Challenges in Identifying Biomarkers of Frailty Syndrome: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Description of Analysis and Presentation of Data
2.5. Qualitative Analysis
2.6. Risk of Bias (Quality) Assessment
3. Results
3.1. Data Search Results and Characteristics of Included Studies
3.1.1. Challenges in FS Biomarker Research
Blood Biomarkers
Genetic, Urine, and Saliva Biomarkers
Risk of Bias (Quality) Assessment Results
4. Discussion
- Developing standardized protocols for laboratory biomarker measurement and FS assessment to enhance comparability across studies.
- Conducting large-scale, longitudinal studies to elucidate causal relationships and the temporal dynamics of biomarkers in FS development.
- Incorporating multifactorial analyses that account for confounding variables and explore interactions between biomarkers, comorbidities, and lifestyle factors.
- Exploring the biological pathways linking biomarkers to FS to inform targeted therapeutic strategies.
- Including diverse ethnicities and considering various aspects of FS (psychological, social, biological, environmental factors) to enhance the generalizability of findings.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| FS | Frailty syndrome |
| FI | Frailty index |
| CRP | C-reactive protein |
| IL-6 | Interleukin 6 |
| TNF-α | Tumor necrosis factor |
| RNA | Ribonucleic acid |
| DNA | Deoxyribonucleic acid |
| NLR | Neutrophil–lymphocyte ratio |
| GDF-15 | Growth Differentiation Factor-15 |
References
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef]
- British Geriatrics Society. Frailty: What’s It All. 2018. Available online: https://www.bgs.org.uk/resources/frailty-what’s-it-all-about (accessed on 1 July 2024).
- World Health Organization. World Report on Ageing and Health. 2015. Available online: https://apps.who.int/iris/handle/10665/186463 (accessed on 1 July 2024).
- Buchner, D.M.; Wagner, E.H. Preventing Frail Health. Clin. Geriatr. Med. 1992, 8, 1–18. [Google Scholar] [CrossRef]
- Sciacchitano, S.; Carola, V.; Nicolais, G.; Sciacchitano, S.; Napoli, C.; Mancini, R.; Rocco, M.; Coluzzi, F. To Be Frail or Not to Be Frail: This Is the Question—A Critical Narrative Review of Frailty. J. Clin. Med. 2024, 13, 721. [Google Scholar] [CrossRef]
- Rane, M.; Orkaby, A.R. Considerations for carotid artery disease management in a frail population. Exp. Gerontol. 2021, 152, 111426. [Google Scholar] [CrossRef]
- Rodríguez-Mañas, L.; Rodriguez-Sánchez, I. Research on Frailty: Where We Stand and Where We Need to Go. J. Am. Med. Dir. Assoc. 2021, 22, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Travers, J.; Romero-Ortuno, R.; Bailey, J.; Cooney, M.T. Delaying and reversing frailty: A systematic review of primary care interventions. Br. J. Gen. Pract. 2019, 69, E61–E69. [Google Scholar] [CrossRef] [PubMed]
- Dent, E.; Martin, C.; Bergman, H.; Woo, J.; Romero-Ortuno, R.; Walston, J.D. Series Frailty 2 Management of frailty: Opportunities, challenges, and future directions. Lancet 2019, 394, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Hoogendijk, E.O.; Afilalo, J.; Ensrud, K.E.; Kowal, P.; Onder, G.; Fried, L.P. Frailty 1 Frailty: Implications for clinical practice and public health. Lancet 2019, 394, 1365–1375. [Google Scholar] [CrossRef]
- Hoogendijk, E.O.; Dent, E. Trajectories, Transitions, and Trends in Frailty among Older Adults: A Review. Ann. Geriatr. Med. Res. 2022, 26, 289–295. [Google Scholar] [CrossRef]
- Dent, E.; Hanlon, P.; Sim, M.; Jylhävä, J.; Liu, Z.; Vetrano, D.L.; Stolz, E.; Pérez-Zepeda, M.U.; Crabtree, D.; Nicholson, C.; et al. Recent developments in frailty identification, management, risk factors and prevention: A narrative review of leading journals in geriatrics and gerontology. Ageing Res. Rev. 2023, 91, 102082. [Google Scholar] [CrossRef]
- Angioni, D.; Lu, W.H.; Sourdet, S.; Macaron, T.; Takeda, C.; Guyonnet, S.; Mangin, J.F.; Rolland, Y.; de Souto Barreto, P.; Vellas, B. Biomarkers of Age-Related Frailty and Frailty Related to Diseases: An Exploratory, Cross-Sectional Analysis from the MAPT Study. J. Nutr. Health Aging 2022, 26, 545–551. [Google Scholar] [CrossRef]
- Chainani, V.; Shaharyar, S.; Dave, K.; Choksi, V.; Ravindranathan, S.; Hanno, R.; Jamal, O.; Abdo, A.; Rafeh, N.A. Objective measures of the frailty syndrome (hand grip strength and gait speed) and cardiovascular mortality: A systematic review. Int. J. Cardiol. 2016, 215, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Maden-Wilkinson, T.M.; Degens, H.; Jones, D.A.; McPhee, J.S. Comparison of MRI and DXA to measure muscle size and age-related atrophy in thigh muscles. J. Musculoskelet. Neuronal Interact. 2013, 13, 320–328. [Google Scholar] [PubMed]
- Sepúlveda, M.; Arauna, D.; García, F.; Albala, C.; Palomo, I.; Fuentes, E. Frailty in Aging and the Search for the Optimal Biomarker: A Review. Biomedicines 2022, 10, 1426. [Google Scholar] [CrossRef] [PubMed]
- Dent, E.; Kowal, P.; Hoogendijk, E.O. Frailty measurement in research and clinical practice: A review. Eur. J. Intern. Med. 2016, 31, 3–10. [Google Scholar] [CrossRef]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. C. Can. Med. Assoc. J. 2005, 173, 489–495. [Google Scholar] [CrossRef]
- Mitnitski, A.B.; Mogilner, A.J.; Rockwood, K. Accumulation of deficits as a proxy measure of aging. Sci. World J. 2001, 1, 323–336. [Google Scholar] [CrossRef]
- Morley, J. Frailty consensus: A call to action. J. Am. Med. Dir. Assoc. 2014, 14, 392–397. [Google Scholar] [CrossRef]
- Thomas, J.; Harden, A. Methods for the thematic synthesis of qualitative research in systematic reviews. BMC Med. Res. Methodol. 2008, 8, 45. [Google Scholar] [CrossRef]
- Tableau Business Intelligence and Analytics Software n.d. Available online: www.tableau.com (accessed on 23 October 2024).
