Pre- and Post- COVID-19 Pandemic Pneumonia Rates in Hospitalized Schizophrenia Patients
Abstract
1. Introduction
2. Aims
3. Materials and Methods
3.1. Data Source
3.2. Study Design
3.3. Statistical Analysis
4. Results
4.1. General Findings
4.2. Findings Related to Antipsychotic Treatments
4.3. Findings Related to Concomitant Medication
4.3.1. Anticholinergic Treatment
4.3.2. Benzodiazepines
4.3.3. Hypnotic Medication
4.3.4. Mood Stabilizers
4.3.5. Antibiotics and Antiviral Medication
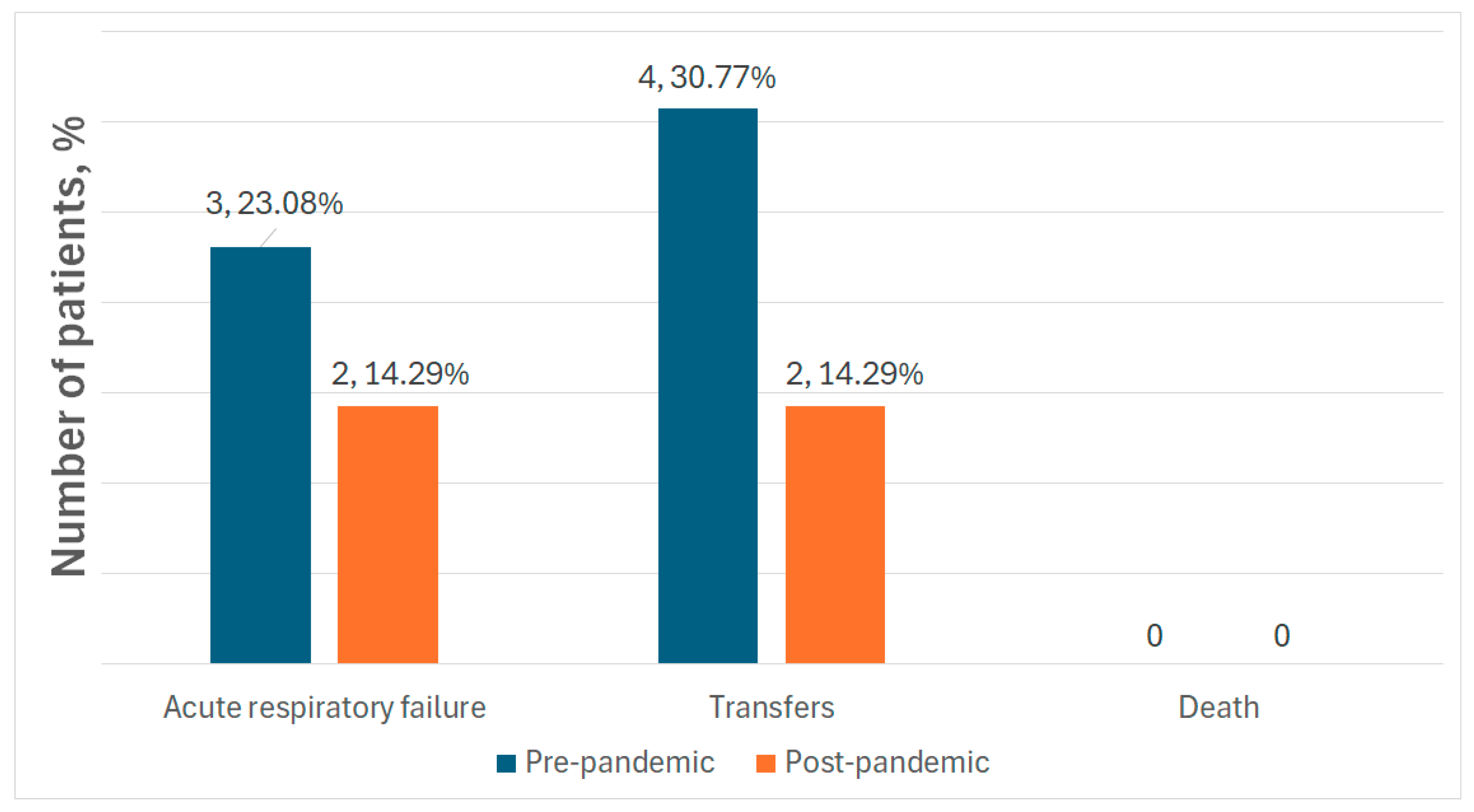
4.4. Complications
5. Discussion
Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COVID-19 | Coronavirus Disease 2019 |
| DSM-5 | Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition |
| EPS | Extrapyramidal symptom |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| SPSS | Statistical Package for the Social Sciences |
| SD | Standard deviation |
| CPZ | Chlorpromazine |
| OAP | Oral antipsychotic |
| LAI | Long-acting injectable antipsychotic |
| OLZ | Olanzapine |
| RIS | Risperidone |
| ARI | Aripiprazole |
| QUE | Quetiapine |
| HAL | Haloperidol |
| FLX | Flupenthixol |
| ZUC | Zuclopenthixol |
| CLZ | Clozapine |
| BZD | Benzodiazepine |
| HAP | Hospital-acquired pneumonia |
| SMI | Severe mental illness |
| UK | United Kingdom |
| MECT | Modified electroconvulsive therapy |
| COPD | Chronic obstructive pulmonary disease |
| CYP2C19 | Cytochrome P450 2C19 |
| CYP1A2 | Cytochrome P450 1A2 |
References
- Tandon, R.; Nasrallah, H.; Akbarian, S.; Carpenter, W.T., Jr.; DeLisi, L.E.; Gaebel, W.; Green, M.F.; Gur, R.E.; Heckers, S.; Kane, J.M.; et al. The schizophrenia syndrome, circa 2024: What we know and how that informs its nature. Schizophr. Res. 2024, 264, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Hany, M.; Rehman, B.; Rizvi, A.; Chapman, J. Schizophrenia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Šimunović Filipčić, I.; Filipčić, I. Schizophrenia and Physical Comorbidity. Psychiatr. Danub. 2018, 30 (Suppl. S4), 152–157. [Google Scholar]
- Correll, C.U.; Solmi, M.; Croatto, G.; Schneider, L.K.; Rohani-Montez, S.C.; Fairley, L.; Smith, N.; Bitter, I.; Gorwood, P.; Taipale, H.; et al. Mortality in people with schizophrenia: A systematic review and meta-analysis of relative risk and aggravating or attenuating factors. World Psychiatry Off. J. World Psychiatr. Assoc. 2022, 21, 248–271. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Ren, C.Y.; Liao, Y. Analysis of risk factors for hospital-acquired pneumonia in schizophrenia. Front. Psychiatry 2024, 15, 1414332. [Google Scholar] [CrossRef]
