Developing a Consensus-Based POCUS Protocol for Critically Ill Patients During Pandemics: A Modified Delphi Study
Abstract
1. Introduction
2. Materials and Methods
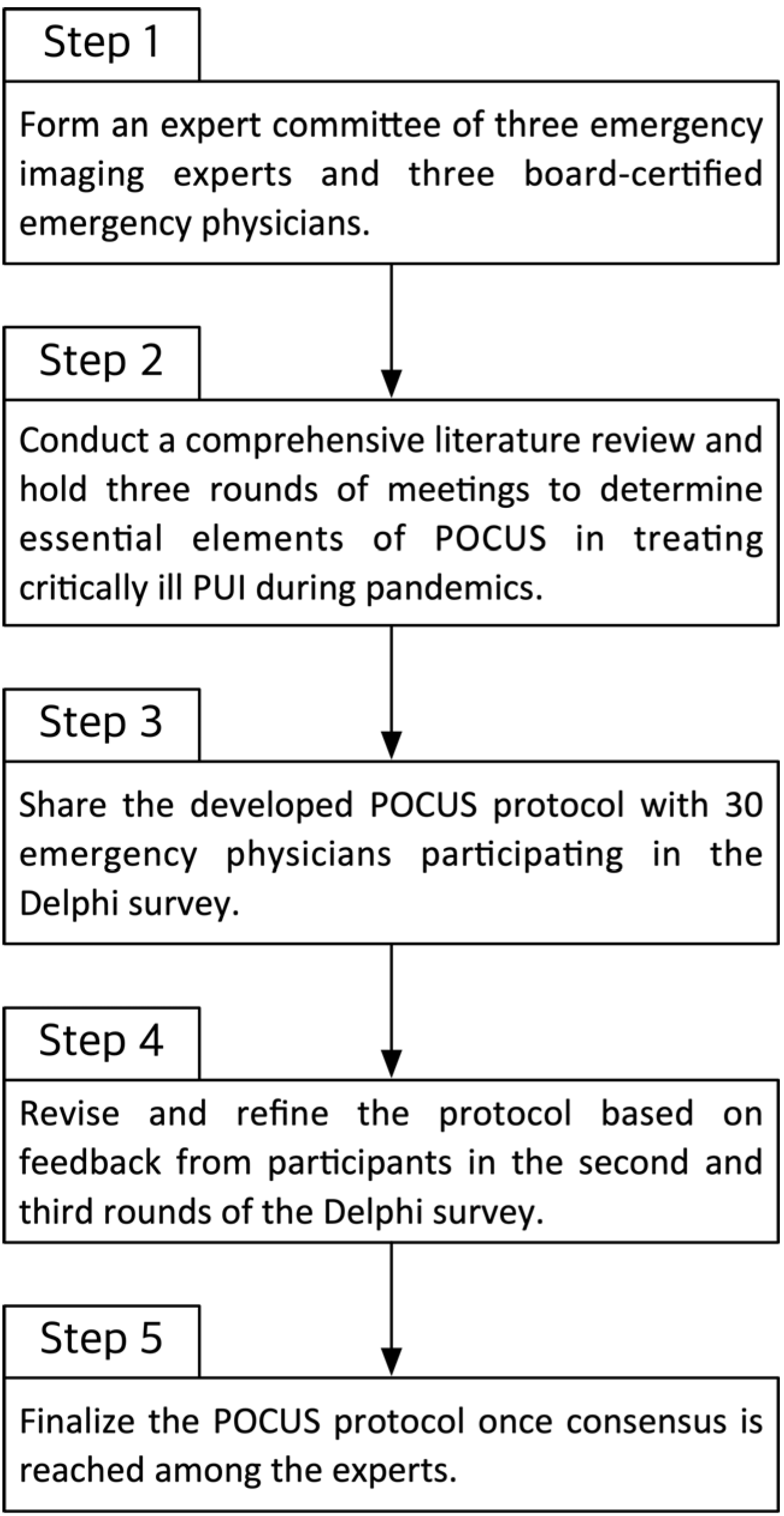
2.1. Assembly of POCUS Committee and Development of POCUS Protocol
2.2. Definition of Target Patient Population
2.3. Participants
2.4. The Modified Delphi Survey Methods
- Round 1: Participants reviewed the initial POCUS protocol and rated their level of agreement with each item (1 = strongly disagree, 9 = strongly agree). They also provided qualitative feedback and suggestions for changes. The committee analyzed the Round 1 responses, identifying items with high agreement and those without consensus, and then revised the protocol accordingly.