- Lunny, C.; Kanji, S.; Thabet, P.; Haidich, A.-B.; Bougioukas, K.I.; Pieper, D. Assessing the methodological quality and risk of bias of systematic reviews: Primer for authors of overviews of systematic reviews. BMJ Med. 2024, 3, e000604. [Google Scholar] [CrossRef]
- Hsu, B.; Hirani, V.; Cumming, R.G.; Naganathan, V.; Blyth, F.M.; Wright, F.C.; Waite, L.M.; Seibel, M.J.; Handelsman, D.J.; Le Couteur, D.G. Cross-sectional and longitudinal relationships between inflammatory biomarkers and frailty in community-dwelling older men: The concord health and ageing in men project. J. Gerontol. 2019, 74, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Buondonno, I.; Sassi, F.; Cattaneo, F.; D’Amelio, P. Association between Immunosenescence, Mitochondrial Dysfunction and Frailty Syndrome in Older Adults. Cells 2023, 12, 44. [Google Scholar] [CrossRef]
- Pansarasa, O.; Mimmi, M.C.; Davin, A.; Giannini, M.; Guaita, A.; Cereda, C. Inflammation and cell-to-cell communication, two related aspects in frailty. Immun. Ageing 2022, 19, 49. [Google Scholar] [CrossRef]
- Samson, L.D.; Engelfriet, P.; Verschuren, W.M.M.; Picavet, H.S.J.; Ferreira, J.A.; de Zeeuw-Brouwer, M.-L.; Buisman, A.M.; Boots, A.M.H. Impaired JAK-STAT pathway signaling in leukocytes of the frail elderly. Immun. Ageing 2022, 19, 5. [Google Scholar] [CrossRef] [PubMed]
- Semmarath, W.; Seesen, M.; Yodkeeree, S.; Sapbamrer, R.; Ayood, P.; Malasao, R.; Siviroj, P.; Limtrakul (Dejkriengkraikul), P. The association between frailty indicators and blood-based biomarkers in early-old community dwellers of Thailand. Int. J. Environ. Res. Public Health 2019, 16, 3457. [Google Scholar] [CrossRef]
- van Sleen, Y.; Shetty, S.A.; van der Heiden, M.; Venema, M.C.A.; Gutiérrez-Melo, N.; Toonen, E.J.M.; van Beek, J.; Buisman, A.M.; van Baarle, D.; Sauce, D. Frailty is related to serum inflammageing markers: Results from the VITAL study. Immun. Ageing 2023, 20, 68. [Google Scholar] [CrossRef]
- Teixeira-Gomes, A.; Laffon, B.; Valdiglesias, V.; Gostner, J.M.; Felder, T.; Costa, C.; Madureira, J.; Fuchs, D.; Teixeira, J.P.; Costa, S. Exploring early detection of frailty syndrome in older adults: Evaluation of Oxi-immune markers, clinical parameters and modifiable risk factors. Antioxidants 2021, 10, 1975. [Google Scholar] [CrossRef]
- Welstead, M.; Muniz-Terrera, G.; Russ, T.C.; Corley, J.; Taylor, A.M.; Gale, C.R.; Luciano, M. Inflammation as a risk factor for the development of frailty in the Lothian Birth Cohort 1936. Exp. Gerontol. 2020, 139, 111005. [Google Scholar] [CrossRef]
- Xu, W.; Liang, Y.; Lin, Z. Association Between Neutrophil–Lymphocyte Ratio and Frailty: The Chinese Longitudinal Healthy Longevity Survey. Front. Med. 2022, 8, 783077. [Google Scholar] [CrossRef]
- Zhang, H.; Hao, M.; Hu, Z.; Li, Y.; Jiang, X.; Wang, J.; Jin, L.; Liu, Z.; Wang, X.; Sun, X. Association of immunity markers with the risk of incident frailty: The Rugao longitudinal aging study. Immun. Ageing 2022, 19, 1–9. [Google Scholar] [CrossRef]
- Arauna, D.; García, F.; Rodríguez-Mañas, L.; Marrugat, J.; Sáez, C.; Alarcón, M.; Wehinger, S.; Espinosa-Parrilla, Y.; Palomo, I.; Fuentes, E. Older adults with frailty syndrome present an altered platelet function and an increased level of circulating oxidative stress and mitochondrial dysfunction biomarker GDF-15. Free Radic. Biol. Med. 2020, 149, 64–71. [Google Scholar] [CrossRef]
- Kamper, R.S.; Nygaard, H.; Praeger-Jahnsen, L.; Ekmann, A.; Ditlev, S.B.; Schultz, M.; Hansen, S.K.; Hansen, P.; Pressel, E.; Suetta, C. GDF-15 is associated with sarcopenia and frailty in acutely admitted older medical patients. J. Cachexia. Sarcopenia Muscle 2024, 15, 1549–1557. [Google Scholar] [CrossRef]
- Castro-Herrera, V.M.; Lown, M.; Fisk, H.L.; Owen-Jones, E.; Lau, M.; Lowe, R.; Hood, K.; Gillespie, D.; Hobbs, F.D.R.; Little, P.; et al. Relationships Between Age, Frailty, Length of Care Home Residence and Biomarkers of Immunity and Inflammation in Older Care Home Residents in the United Kingdom. Front. Aging 2021, 2, 599084. [Google Scholar] [CrossRef] [PubMed]
- Landino, K.; Tanaka, T.; Fantoni, G.; Candia, J.; Bandinelli, S.; Ferrucci, L. Characterization of the plasma proteomic profile of frailty phenotype. GeroScience 2021, 43, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chen, X.; Han, B. Effect modification by sex of the hemoglobin concentration on frailty risk in hospitalized older patients. Clin. Interv. Aging 2021, 16, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Roh, E.; Hwang, S.Y.; Song, E.; Park, M.J.; Yoo, H.J.; Baik, S.H.; Kim, M.; Won, C.W.; Choi, K.M. Association of plasma brain-derived neurotrophic factor levels and frailty in community-dwelling older adults. Sci. Rep. 2022, 12, 1–8. [Google Scholar] [CrossRef]
- Sanz, B.; Arrieta, H.; Hervás, G.; Rezola-Pardo, C.; Ruiz-Litago, F.; Iturburu, M.; Gil, S.M.; Rodríguez-Larrad, A.; Irazusta, J. Serum adiponectin is associated with body composition and cognitive and psychological status in older adults living in long-term nursing homes. Exp. Gerontol. 2019, 121, 1–9. [Google Scholar] [CrossRef]
