Non-COVID (n = 327, 933) | SMD |
| Age | ||||||
| Age 50–65 | 20,651 (5) | 38,540 (7) | 0.07 | 16,747 (5) | 15,965 (5) | 0.01 |
| Age 65–85 | 209,651 (54) | 299,712 (55) | 0.02 | 178,842 (55) | 179,264 (55) | 0.00 |
| Age 85–100 | 155,684 (41) | 203,222 (38) | 0.06 | 132,344 (40) | 132,704 (40) | 0.00 |
| Sex | ||||||
| Female | 217,901 (56) | 301,072 (56) | 0.02 | 184,563 (56) | 184,109 (56) | 0.00 |
| Male | 161,826 (42) | 230,671 (43) | 0.01 | 138,204 (42) | 138,857 (42) | 0.00 |
| Unknown | 7720 (2) | 6830 (1) | 0.01 | 6539 (2) | 6560 (2) | 0.00 |
| Race | ||||||
| White | 238,520 (62) | 317,132 (59) | 0.07 | 201,171 (61) | 201,305 (61) | 0.00 |
| Black | 68,843 (18) | 97,046 (18) | 0.00 | 58,527 (18) | 58,262 (18) | 0.00 |
| Asian | 21,463 (6) | 30,983 (6) | 0.01 | 19,089 (6) | 19,398 (6) | 0.00 |
| Other | 21,816 (6) | 35,720 (7) | 0.04 | 18,836 (6) | 19,375 (6) | 0.01 |
| Unknown | 31,352 (8) | 55,319 (10) | 0.07 | 29,927 (9) | 29,514 (9) | 0.01 |
| Ethnicity | ||||||
| Hispanic or Latino | 46,352 (12) | 66,500 (12) | 0.01 | 38,984 (12) | 40,295 (12) | 0.01 |
| Not Hispanic or Latino | 300,903 (78) | 399,148 (74) | 0.10 | 255,300 (78) | 256,018 (78) | 0.00 |
| Unknown | 38,644 (10) | 75,826 (14) | 0.12 | 33,649 (10) | 31,620 (10) | 0.02 |
| Basic laboratory data | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| Blood pressure, systolic, mean (SD), mmHg | 128 (19) | 128 (19) | 0.02 | 128 (19) | 128 (19) | 0.02 |
| Blood pressure, diastolic, mean (SD), mmHg | 76 (12) | 77 (12) | 0.03 | 77 (12) | 77 (12) | 0.01 |
| Calcium, mean (SD), mg/dL | 9 (1) | 9 (1) | 0.08 | 9 (1) | 9 (1) | 0.04 |
| Glomerular filtration rate, mean (SD), mL/min/1.73 m2 | 79 (26) | 79 (25) | 0.03 | 79 (26) | 79 (25) | 0.02 |
| Hemoglobin, mean (SD), g/dL | 13 (2) | 13 (2) | 0.07 | 13 (2) | 13 (2) | 0.03 |
| Albumin, mean (SD), g/dL | 4 (0) | 4 (0) | 0.11 | 4 (0) | 4 (0) | 0.06 |
| Cholesterol in HDL, mean (SD), mg/dL | 52 (16) | 53 (16) | 0.03 | 52 (16) | 53 (16) | 0.03 |
| Cholesterol, mean (SD), mg/dL | 180 (45) | 182 (45) | 0.05 | 180 (46) | 182 (45) | 0.04 |
| Triglyceride, mean (SD), mg/dL | 135 (93) | 134 (91) | 0.01 | 136 (93) | 134 (92) | 0.02 |
| Cholesterol in LDL, mean (SD), mg/dL | 104 (36) | 107 (36) | 0.07 | 104 (36) | 106 (36) | 0.05 |
| Thyrotropin, mean (SD), U/L | 4 (31) | 4 (30) | 0.00 | 4 (32) | 4 (30) | 0.01 |
| Hemoglobin A1c, mean (SD), % | 6 (1) | 6 (1) | 0.05 | 6 (1) | 6 (1) | 0.13 |
| Phosphate, mean (SD), mg/dL | 4 (1) | 4 (1) | 0.04 | 4 (1) | 4 (1) | 0.02 |
| Parathyrin. intact, mean (SD), pg/mL | 111 (189) | 101 (158) | 0.06 | 109 (196) | 101 (158) | 0.05 |
| Left ventricle ejection fraction, mean (SD), % | 58 (11) | 59 (11) | 0.02 | 58 (11) | 58 (11) | 0.01 |
| Body mass index percentile, mean (SD), % | 73 (30) | 79 (27) | 0.20 | 74 (30) | 73 (30) | 0.06 |
| Albumin/creatinine ratio, mean (SD), mg/g | 246 (6337) | 99 (454) | 0.03 | 276 (6907) | 97 (465) | 0.04 |
| Cortisol, mean (SD), ug/dL | 11 (7) | 11 (7) | 0.08 | 11 (7) | 11 (7) | 0.01 |
| Calcitonin, mean (SD), pg/mL | 1400 (6408) | 138 (480) | 0.28 | 172 (417) | 97 (180) | 0.23 |
| Comorbidities and condition | ||||||
| Endocrine, nutritional, and metabolic diseases | 152,109 (39) | 117,614 (22) | 0.39 | 99,488 (30) | 97,788 (30) | 0.01 |