- Round 2: The modified protocol (after Round 1 revisions) was redistributed with emphasis on the items that did not reach consensus in Round 1. Participants re-rated these items and could see a summary of the group’s prior responses (anonymously) to inform their reconsideration. Further modifications were made based on Round 2 feedback.
- Round 3: A final round was conducted for any remaining items lacking consensus after Round 2. Participants rated these final items, and the protocol was finalized based on the results. Throughout all rounds, a predefined consensus threshold was used: if ≥70% of participants rated an item 7–9 (agree to strongly agree), that item was considered to have achieved consensus and was adopted. Items not meeting this threshold were either revised or omitted from the final protocol, according to expert feedback.
2.5. Statistical Analysis
3. Results
3.1. Study Participants
3.2. Delphi Survey Results
3.2.1. First Survey Result (Supplementary Table S1)
3.2.2. Second Survey Result (Supplementary Table S2)
3.2.3. Third Survey Result
3.3. Protocol Development
- Step 1: POCUS-Echocardiography. The protocol begins with focused cardiac ultrasound given the critical importance of identifying cardiac causes of shock or instability. The echocardiography component directs the clinician to assess for (a) new or decompensated LV dysfunction (indicative of myocarditis, cardiomyopathy, or ischemia), (b) RV dilation or strain (suggestive of pulmonary embolism or acute cor pulmonale), (c) significant pericardial effusion or tamponade physiology (a reversible cause of shock), (d) hyperdynamic LV with IVC collapse (consistent with hypovolemia or distributive shock), or (e) the absence of major abnormalities (“normal” cardiac ultrasound for the context). Each of these findings leads to branch points in the protocol algorithm. For example, a positive finding of RWMA with LV dysfunction steers the provider to consider ACS and obtain cardiology input, whereas a hyperdynamic, underfilled heart would prompt aggressive volume resuscitation and investigation for underlying distributive shock causes. If the cardiac POCUS is essentially normal (no significant wall motion abnormalities, normal systolic function, no large effusion), the protocol notes this but advises not to stop there—one must then turn to the lungs for further clues.
- Step 2: POCUS-Lung Ultrasound. Following the cardiac assessment, a focused lung ultrasound exam is performed. The protocol calls for the evaluation of bilateral anterior and lateral lung fields (with posterior fields as feasible or if the anterior exam is unrevealing and clinical suspicion remains high). Key lung ultrasound findings are checked: the presence of A-lines (which, along with lung sliding, indicate aerated lung), lung sliding itself (absence of which could indicate pneumothorax if lung points or other signs present), the number and distribution of B-lines (which, if diffuse, suggest pulmonary edema or viral pneumonia, whereas focal B-lines might suggest early pneumonia or atelectasis), pleural effusions (which, if moderate or large, might warrant drainage or further imaging), and consolidations (which indicate pneumonia or ARDS). Pleural line abnormalities (irregular, thickened pleura) are also noted as they can be seen in COVID-19 pneumonia and other pneumonias. The protocol emphasizes that lung findings must be interpreted in conjunction with the cardiac findings. For instance, if the heart appears normal but lung ultrasound shows multiple B-lines and patchy consolidation, a primary pulmonary pathology (like COVID-19 pneumonia or ARDS) is likely. Conversely, if the heart shows acute RV strain and the lungs have minimal findings aside from maybe a small pleural effusion, one should suspect a pulmonary embolism.
- Step 3: Integrated Assessment and Further Actions. The final part of the protocol is a decision aid that combines the cardiac and lung POCUS results to guide next steps. Several common combinations are covered: (a) Cardiac abnormal, Lung abnormal—e.g., depressed LV function with diffuse B-lines might point to acute heart failure exacerbated by infection, or cardiogenic shock plus pneumonia, prompting both cardiology and infectious workups; (b) Cardiac abnormal, Lung relatively normal—e.g., RV strain with A-line lungs suggests possible pulmonary embolism (consider confirmatory CT angiography if the patient is stable or treat empirically if unstable); or isolated tamponade on cardiac POCUS suggests immediate pericardiocentesis; (c) Cardiac normal, Lung abnormal—e.g., normal heart with focal unilateral B-lines and consolidation implies primary pneumonia (treat infection, consider antibiotics, respiratory support), whereas diffuse B-lines with normal heart might suggest early ARDS from sepsis (manage oxygenation, consider lung-protective ventilation); (d) Cardiac normal, Lung normal—if both POCUS exams are largely unremarkable but the patient is still unwell, the protocol advises clinicians to look beyond ultrasound: perform thorough auscultation (for wheezes, stridor, etc.), check for signs of other pathologies (e.g., abdominal source of sepsis), and consider laboratory tests for metabolic or toxic causes of shock. This acknowledges that POCUS, while powerful, will not identify all problems (for example, an acute asthma exacerbation or cyanide toxicity would yield normal ultrasounds yet critical illness).