- Barnett, M.J.; Perry, P.J.; Alexander, B.; Kaboli, P.J. Risk of mortality associated with antipsychotic and other neuropsychiatric drugs in pneumonia patients. J. Clin. Psychopharmacol. 2006, 26, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.J.; Yang, S.Y.; Liao, Y.T.; Chen, W.J.; Lee, W.C.; Shau, W.Y.; Chang, Y.T.; Tsai, S.Y.; Chen, C.C. Second-generation antipsychotic medications and risk of pneumonia in schizophrenia. Schizophr. Bull. 2013, 39, 648–657. [Google Scholar] [CrossRef]
- Goff, D.C.; Cather, C.; Evins, A.E.; Henderson, D.C.; Freudenreich, O.; Copeland, P.M.; Bierer, M.; Duckworth, K.; Sacks, F.M. Medical morbidity and mortality in schizophrenia: Guidelines for psychiatrists. J. Clin. Psychiatry 2005, 66, 183–274. [Google Scholar] [CrossRef]
- Suetani, S.; Honarparvar, F.; Siskind, D.; Hindley, G.; Veronese, N.; Vancampfort, D.; Allen, L.; Solmi, M.; Lally, J.; Gaughran, F.; et al. Increased rates of respiratory disease in schizophrenia: A systematic review and meta-analysis including 619,214 individuals with schizophrenia and 52,159,551 controls. Schizophr. Res. 2021, 237, 131–140. [Google Scholar] [CrossRef]
- Volavka, J.; Vevera, J. Very long-term outcome of schizophrenia. Int. J. Clin. Pract. 2018, 72, e13094. [Google Scholar] [CrossRef]
- Emsley, R.; Chiliza, B.; Asmal, L.; Harvey, B.H. The nature of relapse in schizophrenia. BMC Psychiatry 2013, 13, 50. [Google Scholar] [CrossRef]
- Ramachandraiah, C.T.; Subramaniam, N.; Tancer, M. The story of antipsychotics: Past and present. Indian J. Psychiatry 2009, 51, 324–326. [Google Scholar] [CrossRef] [PubMed]
- Haddad, P.M.; Correll, C.U. The acute efficacy of antipsychotics in schizophrenia: A review of recent meta-analyses. Ther. Adv. Psychopharmacol. 2018, 8, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Weston-Green, K. Antipsychotic Drug Development: From Historical Evidence to Fresh Perspectives. Front. Psychiatry 2022, 13, 903156. [Google Scholar] [CrossRef] [PubMed]
- Horacek, J.; Bubenikova-Valesova, V.; Kopecek, M.; Palenicek, T.; Dockery, C.; Mohr, P.; Höschl, C. Mechanism of action of atypical antipsychotic drugs and the neurobiology of schizophrenia. CNS Drugs 2006, 20, 389–409. [Google Scholar] [CrossRef]