- Sanz, B.; Arrieta, H.; Rezola-Pardo, C.; Fernández-Atutxa, A.; Garin-Balerdi, J.; Arizaga, N.; Rodriguez-Larrad, A.; Irazusta, J. Low serum klotho concentration is associated with worse cognition, psychological components of frailty, dependence, and falls in nursing home residents. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Sanz, B.; Rezola-Pardo, C.; Arrieta, H.; Fraile-Bermúdez, A.B.; Alonso-Puyo, J.; Molano, I.; Rodriguez-Larrad, A.; Irazusta, J. Serum Sestrin-1 Concentration Is Higher in Frail than Non-Frail Older People Living in Nursing Homes. Int. J. Environ. Res. Public Health 2022, 19, 1079. [Google Scholar] [CrossRef]
- Shardell, M.; Semba, R.D.; Kalyani, R.R.; Bandinelli, S.; Prather, A.A.; Chia, C.W.; Ferrucci, L. Plasma Klotho and Frailty in Older Adults: Findings from the InCHIANTI Study. J. Gerontol. 2019, 74, 1052–1058. [Google Scholar] [CrossRef]
- Valentini, A.; Cianfarani, M.A.; Tarantino, U.; Di Daniele, N.; Bertoli, A. Osteoprotegerin as a biomarker of geriatric frailty syndrome. Aging 2019, 11, 4900–4909. [Google Scholar] [CrossRef]
- Gomez-Cabrero, D.; Walter, S.; Abugessaisa, I.; Miñambres-Herraiz, R.; Palomares, L.B.; Butcher, L.; Erusalimsky, J.D.; Garcia-Garcia, F.J.; Carnicero, J.; Hardman, T.C.; et al. A robust machine learning framework to identify signatures for frailty: A nested case-control study in four aging European cohorts. GeroScience 2021, 43, 1317–1329. [Google Scholar] [CrossRef]
- Henning, T.; Kochlik, B.; Ara, I.; González-Gross, M.; Fiorillo, E.; Marongiu, M.; Cucca, F.; Rodriguez-Artalejo, F.; Carnicero Carreño, J.A.; Rodriguez-Mañas, L.; et al. Patterns of Dietary Blood Markers Are Related to Frailty Status in the FRAILOMIC Validation Phase. Nutrients 2023, 15, 1142. [Google Scholar] [CrossRef] [PubMed]
- Chew, J.; Tay, L.; Lim, J.P.; Leung, B.P.; Yeo, A.; Yew, S.; Ding, Y.Y.; Lim, W.S. Serum Myostatin and IGF-1 as Gender-Specific Biomarkers of Frailty and Low Muscle Mass in Community-Dwelling Older Adults. J. Nutr. Heal Aging 2019, 23, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Kochlik, B.; Stuetz, W.; Pérès, K.; Pilleron, S.; Féart, C.; García García, F.J.; Bandinelli, S.; Gomez-Cabrero, D.; Rodriguez-Mañas, L.; Grune, T.; et al. Associations of fat-soluble micronutrients and redox biomarkers with frailty status in the FRAILOMIC initiative. J. Cachexia. Sarcopenia Muscle 2019, 10, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Machado-Fragua, M.D.; Hoogendijk, E.O.; Struijk, E.A.; Rodriguez-Artalejo, F.; Lopez-Garcia, E.; Beulens, J.W.; van Ballegooijen, A.J. High dephospho-uncarboxylated matrix Gla protein concentrations, a plasma biomarker of vitamin K, in relation to frailty: The Longitudinal Aging Study Amsterdam. Eur. J. Nutr. 2020, 59, 1243–1251. [Google Scholar] [CrossRef]
- Malaguarnera, G.; Catania, V.E.; Bonfiglio, C.; Bertino, G.; Vicari, E.; Malaguarnera, M. Carnitine serum levels in frail older subjects. Nutrients 2020, 12, 3887. [Google Scholar] [CrossRef]
- Ngestiningsih, D.; Dayanti, J.K.; Batubara, L. Relationship between il-6, il-1β, and vitamin d on frailty status in elderly women. Bali Med. J. 2021, 10, 336–339. [Google Scholar] [CrossRef]
- Pillatt, A.P.; Da Silva, B.; Franz, L.B.B.; Berlezi, E.M.; Schneider, R.H. Muscle, endocrine, and immunological markers of frailty in older people. Exp. Gerontol. 2021, 151, 111405. [Google Scholar] [CrossRef]
- Pilleron, S.; Weber, D.; Pérès, K.; Colpo, M.; Gomez-Cabrero, D.; Stuetz, W.; Dartigues, J.F.; Ferrucci, L.; Bandinelli, S.; Garcia-Garcia, F.J.; et al. Patterns of circulating fat-soluble vitamins and carotenoids and risk of frailty in four European cohorts of older adults. Eur. J. Nutr. 2019, 58, 379–389. [Google Scholar] [CrossRef]
- Rattray, N.J.W.; Trivedi, D.K.; Xu, Y.; Chandola, T.; Johnson, C.H.; Marshall, A.D.; Mekli, K.; Rattray, Z.; Tampubolon, G.; Vanhoutte, B.; et al. Metabolic dysregulation in vitamin E and carnitine shuttle energy mechanisms associate with human frailty. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Vaes, A.M.M.; Brouwer-Brolsma, E.M.; Toussaint, N.; de Regt, M.; Tieland, M.; van Loon, L.J.C.; de Groot, L.C.P.G.M. The association between 25-hydroxyvitamin D concentration, physical performance and frailty status in older adults. Eur. J. Nutr. 2019, 58, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Wu, M.; Cui, J.; Yuan, M.; Chen, Y.; Zeng, T. Plasma 25-hydroxyvitamin D level and the risk of frailty among Chinese community-based oldest-old: Evidence from the CLHLS study. BMC Geriatr. 2020, 20, 1–8. [Google Scholar] [CrossRef]
- Arauna, D.; Chiva-Blanch, G.; Padró, T.; Fuentes, E.; Palomo, I.; Badimon, L. Frail older adults show a distinct plasma microvesicle profile suggesting a prothrombotic and proinflammatory phenotype. J. Cell. Physiol. 2021, 236, 2099–2108. [Google Scholar] [CrossRef] [PubMed]
- Hammami, S.; Ghzaiel, I.; Hammouda, S.; Sakly, N.; Hammami, M.; Zarrouk, A. Evaluation of pro-inflammatory cytokines in frail Tunisian older adults. PLoS ONE 2020, 15, e0242152. [Google Scholar] [CrossRef]