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACS | Acute Coronary Syndrome |
| COPD | Chronic Obstructive Pulmonary Disease |
| COVID-19 | Coronavirus Disease 2019 |
| CT | Computed Tomography |
| ED | Emergency Department |
| ICU | Intensive Care Unit |
| IVC | Inferior Vena Cava |
| LUS | Lung Ultrasound |
| LV | Left Ventricle |
| POCUS | Point-of-Care Ultrasound |
| PUI | Person Under Investigation |
| RWMA | Regional Wall Motion Abnormality |
| RV | Right Ventricle |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| SECCI | Society of Emergency and Critical Care Imaging |
| STATA | Statistical Software (STATA Corporation) |
References
- Biddinger, P.D.; Shenoy, E.S. Evaluation of the Person Under Investigation. In Bioemergency Planning: A Guide for Healthcare Facilities; Hewlett, A., Murthy, A.R.K., Eds.; Springer: Cham, Switzerland, 2018; pp. 143–156. [Google Scholar]
- Lin, M.; Beliavsky, A.; Katz, K.; Powis, J.E.; Ng, W.; Williams, V.; Michelle Science, M.; Groves, H.; Muller, M.P.; Vaisman, A.; et al. What can early Canadian experience screening for COVID-19 teach us about how to prepare for a pandemic? CMAJ 2020, 192, E314–E318. [Google Scholar] [CrossRef]
- Hanson, K.E.; Caliendo, A.M.; Arias, C.A.; Englund, J.A.; Lee, M.J.; Loeb, M.; Patel, R.; El Alayli, A.; Kalot, M.A.; Falck-Ytter, Y. Infectious Diseases Society of America guidelines on the diagnosis of coronavirus disease 2019. Clin. Infect. Dis. 2020, 71, e1468–e1478. [Google Scholar]
- Abrams, E.R.; Rose, G.; Fields, J.M.; Esener, D. Point-of-care ultrasound in the evaluation of COVID-19. J. Emerg. Med. 2020, 59, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Musa, M.J.; Yousef, M.; Adam, M.; Wagealla, A.; Boshara, L.; Belal, D.; Abukonna, A. The role of lung ultrasound before and during the COVID-19 pandemic: A review article. Curr. Med. Imaging 2022, 18, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Perrone, T.; Soldati, G.; Padovini, L.; Fiengo, A.; Lettieri, G.; Sabatini, U.; Gori, G.; Lepore, F.; Garolfi, M.; Palumbo, I.; et al. A new lung ultrasound protocol able to predict worsening in patients affected by SARS-CoV-2 pneumonia. J. Ultrasound Med. 2021, 40, 1627–1635. [Google Scholar] [CrossRef]
- Dacrema, A.; Silva, M.; Rovero, L.; Vertemati, V.; Losi, G.; Piepoli, M.F.; Sacchi, R.; Mangiacotti, M.; Nazerian, P.; Pagani, L.; et al. A simple lung ultrasound protocol for the screening of COVID-19 pneumonia in the emergency department. Intern. Emerg. Med. 2021, 16, 1965–1973. [Google Scholar] [CrossRef]
- Narasimhan, M.; Koenig, S.J.; Mayo, P.H. A whole-body approach to point-of-care ultrasound. Chest 2016, 150, 772–776. [Google Scholar] [CrossRef]
- Karagöz, A.; Saglam, C.; Demirbas, H.B.; Korkut, S.; Ünlüer, E.E. Accuracy of bedside lung ultrasound as a rapid triage tool for suspected COVID-19 cases. Ultrasound Q. 2020, 36, 339–344. [Google Scholar] [CrossRef]
- Xie, M.; Chou, Y.-H.; Zhang, L.; Zhang, D.; Tiu, C.-M. Application of point-of-care cardiac ultrasonography in COVID-19 infection: Lessons learned from the early experience. J. Med. Ultrasound 2021, 29, 3–6. [Google Scholar] [CrossRef]
- Chua, M.T.; Boon, Y.; Yeoh, C.K.; Li, Z.; Goh, C.J.M.; Kuan, W.S. Point-of-care ultrasound use in COVID-19: A narrative review. Ann. Transl. Med. 2024, 12, 13. [Google Scholar] [CrossRef]
- Matthies, A.; Trauer, M.; Chopra, K.; Jarman, R.D. Diagnostic accuracy of point-of-care lung ultrasound for COVID-19: A systematic review and meta-analysis. Emerg. Med. J. 2023, 40, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Brahier, T.; Meuwly, J.-Y.; Pantet, O.; Brochu Vez, M.-J.; Gerhard Donnet, H.; Hartley, M.-A.; Hugli, O.; Boillat-Blanco, N. Lung ultrasonography for risk stratification in patients with COVID-19: A prospective observational cohort study. Clin. Infect. Dis. 2021, 73, e4189–e4196. [Google Scholar] [CrossRef] [PubMed]