- Muench, J.; Hamer, A.M. Adverse effects of antipsychotic medications. Am. Fam. Phys. 2010, 81, 617–622. [Google Scholar]
- Eby, A.; Jacob, E.; George, P.S.G. First-generation antipsychotics use and reduced risk of pneumonia- Clinical implications in SARS-CoV2 treatment: A systematic review and meta-analysis of observational studies. J. Appl. Pharm. Sci. 2022, 12, 146–156. [Google Scholar] [CrossRef]
- Parsa, M.A.; Bastani, B. Quetiapine (Seroquel) in the treatment of psychosis in patients with Parkinson’s disease. J. Neuropsychiatry Clin. Neurosci. 1998, 10, 216–219. [Google Scholar] [CrossRef]
- Sveinbjornsdottir, S. The clinical symptoms of Parkinson’s disease. J. Neurochem. 2016, 139 (Suppl. S1), 318–324. [Google Scholar] [CrossRef]
- Young, B.K.; Camicioli, R.; Ganzini, L. Neuropsychiatric adverse effects of antiparkinsonian drugs. Characteristics, evaluation and treatment. Drugs Aging 1997, 10, 367–383. [Google Scholar] [CrossRef]
- Shotbolt, P.; Samuel, M.; David, A. Quetiapine in the treatment of psychosis in Parkinson’s disease. Ther. Adv. Neurol. Disord. 2010, 3, 339–350. [Google Scholar] [CrossRef]
- Luykx, J.J.; Correll, C.U.; Manu, P.; Tanskanen, A.; Hasan, A.; Tiihonen, J.; Taipale, H. Pneumonia Risk, Antipsychotic Dosing, and Anticholinergic Burden in Schizophrenia. JAMA Psychiatry 2024, 81, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.M.; Barnes, T.R.E.; Young, A.H. The Maudsley Prescribing Guidelines in Psychiatry, 14th ed.; John Wiley and Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Sheikh, W.A. Prophylactic use of trihexyphenidyl (artane) in schizophrenia and psychosis: A critical review of literature to guide for evidence based practice in Zambia. Med. J. Zamb. 2019, 46, 133–139. [Google Scholar] [CrossRef]
- Wu, M.; Li, Z.; Zheng, W.; Zhuang, J.; Wu, S.; Zhou, Q.; Cai, J.; Zheng, H.; Zeng, G.; Zhang, W.; et al. Association between anticholinergic medication uses and the risk of pneumonia in elderly adults: A meta-analysis and systematic review. Ann. Med. 2023, 55, 2209736. [Google Scholar] [CrossRef] [PubMed]
- Ghossein, N.; Kang, M.; Lakhkar, A.D. Anticholinergic Medications. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK555893/ (accessed on 8 May 2023).