- Yin, M.; Zhang, X.; Zheng, X.; Chen, C.; Tang, H.; Yu, Z.; He, X.; Jing, W.; Tang, X.; Xu, X.; et al. Cholesterol alone or in combination is associated with frailty among community-dwelling older adults: A cross-sectional study. Exp. Gerontol. 2023, 180, 112254. [Google Scholar] [CrossRef]
- Brunelli, L.; Davin, A.; Sestito, G.; Mimmi, M.C.; De Simone, G.; Balducci, C.; Pansarasa, O.; Forloni, G.; Cereda, C.; Pastorelli, R.; et al. Plasmatic Hippuric Acid as a Hallmark of Frailty in an Italian Cohort: The Mediation Effect of Fruit-Vegetable Intake. J. Gerontol 2021, 76, 2081–2089. [Google Scholar] [CrossRef]
- Jang, I.-Y.; Park, J.H.; Kim, J.H.; Lee, S.; Lee, E.; Lee, J.Y.; Park, S.J.; Kim, D.A.; Hamrick, M.W.; Kim, B.-J. The association of circulating kynurenine, a tryptophan metabolite, with frailty in older adults. Aging 2020, 12, 22253–22265. [Google Scholar] [CrossRef]
- Wei, Y.; Zhou, J.; Zhao, F.; Chen, C.; Wang, J.; Luo, Y.; Li, C.; Xiong, J.; Lv, Y.; Li, J.; et al. Association of blood lead exposure with frailty and its components among the Chinese oldest old. Ecotoxicol. Environ. Saf. 2022, 242, 113959. [Google Scholar] [CrossRef]
- Zawadzki, B.; Mazur, G.; Butrym, A. Iron dysregulation and frailty syndrome. J. Clin. Med. 2021, 10, 5596. [Google Scholar] [CrossRef]
- Sanz, B.; Rezola-Pardo, C.; Arrieta, H.; Fernández-Atutxa, A.; Lora-Diaz, I.; Gil-Goikouria, J.; Rodriguez-Larrad, A.; Irazusta, J. High serum angiotensin-converting enzyme 2 activity as a biomarker of frailty in nursing home residents. Exp. Gerontol. 2022, 158, 111655. [Google Scholar] [CrossRef]
- Hammami, S.; Zarrouk, A.; Piron, C.; Almas, I.; Sakly, N.; Latteur, V. Prevalence and factors associated with frailty in hospitalized older patients. BMC Geriatr. 2020, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Yan, J.; Xu, L.; Shen, S.; Zeng, X.; Chen, L. Relationship between nutritional status and frailty in hospitalized older patients. Clin. Interv. Aging 2019, 14, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, W.; Zhu, M.; Wen, X.; Jin, J.; Wang, H.; Lv, D.; Zhao, S.; Wu, X.; Jiao, J. Myokines and Biomarkers of Frailty in Older Inpatients with Undernutrition: A Prospective Study. J. Frailty Aging 2024, 13, 82–90. [Google Scholar] [CrossRef] [PubMed]
- McKechnie, D.G.J.; Olia Papacosta, A.; Lennon, L.T.; Ramsay, S.E.; Whincup, P.H.; Goya Wannamethee, S. Associations between inflammation, cardiovascular biomarkers and incident frailty: The British Regional Heart Study. Age Ageing 2021, 50, 1979–1987. [Google Scholar] [CrossRef]
- Agostini, S.; Mancuso, R.; Citterio, L.A.; Mihali, G.A.; Arosio, B.; Clerici, M. Evaluation of serum miRNAs expression in frail and robust subjects undergoing multicomponent exercise protocol (VIVIFRAIL). J. Transl. Med. 2023, 21, 1–9. [Google Scholar] [CrossRef]
- Carini, G.; Mingardi, J.; Bolzetta, F.; Cester, A.; Bolner, A.; Nordera, G.; La Via, L.; Ieraci, A.; Russo, I.; Maggi, S.; et al. miRNome Profiling Detects miR-101-3p and miR-142-5p as Putative Blood Biomarkers of Frailty Syndrome. Genes 2022, 13, 231. [Google Scholar] [CrossRef]
- Selenius, J.S.; Silveira, P.P.; Haapanen, M.J.; Von Bonsdorff, M.; Lahti, J.; Eriksson, J.G.; Wasenius, N.S. The brain insulin receptor gene network and associations with frailty index. Age Ageing 2024, 53, afae091. [Google Scholar] [CrossRef]
- Grasselli, C.; Bombelli, S.; Eriani, S.; Domenici, G.; Galluccio, R.; Tropeano, C.; De Marco, S.; Bolognesi, M.M.; Torsello, B.; Bianchi, C.; et al. DNA Damage in Circulating Hematopoietic Progenitor Stem Cells as Promising Biological Sensor of Frailty. J. Gerontol 2022, 77, 1279–1286. [Google Scholar] [CrossRef]
- Inglés, M.; Mas-Bargues, C.; Gimeno-Mallench, L.; Cruz-Guerrero, R.; García-García, F.J.; Gambini, J.; Borrás, C.; Rodríguez-Mañas, L.; Viña, J. Relation Between Genetic Factors and Frailty in Older Adults. J. Am. Med. Dir. Assoc. 2019, 20, 1451–1457. [Google Scholar] [CrossRef]
- Iparraguirre, L.; Alberro, A.; Iñiguez, S.G.; Muñoz-Culla, M.; Vergara, I.; Matheu, A.; Otaegui, D. Blood RNA-Seq profiling reveals a set of circular RNAs differentially expressed in frail individuals. Immun. Ageing 2023, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Juárez-Cedillo, T.; Vargas-Alarcón, G.; Martínez-Rodríguez, N.; Juárez-Cedillo, E.; Fragoso, J.M.; Escobedo-de-la-Peña, J. Interleukin 10 gene polymorphisms and frailty syndrome in elderly Mexican people: (Sadem study). Mol. Genet. Genomic Med. 2019, 7, e918. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Chang, C.H.; Chen, C.Y.; Shih, C.Y.; Peng, J.K.; Huang, H.L.; Chen, P.Y.; Huang, T.-L.; Chen, C.Y.; Tsai, J.S. Upregulation of cluster of differentiation 36 mRNA expression in peripheral blood mononuclear cells correlates with frailty severity in older adults. J. Cachexia. Sarcopenia Muscle 2022, 13, 1948–1955. [Google Scholar] [CrossRef]
- Martínez-Ezquerro, J.D.; Rodríguez-Castañeda, A.; Ortiz-Ramírez, M.; Sánchez-García, S.; Rosas-Vargas, H.; Sánchez-Arenas, R.; García-de la Torre, P. Oxidative Stress, Telomere Length, and Frailty in an Old Age Population. Rev. Investig. Clin. 2019, 71, 393–401. [Google Scholar] [CrossRef]