- Blair, P.W.; Siddharthan, T.; Liu, G.; Bai, J.; Cui, E.B.; East, J.R.; Herrera, P.; Anova, L.; Mahadevan, V.B.; Hwang, J.; et al. Point-of-care lung ultrasound predicts severe disease and death due to COVID-19: A prospective cohort study. Crit. Care Explor. 2022, 4, e0732. [Google Scholar] [CrossRef] [PubMed]
- Polyzogopoulou, E.; Velliou, M.; Verras, C.; Ventoulis, I.; Parissis, J.; Osterwalder, J.; Hoffmann, B. Point-of-care ultrasound: A multimodal tool for the management of sepsis in the emergency department. Medicina 2023, 59, 1180. [Google Scholar] [CrossRef]
- Coneybeare, D.; Das, D.; Lema, P.; Chang, B.; Ng, L. COVUS: An algorithm to maximize the use of point-of-care ultrasound in the emergency management of COVID-19. J. Emerg. Med. 2021, 61, 61–66. [Google Scholar] [CrossRef]
- Huang, G.; Vengerovsky, A.; Morris, A.; Town, J.; Carlbom, D.; Kwon, Y. Development of a COVID-19 point-of-care ultrasound protocol. J. Am. Soc. Echocardiogr. 2020, 33, 903–905. [Google Scholar] [CrossRef]
- Shrestha, A.P.; Blank, W.; Blank, U.H.; Horn, R.; Morf, S.; Shrestha, S.K.; Shrestha, S.P.; Basnet, S.; Dongol, A.; Dangal, R.K.; et al. Delphi consensus recommendations for the development of the emergency medicine point-of-care ultrasound (POCUS) curriculum in Nepal. POCUS J. 2024, 9, 133–142. [Google Scholar] [CrossRef]
- Wong, J.; Olszynski, P.; Cheung, W.; Pageau, P.; Lewis, D.; Kwan, C.; Woo, M.Y. Position statement: Minimum archiving requirements for emergency medicine PoCUS—A modified Delphi-derived national consensus. CJEM 2021, 23, 450–454. [Google Scholar] [CrossRef]
- Schnittke, N.; Russell, F.M.; Gottlieb, M.; Lam, S.H.F.; Kessler, D.O.; Roppolo, L.P.; Demasi, S.C.; Henwood, P.; Liu, Y.T.; Marin, J.R.; et al. Standards for point-of-care ultrasound research reporting (SPUR): A modified Delphi to develop a framework for reporting POCUS research. Acad. Emerg. Med. 2025. online ahead of print. [Google Scholar] [CrossRef]
- Kok, B.; Schuit, F.; Lieveld, A.; Azijli, K.; Nanayakkara, P.W.; Bosch, F. Comparing lung ultrasound: Extensive versus short in COVID-19 (CLUES): A multicentre, observational study in the ED. BMJ Open 2021, 11, e048795. [Google Scholar] [CrossRef]
- García-Cruz, E.; Manzur-Sandoval, D.; Rascón-Sabido, R.; Gopar-Nieto, R.; Barajas-Campos, R.L.; Jordán-Ríos, A.; Martínez, D.S.; Jiménez-Rodríguez, G.M.; Murillo-Ochoa, A.L.; Díaz-Méndez, A.; et al. Critical care ultrasonography during COVID-19 pandemic: The ORACLE protocol. Echocardiography 2020, 37, 1353–1361. [Google Scholar] [CrossRef]
- Henwood, P.C. Imaging an outbreak—Ultrasound in an Ebola treatment unit. N. Engl. J. Med. 2019, 381, 6–9. [Google Scholar] [CrossRef]

| Category | General Emergency Physician | Intensivist | Pediatric Emergency Physician | Total |
|---|---|---|---|---|
| Years of Experience | ||||
| 1–5 years | 4 (10.3%) | 3 (7.7%) | 3 (7.7%) | 10 (25.6%) |
| 6–10 years | 7 (17.9%) | 4 (10.3%) | 4 (10.3%) | 15 (38.5%) |
| 11–15 years | 5 (12.8%) | 2 (5.1%) | 3 (7.7%) | 10 (25.6%) |
| 16+ years | 4 (10.3%) | 2 (5.1%) | 2 (5.1%) | 8 (20.5%) |
| Total | 20 (51.3%) | 9 (23.1%) | 10 (25.6%) | 39 (100%) |
| Hospital Type | ||||
| Secondary Hospital | 10 (25.6%) | 6 (15.4%) | 5 (12.8%) | 21 (53.8%) |
| Tertiary Hospital | 9 (23.1%) | 3 (7.7%) | 6 (15.4%) | 18 (46.2%) |
| Academic Hospital | 8 (20.5%) | 5 (12.8%) | 6 (15.4%) | 19 (48.7%) |