- Lee, C.Y.; Cheng, Y.D.; Cheng, W.Y.; Tsai, T.H.; Huang, K.H. The Prevalence of Anticholinergic Drugs and Correlation with Pneumonia in Elderly Patients: A Population-Based Study in Taiwan. Int. J. Environ. Res. Public Health 2020, 17, 6260. [Google Scholar] [CrossRef]
- Chatterjee, S.; Carnahan, R.M.; Chen, H.; Holmes, H.M.; Johnson, M.L.; Aparasu, R.R. Anticholinergic Medication Use and Risk of Pneumonia in Elderly Adults: A Nested Case-Control Study. J. Am. Geriatr. Soc. 2016, 64, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, R.; Torres, A.; El-Ebiary, M.; de la Bellacasa, J.P.; Estruch, R.; Mensa, J.; Fernández-Solá, J.; Hernández, C.; Rodriguez-Roisin, R. Community-acquired pneumonia in the elderly: A multivariate analysis of risk and prognostic factors. Am. J. Respir. Crit. Care Med. 1996, 154, 1450–1455. [Google Scholar] [CrossRef]
- Meyer, R.; Skov, K.; Dhillon, I.K.; Olsson, E.; Graudal, N.A.; Baandrup, L.; Jürgens, G. Onset of Action of Selected Second-Generation Antipsychotics (Pines)-A Systematic Review and Meta-Analyses. Biomedicines 2022, 11, 82. [Google Scholar] [CrossRef]
- Taipale, H.; Tolppanen, A.M.; Koponen, M.; Tanskanen, A.; Lavikainen, P.; Sund, R.; Tiihonen, J.; Hartikainen, S. Risk of pneumonia associated with incident benzodiazepine use among community-dwelling adults with Alzheimer disease. CMAJ Can. Med. Assoc. J. = J. L’association Medicale Can. 2017, 189, E519–E529. [Google Scholar] [CrossRef]
- De Berardis, D.; Ventriglio, A.; Fornaro, M.; Vellante, F.; Martinotti, G.; Fraticelli, S.; Di Giannantonio, M. Overcoming the Use of Mechanical Restraints in Psychiatry: A New Challenge in the Everyday Clinical Practice at the Time of COVID-19. J. Clin. Med. 2020, 9, 3774. [Google Scholar] [CrossRef]
- Aguilera-Serrano, C.; Goodman-Casanova, J.M.; Bordallo-Aragón, A.; García-Sánchez, J.A.; Mayoral-Cleries, F.; Guzmán-Parra, J. Attitudes about Mechanical Restraint Use in Mental Health Hospitalization Services: A Spanish Survey. Healthcare 2023, 11, 1909. [Google Scholar] [CrossRef]
- Vergis, E.N.; Brennen, C.; Wagener, M.; Muder, R.R. Pneumonia in Long-term Care: A Prospective Case-Control Study of Risk Factors and Impact on Survival. Arch. Intern. Med. 2001, 161, 2378–2381. [Google Scholar] [CrossRef]
- Torres, A.; Cilloniz, C.; Niederman, M.S.; Menéndez, R.; Chalmers, J.D.; Wunderink, R.G.; van der Poll, T. Pneumonia. Nat. Rev. Dis. Prim. 2021, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.S. Pneumonia—Overview. In Encyclopedia of Respiratory Medicine; Academic Press: Cambridge, MA, USA, 2022; pp. 185–197. [Google Scholar] [CrossRef]
- Ufuk, F.; Savaş, R. COVID-19 pneumonia: Lessons learned, challenges, and preparing for the future. Diagn. Interv. Radiol. 2022, 28, 576–585. [Google Scholar] [CrossRef]