- Mourtzi, N.; Ntanasi, E.; Yannakoulia, M.; Kosmidis, M.; Anastasiou, C.; Dardiotis, E.; Hadjigeorgiou, G.; Sakka, P.; Scarmeas, N. Apolipoprotein ϵ4 allele is associated with frailty syndrome: Results from the hellenic longitudinal investigation of ageing and diet study. Age Ageing 2019, 48, 917–921. [Google Scholar] [CrossRef]
- Rabaneda-Bueno, R.; Torres-Carrillo, N.; Ávila-Funes, J.A.; Gutiérrez-Robledo, L.M.; Pérez-Suárez, T.G.; Acosta, J.L.; Torres-Castro, S.; Fletes-Rayas, A.L.; Gutierrez-Hurtado, I.; Sandoval-Pinto, E.; et al. PTPN22 gene functional polymorphism (rs2476601) in older adults with frailty syndrome. Mol. Biol. Rep. 2021, 48, 1193–1204. [Google Scholar] [CrossRef]
- Jiang, S.; Kang, L.; Zhu, M.; Zhang, X.; Wang, C.; Cai, J.; Liu, X. A potential urinary biomarker to determine frailty status among older adults. Arch. Gerontol. Geriatr. 2020, 88, 104038. [Google Scholar] [CrossRef]
- Liang, Y.D.; Liu, Q.; Du, M.H.; Liu, Z.; Yao, S.M.; Zheng, P.P.; Wan, Y.H.; Sun, N.; Li, Y.Y.; Liu, J.P.; et al. Urinary 8-oxo-7,8-dihydroguanosine as a potential biomarker of frailty for elderly patients with cardiovascular disease. Free Radic. Biol. Med. 2020, 152, 248–254. [Google Scholar] [CrossRef]
- Furtado, G.E.; Uba-Chupel, M.; Minuzzi, L.; Patrício, M.; Loureiro, M.; Bandelow, S.; Hogervorst, E.; Ferreira, J.P.; Teixeira, A.M. Exploring the potential of salivary and blood immune biomarkers to elucidate physical frailty in institutionalized older women. Exp. Gerontol. 2020, 129, 110759. [Google Scholar] [CrossRef]
- Gómez-Rubio, P.; Trapero, I.; Cauli, O.; Buigues, C. Salivary IL-6 Concentration Is Associated with Frailty Syndrome in Older Individuals. Diagnostics 2022, 12, 117. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, M.; Chen, D.; Jiang, X.; Xiong, Z. Inflammatory biomarkers in older adults with frailty: A systematic review and meta-analysis of cross-sectional studies. Aging Clin. Exp. Res. 2022, 34, 971–987. [Google Scholar] [CrossRef]
- Prommaban, A.; Moonkayaow, S.; Phinyo, P.; Siviroj, P.; Sirikul, W.; Lerttrakarnnon, P. The Effect of Exercise Program Interventions on Frailty, Clinical Outcomes, and Biomarkers in Older Adults: A Systematic Review. J. Clin. Med. 2024, 13, 6570. [Google Scholar] [CrossRef]
- Pothier, K.; Gana, W.; Bailly, N.; Fougère, B. Associations Between Frailty and Inflammation, Physical, and Psycho-Social Health in Older Adults: A Systematic Review. Front. Psychol. 2022, 13, 805501. [Google Scholar] [CrossRef]
- Pan, Y.; Ji, T.; Li, Y.; Ma, L. Omics biomarkers for frailty in older adults. Clin. Chim. Acta 2020, 510, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, M.; Solfrizzi, V.; Sardone, R.; Dibello, V.; Castellana, F.; Zupo, R.; Lampignano, L.; Bortone, I.; Daniele, A.; Panza, F. The epigenetics of frailty. Epigenomics 2024, 16, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Tomkova, K.; Pathak, S.; Abbasciano, R.; Wozniak, M.; Murphy, G.J. A systematic review and meta-analysis of studies that have evaluated the role of mitochondrial function and iron metabolism in frailty. Clin. Transl. Sci. 2021, 14, 2370–2378. [Google Scholar] [CrossRef] [PubMed]
- Salvioli, S.; Basile, M.S.; Bencivenga, L.; Carrino, S.; Conte, M.; Damanti, S.; De Lorenzo, R.; Fiorenzato, E.; Gialluisi, A.; Ingannato, A.; et al. Biomarkers of aging in frailty and age-associated disorders: State of the art and future perspective. Ageing Res. Rev. 2023, 91, 102044. [Google Scholar] [CrossRef]
- Gupta, S.; Venkatesh, A.; Ray, S.; Srivastava, S. Challenges and prospects for biomarker research: A current perspective from the developing world. Biochim. Biophys. Acta 2014, 1844, 899–908. [Google Scholar] [CrossRef]
- Gale, C.R.; Westbury, L.; Cooper, C. Social isolation and loneliness as risk factors for the progression of frailty: The English Longitudinal Study of Ageing. Age Ageing 2018, 47, 392–397. [Google Scholar] [CrossRef]
- Kojima, G.; Aoyama, R.; Tanabe, M. Associations Between Social Isolation and Physical Frailty in Older Adults: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2022, 23, e3–e6. [Google Scholar] [CrossRef]
- Eppler-Hattab, R.; Doron, I.; Meshoulam, I. Development and validation of a workplace age-friendliness measure. Innov. Aging 2020, 4, igaa024. [Google Scholar] [CrossRef]
- Nilsson, C.J.; Nørgaard, S.; Foverskov, E.; Bruunsgaard, H.; Andersen, P.K.; Lund, R. Positive and negative aspects of social relations and low-grade inflammation in Copenhagen Aging and Midlife Biobank. Eur. J. Ageing 2020, 17, 531–546. [Google Scholar] [CrossRef]
- Lubben, J.; Blozik, E.; Gillmann, G.; Iliffe, S.; Von Kruse, W.R.; Beck, J.C.; Stuck, A.E. Performance of an abbreviated version of the lubben social network scale among three European community-dwelling older adult populations. Gerontologist 2006, 46, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; He, Z.; Yun, H.; Wang, R.; Liu, C. A Meta-Analysis of the Effects of Different Exercise Modes on Inflammatory Response in the Elderly. Int. J. Environ. Res. Public Health 2022, 19, 10451. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.V.; Wu, W.T.; Chen, Y.H.; Chen, L.R.; Hsu, W.H.; Lin, Y.L.; Han, D.S. Enhanced serum levels of tumor necrosis factor-α, interleukin-1β, and -6 in sarcopenia: Alleviation through exercise and nutrition intervention. Aging 2023, 15, 13471–13485. [Google Scholar] [CrossRef]