| Expert Delphi Survey on Items to be Included in the Point-of-Care Ultrasound (POCUS) Protocol in the Event of an Infectious Disaster | |||||||||
| Please indicate the degree of consent to the questions below in V [1 = Very disagree~9 = Very agree] | |||||||||
| POCUS-echocardiography | |||||||||
| Q1 | POCUS-echocardiography shall be included within this protocol. | ||||||||
| A1 | Strongly disagree | Strongly agree | |||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| 1/39 (3%) | 1/39 (3%) | 10/39 (26%) | 27/39 (69%) | ||||||
| Q2,3_1 | ACS w/u is considered when RWMA is shown in the presence of new or aggravated left ventricle dysfunction or RV dilatation or strain with POCUS-echocardiography. | ||||||||
| A2 | Strongly disagree | Strongly agree | |||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| 4/39 (10%) | 1/39 (3%) | 7/39 (18%) | 14/39 (36%) | 13/39 (33%) | |||||
| Q4 | Evaluate the presence of large amounts of pericardial effusion and tamponade features with POCUS-echocardiography. | ||||||||
| A4 | Strongly disagree | Strongly agree | |||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| 5/39 (13%) | 7/39 (18%) | 27/39 (69%) | |||||||
| Q5_1 | If POCUS-echocardiography is normal and A-line + long sliding is seen in LUS, but the patient’s dyspnea is continued, auscultation is performed to check COPD, asthma, etc., and additional tests are considered if necessary to confirm other metabolic causes. | ||||||||
| A5 | Strongly disagree | Strongly agree | |||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| 1/39 (3%) | 3/39 (3%) | 3/39 (8%) | 6/39 (15%) | 12/39 (31%) | 14/39 (36%) | ||||
| POCUS-lung ultrasound | |||||||||
| Q7 | POCUS-lung ultrasound should be included within this protocol. | ||||||||
| A7 | Strongly disagree | Strongly agree | |||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| 3/39 (8%) | 4/39 (10%) | 5/39 (13%) | 27/39 (69%) | ||||||
| Q8_1 | POCUS-lung ultrasound scans six areas of the chest (see attachment 2) but considers adding dorsal if six areas are normal or highly suspected of pneumonia. | ||||||||
| A8 | Strongly disagree | Strongly agree | |||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| 1/39 (3%) | 2/39 (5%) | 4/39 (10%) | 2/39 (5%) | 18/39 (46%) | 6/39 (15%) | 6/39 (15%) | |||
| Q9 | Check if A-line and Lung sliding are present with POCUS-lung ultrasound. | ||||||||
| A9 | Strongly disagree | Strongly agree | |||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| 1/39 (3%) | 6/39 (15%) | 7/39 (18%) | 25/39 (64%) | ||||||
| Q10 | The presence, distribution, and density of B-line are evaluated with POCUS-lung ultrasound. | ||||||||
| A10 | Strongly disagree | Strongly agree | |||||||
| 1 | 2 | 3 | 4 | 5 | 6 (10%) | 7 | 8 | 9 | |
| 1/39 (3%) | 4/39 (10%) | 5/39 (13%) | 9/39 (23%) | 20/39 (51%) | |||||
| Q11 | Evaluate the presence or absence of pleural effusion with POCUS-lung ultrasound. | ||||||||
| A11 | Strongly disagree | Strongly agree | |||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| 1/39 (3%) | 1/39 (3%) | 4/39 (10%) | 11/39 (28%) | 22/39 (56%) | |||||
| Q12 | Evaluate the presence or absence of consolidation with POCUS-lung ultrasound. | ||||||||
| A12 | Strongly disagree | Strongly agree | |||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| 1/39 (3%) | 1/39 (3%) | 1/39 (3%) | 3/39 (8%) | 5/39(13%) | 8/39 (21%) | 8/39 (21%) | 12/39 (31%) | ||
| Q13 | Evaluate the presence or absence of pneumothorax with POCUS-lung ultrasound. | ||||||||
| A13 | Strongly disagree | Strongly agree | |||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| 1/39 (3%) | 2/39 (5%) | 8/39 (21%) | 10/39 (26%) | 18/39 (46%) | |||||