| Hyperlipidemia, unspecified | 51,422 (13) | 42,619 (8) | 0.18 | 36,932 (11) | 36,096 (11) | 0.01 |
| Body mass index | 44,847 (12) | 31,356 (6) | 0.21 | 24,912 (8) | 24,728 (8) | 0.00 |
| Other long-term drug therapies | 34,679 (9) | 28,356 (5) | 0.15 | 24,448 (7) | 24,187 (7) | 0.00 |
| Other symptoms and signs involving the digestive system | 13,771 (4) | 11,336 (2) | 0.09 | 10,240 (3) | 8875 (3) | 0.02 |
| Nicotine dependence, unspecified | 8013 (2) | 7067 (1) | 0.06 | 5597 (2) | 5555 (2) | 0.00 |
| Dyspnea, unspecified | 7369 (2) | 5418 (1) | 0.08 | 5218 (2) | 4382 (1) | 0.02 |
| Gout | 5387 (1) | 4435 (1) | 0.06 | 3771 (1) | 3686 (1) | 0.00 |
| Alcohol-related disorders | 5197 (1) | 3926 (1) | 0.06 | 3413 (1) | 3325 (1) | 0.00 |
| Systemic connective tissue disease | 4416 (1) | 4128 (1) | 0.04 | 3305 (1) | 3243 (1) | 0.00 |
| Long-term use of steroids | 4245 (1) | 3001 (1) | 0.06 | 2691 (1) | 2654 (1) | 0.00 |
| Exercise counseling | 3852 (1) | 2888 (1) | 0.05 | 2436 (1) | 2433 (1) | 0.00 |
| Alcohol abuse | 2945 (1) | 2148 (1) | 0.05 | 1870 (1) | 1808 (1) | 0.00 |
| Long-term use of non-steroidal anti-inflammatories | 2556 (1) | 2020 (0) | 0.04 | 1747 (1) | 1734 (1) | 0.00 |
| Long-term use of systemic steroids | 2421 (1) | 1741 (0) | 0.04 | 1563 (0) | 1526 (0) | 0.00 |
| Long-term use of inhaled steroids | 2180 (1) | 1486 (0) | 0.04 | 1329 (0) | 1326 (0) | 0.00 |
| Influenza due to influenza virus | 1491 (0) | 1360 (0) | 0.02 | 1073 (0) | 1016 (0) | 0.00 |
| Body mass index of 19.9 or less, adult a | 1109 (0) | 733 (0) | 0.03 | 698 (0) | 673 (0) | 0.00 |
| Overweight and obesity | 39,870 (10) | 589 (0) | 0.47 | 599 (0) | 589 (0) | 0.00 |
| Pressure ulcer | 1140 (0) | 480 (0) | 0.05 | 465 (0) | 457 (0) | 0.00 |
| Abnormal findings on diagnostic imaging of limbs | 368 (0) | 342 (0) | 0.01 | 272 (0) | 280 (0) | 0.00 |
| Abnormal results of pulmonary function studies | 331 (0) | 289 (0) | 0.01 | 240 (0) | 231 (0) | 0.00 |
| Morbid (severe) obesity due to excess calories | 6089 (2) | 191 (0) | 0.17 | 204 (0) | 179 (0) | 0.00 |
| Pneumonia, unspecified organism | 4125 (1) | 74 (0) | 0.14 | 79 (0) | 74 (0) | 0.00 |
| Procedure | ||||||
| Immunology procedures | 31,913 (8) | 29,034 (5) | 0.12 | 23,607 (7) | 23,439 (7) | 0.00 |
| Vaccination for COVID-19 b | 17,296 (4) | 11,201 (2) | 0.14 | 11,030 (3) | 10,669 (3) | 0.01 |
| Immunization for COVID-19 b | 12,484 (3) | 7709 (1) | 0.12 | 7667 (2) | 7344 (2) | 0.01 |
| Dual-energy X-ray absorptiometry (DXA), bone density study | 6704 (2) | 5686 (1) | 0.06 | 5005 (2) | 4961 (2) | 0.00 |
| Smoking and tobacco use cessation counseling visit | 997 (0) | 867 (0) | 0.02 | 699 (0) | 689 (0) | 0.00 |
| Smoking and tobacco use cessation counseling visit; intermediate | 861 (0) | 752 (0) | 0.02 | 607 (0) | 598 (0) | 0.00 |
| Dual-energy X-ray absorptiometry (DXA), appendicular skeleton | 275 (0) | 242 (0) | 0.01 | 216 (0) | 200 (0) | 0.00 |
| Dual-energy X-ray absorptiometry (DXA), axial skeleton | 254 (0) | 259 (0) | 0.01 | 194 (0) | 173 (0) | 0.00 |
| Admission procedure | 110 (0) | 244 (0) | 0.01 | 93 (0) | 94 (0) | 0.00 |
| Admission to establishment | 110 (0) | 244 (0) | 0.01 | 93 (0) | 94 (0) | 0.00 |
| Hospital admission | 52 (0) | 170 (0) | 0.01 | 48 (0) | 57 (0) | 0.00 |
| Emergency room admission | 50 (0) | 143 (0) | 0.01 | 46 (0) | 56 (0) | 0.00 |