- Pereira, D.S.; Mateo, E.C.C.; De Queiroz, B.Z.; Assumpção, A.M.; Miranda, A.S.; Felício, D.C.; Rocha, N.P.; Da Cruz Dos Anjos, D.M.; Pereira, D.A.G.; Teixeira, A.L.; et al. TNF-α, IL6, and IL10 polymorphisms and the effect of physical exercise on inflammatory parameters and physical performance in elderly women. Age 2013, 35, 2455–2463. [Google Scholar] [CrossRef] [PubMed][Green Version]
- El Assar, M.; Rodríguez-Sánchez, I.; Álvarez-Bustos, A.; Rodríguez-Mañas, L. Biomarkers of frailty. Mol. Aspects Med. 2024, 97, 101271. [Google Scholar] [CrossRef]
- Fischer, J.; Hans, D.; Lamy, O.; Marques-Vidal, P.; Vollenweider, P.; Aubry-Rozier, B. “inflammaging” and bone in the OsteoLaus cohort. Immun. Ageing 2020, 17, 5. [Google Scholar] [CrossRef]
- Alberro, A.; Iribarren-Lopez, A.; Sáenz-Cuesta, M.; Matheu, A.; Vergara, I.; Otaegui, D. Inflammaging markers characteristic of advanced age show similar levels with frailty and dependency. Sci. Rep. 2021, 11, 4358. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
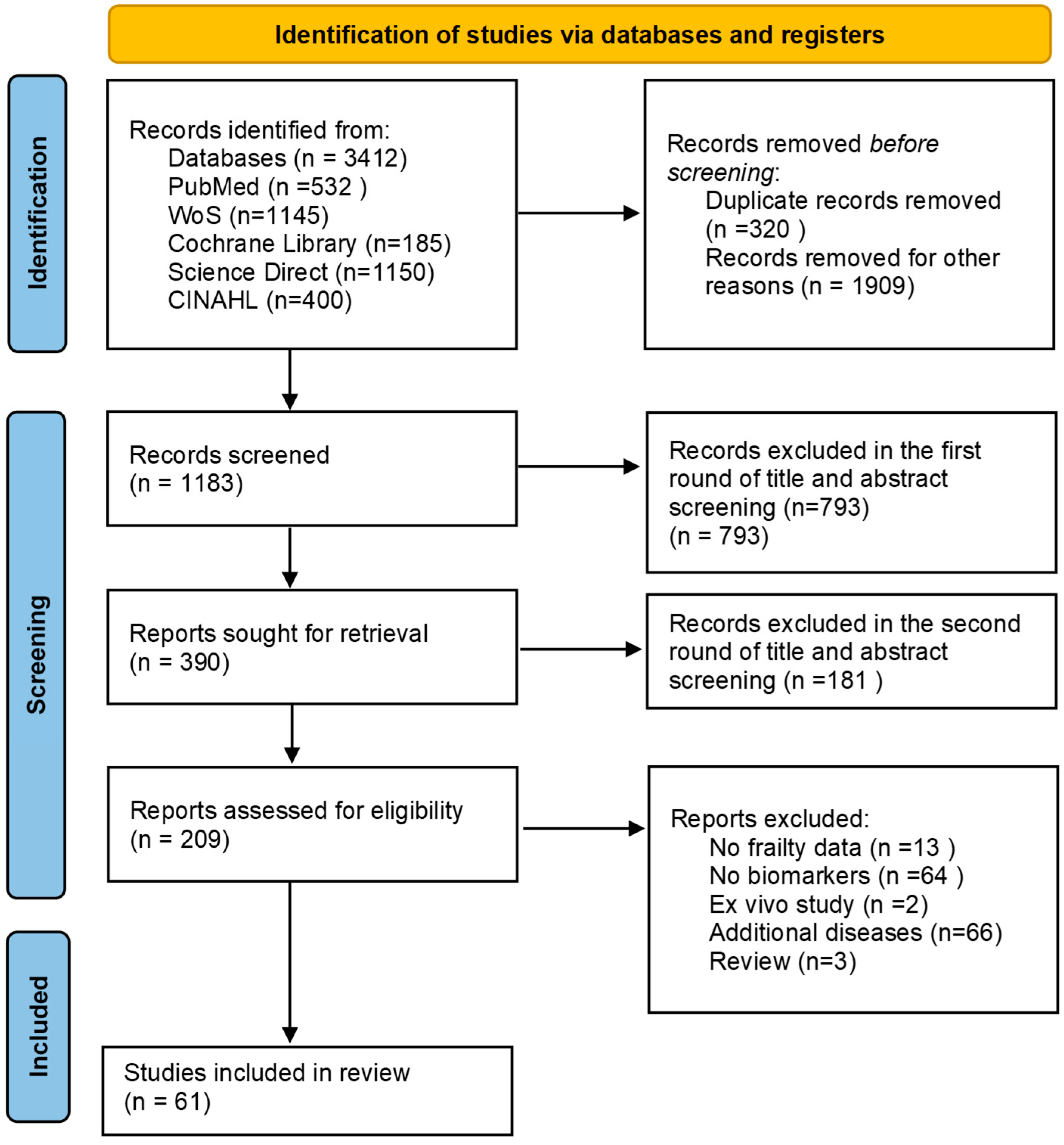
| Challenge Category | Challenge Subcategory | Specific Challenges | Publication |
|---|---|---|---|
| Biomarkers of inflammation | |||
| Study design | Sample size | Small sample size | Buondonno et al., 2023 [25] Chew et al., 2019 [47] Hammami et al., 2020 [58] Hammami et al., 2020 [65] Liu et al., 2024 [67] Pansarasa et al., 2022 [26] Samson et al., 2022 [27] Semmarath et al., 2019 [28] Xu et al., 2022 [32] |
| loss of a part of the cohort | McKechnie et al., 2021 [68] Xu et al., 2022 [32] | ||
| depletion of the sample | Welstead et al., 2020 [31] | ||
| Study design | - | None of the studies identified this theme | |
| Diagnosis | Subjective self-report | McKechnie et al., 2021 [68] | |
| self-reported data | Semmarath et al., 2019 [28] | ||
| only one scale of frailty | Teixeira-Gomes et al., 2021 [30] Xu et al., 2022 [32] | ||
| Incomplete outcome data | Lack of follow-up | Buondonno et al., 2023 [25] | |
| samples from another trial | Castro-Herrera et al., 2021 [36] | ||
| Experimental method | No power calculation | Castro-Herrera et al., 2021 [36] | |
| second kind of error | Chew et al., 2019 [47] | ||
| p-value was not adjusted | van Sleen et al., 2023 [29] | ||
| Confounders | Severity of concomitant disease was not taken into account | Castro-Herrera et al., 2021 [36] McKechnie et al., 2021 [68] Zhang et al., 2022 [33] | |
| effect of drugs on biomarkers was not taken into account | Castro-Herrera et al., 2021 [36] Welstead et al., 2020 [31] | ||
| Study duration | Short follow-up | Hammami et al., 2020 [58] Hammami et al., 2020 [65] Hsu et al., 2019 [24] Zhang et al., 2022 [33] | |
| Sampling | Heterogeneous group | Chew et al., 2019 [47] Hammami et al., 2020 [58] Hammami et al., 2020 [65] Hsu et al., 2019 [24] van Sleen et al., 2023 [29] Xu et al., 2022 [32] | |
| Unclear Pathophysiological Mechanism | Insufficient evidence | No cause-and-effect relationships | Buondonno et al., 2023 [25] |
| Biomarker | Measurement | Not checked entire sample | Castro-Herrera et al., 2021 [36] |
| measured at one point in time | Zhang et al., 2022 [33] | ||
| Outcomes | There is no effect on long-term adverse clinical outcomes | Liu et al., 2024 [67] | |
| Protein biomarkers | |||
| Study design | Sample size | Small sample size | Arauna et al., 2020 [34] Sanz et al., 2021 [41] Valentini et al., 2019 [44] |
| Study design | Cross-sectional data do not allow to establish a causal relationship | Sanz et al., 2019 [40] | |
| Diagnosis | Retrospectively based on clinical files | Angioni et al., 2022 [13] | |
| only one scale of frailty | Li et al., 2021 [38] | ||
| body composition was determined using bioelectric impedance | Sanz et al., 2019 [40] | ||
| Incomplete outcome data | Samples from another trial | Angioni et al., 2022 [13] | |
| data were missing due to lack of response and mortality | Shardell et al., 2019 [43] | ||
| Experimental method | - | None of the studies identified this theme. | |
| Confounders | The effect of drugs on biomarkers was not taken into account | Sanz et al., 2021 [41] | |
| no data on use of anti-inflammatory or steroid drugs | Kamper et al., 2024 [35] | ||
| no information about possible dehydration or fluid overload | Kamper et al., 2024 [35] | ||
| lack of information about the deterioration in cognitive function | Sanz et al., 2021 [41] | ||
| Study duration | - | None of the studies identified this theme | |
| Sampling | Heterogeneous group | Landino et al., 2021 [37] | |
| Unclear Pathophysiological Mechanism | Insufficient evidence | No cause-and-effect relationships | Kamper et al., 2024 [35] Li et al., 2021 [38] Roh et al., 2022 [39] |
| Biomarker | Measurement | Does not reflect all the proteins | Landino et al., 2021 [37] |
| blood samples were taken late | Kamper et al., 2024 [35] | ||
| measured once | Shardell et al., 2019 [43] | ||
| Outcomes | High correlation with the aging | Kamper et al., 2024 [35] | |