- Tan, X.; Zhang, Z.; Zhang, H.; Li, J.; Yang, X.; Wang, L.; Liao, X. Comparative study on the incidence of non-COVID-19 viral pneumonia before and after the COVID-19 pandemic: A retrospective analysis based on respiratory non-COVID viral nucleic acid results. J. Intensiv. Med. 2024, 4, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Ur Rehman, K.; Sivakumar, P. Bacterial pneumonias: Before and after the COVID-19 pandemic. Clin. Probl. 2021, 58 (Suppl. S65), PA289. [Google Scholar] [CrossRef]
- Kaysin, A.; Viera, A.J. Community-Acquired Pneumonia in Adults: Diagnosis and Management. Am. Fam. Physician 2016, 94, 698–706. [Google Scholar]
- Gardner, D.M.; Murphy, A.L.; O′Donnell, H.; Centorrino, F.; Baldessarini, R.J. International consensus study of antipsychotic dosing. Am. J. Psychiatry 2010, 167, 686–693. [Google Scholar] [CrossRef]
- Leucht, S.; Samara, M.; Heres, S.; Patel, M.X.; Furukawa, T.; Cipriani, A.; Geddes, J.; Davis, J.M. Dose Equivalents for Second-Generation Antipsychotic Drugs: The Classical Mean Dose Method. Schizophr. Bull. 2015, 41, 1397–1402. [Google Scholar] [CrossRef]
- Inada, T.; Inagaki, A. Psychotropic dose equivalence in Japan. Psychiatry Clin. Neurosci. 2015, 69, 440–447. [Google Scholar] [CrossRef]
- Langan, J.; Martin, D.; Shajahan, P.; Smith, D.J. Antipsychotic dose escalation as a trigger for neuroleptic malignant syndrome (NMS): Literature review and case series report. BMC Psychiatry 2012, 12, 214. [Google Scholar] [CrossRef]
- Woods, S.W. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J. Clin. Psychiatry 2003, 64, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, Q.; Wang, C.; Li, L.; Xu, M.; Yan, F.; Chen, W.; Wan, Y. Influencing factors of hospital-acquired pneumonia infection in the middle-aged and elderly patients with schizophrenia. Front. Psychiatry 2021, 12, 746791. [Google Scholar] [CrossRef]
- Chan, H.Y.; Lai, C.L.; Lin, Y.C.; Hsu, C.C. Is Antipsychotic Treatment Associated with Risk of Pneumonia in People with Serious Mental Illness? The Roles of Severity of Psychiatric Symptoms and Global Functioning. J. Clin. Psychopharmacol. 2019, 39, 434–440. [Google Scholar] [CrossRef]
- Saqib, K.; Qureshi, A.S.; Butt, Z.A. COVID-19, Mental Health, and Chronic Illnesses: A Syndemic Perspective. Int. J. Environ. Res. Public Health 2023, 20, 3262. [Google Scholar] [CrossRef]
- Al-Beltagi, M.; Saeed, N.K.; Bediwy, A.S. COVID-19 disease and autoimmune disorders: A mutual pathway. World J. Methodol. 2022, 12, 200–223. [Google Scholar] [CrossRef]
- Brand, B.A.; Haveman, Y.R.A.; de Beer, F.; de Boer, J.N.; Dazzan, P.; Sommer, I.E.C. Antipsychotic medication for women with schizophrenia spectrum disorders. Psychol. Med. 2022, 52, 649–663. [Google Scholar] [CrossRef] [PubMed]
- Zhao, V.; Gong, Y.; Thomas, N.; Das, S. Clozapine and Pneumonia: Synthesizing the Link by Reviewing Existing Reports—A Systematic Review and Meta-Analysis. Medicina 2024, 60, 2016. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, S.; Wang, Y.; Liang, J.; Li, H.; Shi, H.; Miao, T.; Wu, S.; Xiao, A.; Ye, J.; et al. Seasonal characteristics of nosocomial infection in a psychiatric hospital in China with different nosocomial prevention and control backgrounds: A retrospective study. Sci. Rep. 2024, 14, 17045. [Google Scholar] [CrossRef]