| Q14 | The status of lung is evaluated by findings such as B-line, lung sliding, pleural irregularities, pleural effect, and consolidation with POCUS-lung ultrasound. | ||||||||
| A14 | Strongly disagree | Strongly agree | |||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| 1/39 (3%) | 1/39 (3%) | 2/39 (5%) | 6/39 (15%) | 8/39 (21%) | 21/39 (54%) | ||||
| Other POCUS or additional imaging tests | |||||||||
| Q16_1 | If you have RV dilatation or strain with POCUS-echocardiography, but you see a B-line in LUS, there is no lung sliding, consider additional w/u | ||||||||
| A16 | Strongly disagree | Strongly agree | |||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| 2/39 (5%) | 4/39 (10%) | 3/39 (8%) | 13/39 (33) | 9/39 (23%) | 8/39 (21%) | ||||
| Q17_1 | Aorta CT is considered if aortic detection is suspected if there is a large amount of pericardial effect and tamponade feature with POCUS-echocardiography. | ||||||||
| A17 | Strongly disagree | Strongly agree | |||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| 1/39 (3%) | 2/39 (5%) | 1/39 (3%) | 2/39 (5%) | 2/39 (5%) | 5/39(13%) | 10/39 (26%) | 16/39 (41%) | ||
| Q18 | If you have any additional comments regarding POCUS, please write them down. (Description) | ||||||||
| A18 | It takes a lot of time to scan six areas to find pneumonia in the field, and there is concern about the efficiency of the extra dorsal scan. | ||||||||
| Additional w/u for chronic RV strain is not likely to be implemented in the field. | |||||||||
| On second thought, looking to the dorsal side in POCUS-lung ultrasound is considered to be a waste of time and excessive. | |||||||||
| POCUS should be carried out in the event of an infection disaster, should be given value as a means to quickly diagnose and treat in the quarantine area. | |||||||||
| It is difficult to understand the expression that B-line is visible in the US, there is no lung sliding, or wheezing in the stethoscope. Change it to a clear expression. | |||||||||
| When you present the protocol in the future, you will need a helper to scan your back in a severely ill patient. | |||||||||
| It would be better to present it only for the US test except for the stethoscope findings. | |||||||||
| Not adding the dorsal side, can be considered without implementation, so I think this can be logically natural regardless of the actual clinical application. | |||||||||
| I wonder how we can evaluate this later. | |||||||||
| If you do a back test, it will take a long time and the accuracy will be low, so it would be better not to do a back ultrasound. | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, H.; Lee, J.H.; Suh, D.; You, K.M.; PULSE Group. Developing a Consensus-Based POCUS Protocol for Critically Ill Patients During Pandemics: A Modified Delphi Study. Medicina 2025, 61, 1319. https://doi.org/10.3390/medicina61081319
Kwon H, Lee JH, Suh D, You KM, PULSE Group. Developing a Consensus-Based POCUS Protocol for Critically Ill Patients During Pandemics: A Modified Delphi Study. Medicina. 2025; 61(8):1319. https://doi.org/10.3390/medicina61081319
Chicago/Turabian StyleKwon, Hyuksool, Jin Hee Lee, Dongbum Suh, Kyoung Min You, and PULSE Group. 2025. "Developing a Consensus-Based POCUS Protocol for Critically Ill Patients During Pandemics: A Modified Delphi Study" Medicina 61, no. 8: 1319. https://doi.org/10.3390/medicina61081319
APA StyleKwon, H., Lee, J. H., Suh, D., You, K. M., & PULSE Group. (2025). Developing a Consensus-Based POCUS Protocol for Critically Ill Patients During Pandemics: A Modified Delphi Study. Medicina, 61(8), 1319. https://doi.org/10.3390/medicina61081319