| Vitamin biomarkers | |||
| Study design | Sample size | Small sample size | Ngestiningsih et al., 2021 [51] Rattray et al., 2019 [54] Pillatt et al., 2021 [52] |
| Study design | Cross-sectional study | Malaguarnera et al., 2020 [50] Xiao et al., 2020 [56] Kochlik et al., 2019 [48] | |
| Diagnosis | Self-reported data | Pilleron et al., 2019 [53] Xiao et al., 2020 [56] | |
| pre-weak condition was not taken | Xiao et al., 2020 [56] | ||
| Incomplete outcome data | - | None of the studies identified this theme | |
| Experimental method | Large confidence intervals of causal estimates | Rattray et al., 2019 [54] | |
| Confounders | No data on concomitant diseases or medication | Henning et al., 2023 [46] Pillatt et al., 2021 [52] | |
| Study duration | Short follow-up | Henning et al., 2023 [46] | |
| Sampling | Heterogeneous groups | Gomez-Cabrero et al., 2021 [45] Kochlik et al., 2019 [48] | |
| exclusion of the weakest participants | Machado-Fragua et al., 2020 [49] | ||
| Unclear Pathophysiological Mechanism | Insufficient evidence | No cause-and-effect relationships | Vaes et al., 2019 [55] |
| Biomarker | Measurement | Measured at one point in time | Machado-Fragua et al., 2020 [49] |
| use only one biomarker of vitamin K | Machado-Fragua et al., 2020 [49] | ||
| no measured carnitine levels | Malaguarnera et al., 2020 [50] | ||
| Outcomes | - | None of the studies identified this theme | |
| Lipid biomarkers | |||
| Study design | Sample size | Small sample size | Arauna et al., 2021 [57] |
| Study design | - | None of the studies identified this theme | |
| Diagnosis | - | None of the studies identified this theme | |
| Incomplete outcome data | - | None of the studies identified this theme | |
| Experimental method | - | None of the studies identified this theme | |
| Confounders | No data on acute infections | Yin et al., 2023 [59] | |
| Study duration | - | None of the studies identified this theme | |
| Sampling | - | None of the studies identified this theme | |
| Unclear Pathophysiological Mechanism | Insufficient evidence | - | None of the studies identified this theme |
| Biomarker | Measurement | Biological markers were measured using various analyzers | Yin et al., 2023 [59] |
| biochemical markers of bone have not been assessed | Yin et al., 2023 [59] | ||
| Outcomes | - | None of the studies identified this theme | |
| Acid biomarkers | |||
| Study design | Sample size | - | None of the studies identified this theme |
| Study design | Cross-sectional study | Jang et al., 2020 [61] | |
| Diagnosis | - | None of the studies identified this theme | |
| Incomplete outcome data | - | None of the studies identified this theme | |
| Experimental method | - | None of the studies identified this theme | |
| Confounders | - | None of the studies identified this theme | |
| Study duration | - | None of the studies identified this theme | |
| Sampling | The average age of the participants was considered relatively young | Jang et al., 2020 [61] | |
| Unclear Pathophysiological Mechanism | Insufficient evidence | - | None of the studies identified this theme |
| Biomarker | Measurement | - | None of the studies identified this theme |
| Outcomes | - | None of the studies identified this theme | |
| Metal biomarkers | |||
| Study design | Sample size | Small sample size | Zawadzki et al., 2021 [63] |
| Study design | - | None of the studies identified this theme | |
| Diagnosis | - | None of the studies identified this theme | |
| Incomplete outcome data | - | None of the studies identified this theme | |
| Experimental method | - | None of the studies identified this theme | |
| Confounders | Characteristics of chronic diseases were not taken into account | Wei et al., 2022 [62] | |
| coexistence of acute inflammatory diseases | Zawadzki et al., 2021 [63] | ||
| Study duration | - | None of the studies identified this theme | |
| Sampling | Only one location | Wei et al., 2022 [62] | |
| Unclear Pathophysiological Mechanism | Insufficient evidence | No cause-and-effect relationships | Wei et al., 2022 [62] Zawadzki et al., 2021 [63] |
| Biomarker | Measurement | The lead content in hair and bones not measured | Wei et al., 2022 [62] |
| Outcomes | - | None of the studies identified this theme | |
| Enzyme biomarkers | |||
| Study design | Sample size | - | None of the studies identified this theme |
| Study design | - | None of the studies identified this theme | |
| Diagnosis | - | None of the studies identified this theme | |
| Incomplete outcome data | - | None of the studies identified this theme | |
| Experimental method | - | None of the studies identified this theme | |
| Confounders | The effect drugs on biomarkers was not taken into account | Sanz et al., 2022 [64] | |
| Study duration | - | None of the studies identified this theme | |
| Sampling | - | None of the studies identified this theme | |
| Unclear Pathophysiological Mechanism | Insufficient evidence | - | None of the studies identified this theme |
| Biomarker | Measurement | - | None of the studies identified this theme |
| Outcomes | - | None of the studies identified this theme | |