| Admission to department | 58 (0) | 81 (0) | 0.00 | 45 (0) | 38 (0) | 0.00 |
| Admission to: | ||||||
| Intensive care unit | 10 (0) | 20 (0) | 0.00 | 10 (0) | 10 (0) | 0.00 |
| Neurology department | 10 (0) | 10 (0) | 0.00 | 10 (0) | 0 (0) | 0.01 |
| Pulmonary medicine department | 14 (0) | 10 (0) | 0.00 | 10 (0) | 10 (0) | 0.00 |
| Intensive care unit | 0 (0) | 0 (0) | 0.00 | 0 (0) | 0 (0) | 0.00 |
| Medications | ||||||
| Central nervous system drugs | 166,002 (43) | 177,022 (33) | 0.21 | 130,697 (40) | 129,547 (40) | 0.01 |
| SARS-CoV-2 (COVID-19) vaccine | 101,880 (26) | 103,641 (19) | 0.17 | 80,137 (24) | 78,305 (24) | 0.01 |
| Antibacterials for systemic use | 102,532 (27) | 107,062 (20) | 0.16 | 79,341 (24) | 79,331 (24) | 0.00 |
| Drug for obstructive lung disease | 93,262 (24) | 95,068 (18) | 0.16 | 71,515 (22) | 71,408 (22) | 0.00 |
| Cardiac therapy | 84,989 (22) | 82,546 (15) | 0.17 | 64,018 (20) | 63,490 (19) | 0.00 |
| Lipid-modifying agent | 66,783 (17) | 74,096 (14) | 0.10 | 54,228 (17) | 52,754 (16) | 0.01 |
| Sedatives or hypnotics | 49,608 (13) | 47,233 (9) | 0.13 | 37,082 (11) | 36,586 (11) | 0.00 |
| Antithrombotic agents | 48,929 (13) | 46,295 (9) | 0.13 | 36,134 (11) | 35,162 (11) | 0.01 |
| Benzodiazepine derivatives | 42,010 (11) | 39,634 (7) | 0.12 | 31,157 (10) | 30,759 (9) | 0.00 |
| Drugs used in diabetes | 28,240 (7) | 35,641 (7) | 0.03 | 23,902 (7) | 22,494 (7) | 0.02 |
| Non-steroidal anti-inflammatory drugs | 25,519 (7) | 28,273 (5) | 0.06 | 19,728 (6) | 19,501 (6) | 0.00 |
| Antihypertensives | 14,446 (4) | 12,877 (2) | 0.08 | 10,140 (3) | 10,054 (3) | 0.00 |
| Endocrine therapy | 11,559 (3) | 13,759 (3) | 0.03 | 9225 (3) | 8987 (3) | 0.00 |
| Immunosuppressants | 8835 (2) | 9376 (2) | 0.04 | 7080 (2) | 6974 (2) | 0.00 |
| Colchicine | 2144 (1) | 2200 (0) | 0.02 | 1673 (1) | 1647 (1) | 0.00 |
| Anti-obesity preparations | 1796 (0) | 1807 (0) | 0.02 | 1344 (0) | 1293 (0) | 0.00 |
| Antimycobacterial | 857 (0) | 959 (0) | 0.01 | 653 (0) | 655 (0) | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, S.-Y.; Sun, Y.-F.; Yeh, J.-J. Long-Term Impact of COVID-19 on Osteoporosis Risk Among Patients Aged ≥50 Years with New-Onset Overweight, Obesity, or Type 2 Diabetes: A Multi-Institutional Retrospective Cohort Study. Medicina 2025, 61, 1320. https://doi.org/10.3390/medicina61081320
Su S-Y, Sun Y-F, Yeh J-J. Long-Term Impact of COVID-19 on Osteoporosis Risk Among Patients Aged ≥50 Years with New-Onset Overweight, Obesity, or Type 2 Diabetes: A Multi-Institutional Retrospective Cohort Study. Medicina. 2025; 61(8):1320. https://doi.org/10.3390/medicina61081320
Chicago/Turabian StyleSu, Sheng-You, Yi-Fan Sun, and Jun-Jun Yeh. 2025. "Long-Term Impact of COVID-19 on Osteoporosis Risk Among Patients Aged ≥50 Years with New-Onset Overweight, Obesity, or Type 2 Diabetes: A Multi-Institutional Retrospective Cohort Study" Medicina 61, no. 8: 1320. https://doi.org/10.3390/medicina61081320
APA StyleSu, S.-Y., Sun, Y.-F., & Yeh, J.-J. (2025). Long-Term Impact of COVID-19 on Osteoporosis Risk Among Patients Aged ≥50 Years with New-Onset Overweight, Obesity, or Type 2 Diabetes: A Multi-Institutional Retrospective Cohort Study. Medicina, 61(8), 1320. https://doi.org/10.3390/medicina61081320