| Challenge Category | Challenge Subcategory | Specific Challenges | Publication |
|---|---|---|---|
| Genetic biomarkers | |||
| Study design | Sample size | Small sample size | Carini et al., 2022 [70] Inglés et al., 2019 [73] Iparraguirre et al., 2023 [74] Lee et al., 2022 [76] |
| loss of a part of the cohort | Selenius et al., 2024 [71] | ||
| insufficient recruitment skills | Martínez-Ezquerro et al., 2019 [77] | ||
| Study design | Cross-sectional study | Lee et al., 2022 [76] | |
| Diagnosis | High heterogeneity that characterizes the frail phenotype | Iparraguirre et al., 2023 [74] | |
| Incomplete outcome data | - | None of the studies identified this theme | |
| Experimental method | - | None of the studies identified this theme | |
| Confounders | The severity of concomitant disease and drugs was not taken into account | Agostini et al., 2023 [69] Grasselli et al., 2022 [72] | |
| results obtained are distorted by subclinical stages of dementia | Mourtzi et al., 2019 [78] | ||
| genetic and environmental factors | Mourtzi et al., 2019 [78] | ||
| Study duration | - | None of the studies identified this theme | |
| Sampling | Conducted in only one location | Juárez-Cedillo et al., 2019 [75] Selenius et al., 2024 [71] | |
| Unclear Pathophysiological Mechanism | Insufficient evidence | - | None of the studies identified this theme. |
| Biomarker | Measurement | Measured at one point in time | Lee et al., 2022 [76] |
| small RNA sequencing was performed on whole blood samples | Carini et al., 2022 [70] | ||
| the allele variant of T is not presented | Rabaneda-Bueno et al., 2021 [79] | ||
| Outcomes | - | None of the studies identified this theme | |
| Urine biomarkers | |||
| Study design | Sample size | - | None of the studies identified this theme |
| Study design | Cross-sectional study | Liang et al., 2020 [81] | |
| Diagnosis | Only one scale of frailty | Liang et al., 2020 [81] | |
| Incomplete outcome data | - | None of the studies identified this theme | |
| Experimental method | - | None of the studies identified this theme | |
| Confounders | - | None of the studies identified this theme | |
| Study duration | - | None of the studies identified this theme | |
| Sampling | Not fully representative sample | Liang et al., 2020 [81] | |
| Unclear Pathophysiological Mechanism | Insufficient evidence | - | None of the studies identified this theme |
| Biomarker | Measurement | Circadian variability of cytokines | Jiang et al., 2020 [80] |
| Outcomes | - | None of the studies identified this theme | |
| Salivary biomarkers | |||
| Study design | Sample size | Small sample size | Furtado et al., 2020 [82] Gómez-Rubio et al., 2022 [83] |
| Study design | Cross-sectional studies are limited in their ability to determine causal relationships | Furtado et al., 2020 [82] Gómez-Rubio et al., 2022 [83] | |
| Diagnosis | - | None of the studies identified this theme | |
| Incomplete outcome data | - | None of the studies identified this theme | |
| Experimental method | Large number of biomarkers adds complexity to analysis | Furtado et al., 2020 [82] | |
| Different biological materials (saliva vs. blood) introduced in the statistical model | Furtado et al., 2020 [82] | ||
| Confounders | The influence of all confounding factors was not eliminated | Gómez-Rubio et al., 2022 [83] | |
| Study duration | - | None of the studies identified this theme | |
| Sampling | - | None of the studies identified this theme | |
| Unclear Pathophysiological Mechanism | Insufficient evidence | - | None of the studies identified this theme |
| Biomarker | Measurement | Individual variability in biomarkers studied | Furtado et al., 2020 [82] |
| Outcomes | Inconsistent findings on the link between salivary IL-6 and dental/periodontal diseases | Gómez-Rubio et al., 2022 [83] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omarova, I.; Yeshmanova, A.; Gabdulina, G.; Tazhiyeva, A.; Ryspekova, S.; Abdykulova, A.; Nuftieva, A.; Abdirova, T.; Sailanova, D.; Mombiyeva, Z.; et al. Challenges in Identifying Biomarkers of Frailty Syndrome: A Systematic Review. Medicina 2025, 61, 1309. https://doi.org/10.3390/medicina61071309
Omarova I, Yeshmanova A, Gabdulina G, Tazhiyeva A, Ryspekova S, Abdykulova A, Nuftieva A, Abdirova T, Sailanova D, Mombiyeva Z, et al. Challenges in Identifying Biomarkers of Frailty Syndrome: A Systematic Review. Medicina. 2025; 61(7):1309. https://doi.org/10.3390/medicina61071309
Chicago/Turabian StyleOmarova, Indira, Ainur Yeshmanova, Gulzhan Gabdulina, Aigul Tazhiyeva, Shynar Ryspekova, Akmaral Abdykulova, Ainur Nuftieva, Tamara Abdirova, Dame Sailanova, Zhanar Mombiyeva, and et al. 2025. "Challenges in Identifying Biomarkers of Frailty Syndrome: A Systematic Review" Medicina 61, no. 7: 1309. https://doi.org/10.3390/medicina61071309
APA StyleOmarova, I., Yeshmanova, A., Gabdulina, G., Tazhiyeva, A., Ryspekova, S., Abdykulova, A., Nuftieva, A., Abdirova, T., Sailanova, D., Mombiyeva, Z., & Karibayeva, I. (2025). Challenges in Identifying Biomarkers of Frailty Syndrome: A Systematic Review. Medicina, 61(7), 1309. https://doi.org/10.3390/medicina61071309