- Filik, R.; Sipos, A.; Kehoe, P.G.; Burns, T.; Cooper, S.J.; Stevens, H.; Laugharne, R.; Young, G.; Perrington, S.; McKendrick, J.; et al. The cardiovascular and respiratory health of people with schizophrenia. Acta Psychiatr. Scand. 2006, 113, 298–305. [Google Scholar] [CrossRef]
- Chou, F.H.; Tsai, K.Y.; Chou, Y.M. The incidence and all-cause mortality of pneumonia in patients with schizophrenia: A nine-year follow-up study. J. Psychiatr. Res. 2013, 47, 460–466. [Google Scholar] [CrossRef]
- Haga, T.; Ito, K.; Sakashita, K.; Iguchi, M.; Ono, M.; Tatsumi, K. Risk factors for pneumonia in patients with schizophrenia. Neuropsychopharmacol. Rep. 2018, 38, 204–209. [Google Scholar] [CrossRef]
- Funayama, M.; Takata, T. Psychiatric inpatients subjected to physical restraint have a higher risk of deep vein thrombosis and aspiration pneumonia. Gen. Hosp. Psychiatry 2020, 62, 1–5. [Google Scholar] [CrossRef]
- Betini, G.S.; Yu, D.; Perez, E.; Sareten, J.; Perlmaln, C.M.; Hirdes, J.P. Changes in psychiatric admissions in the first year of COVID-19 in Ontario, Canada. Int. J. Ment. Health Syst. 2025, 19, 18. [Google Scholar] [CrossRef]
- Miron, A.A.; Petric, P.S.; Teodorescu, A.; Ifteni, P.; Chele, G.; Szalontay, A.S. Benzodiazepines and Mood Stabilizers in Schizophrenia Patients Treated with Oral versus Long-Acting Injectable Antipsychotics-An Observational Study. Brain Sci. 2023, 13, 173. [Google Scholar] [CrossRef] [PubMed]
- Moga, S.; Teodorescu, A.; Ifteni, P.; Gavris, C.; Petric, P.S. Inflammatory Response in SARS-CoV-2 Infection of Patients with Schizophrenia and Long-Term Antipsychotic Treatment. Neuropsychiatr. Dis. Treat. 2021, 17, 3053–3060. [Google Scholar] [CrossRef] [PubMed]
- De Leon, J.; Sanz, E.J.; De Las Cuevas, C. Data from the World Health Organization’s Pharmacovigilance Database Supports the Prominent Role of Pneumonia in Mortality Associated with Clozapine Adverse Drug Reactions. Schizophr. Bull. 2020, 46, 1–3. [Google Scholar] [CrossRef]
- Vermeulen, J.M.; van Rooijen, G.; van de Kerkhof, M.P.J.; Sutterland, A.L.; Correll, C.U.; De Haan, L. Clozapine and Long-Term Mortality Risk in Patients with Schizophrenia: A Systematic Review and Meta-analysis of Studies Lasting 1.1–12.5 Years. Schizophr. Bull. 2019, 45, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Partanen, J.J.; Häppölä, P.; Kämpe, A.; Ahola-Olli, A.; Hellsten, A.; Rask, S.M.; Koskela, J.T. High Burden of Ileus and Pneumonia in Clozapine-Treated Individuals with Schizophrenia: A Finnish 25-Year Follow-Up Register Study. Am. J. Psychiatry 2024, 181, 879–892. [Google Scholar] [CrossRef]
- Chen, T.Y.; Winkelman, J.W.; Mao, W.C.; Liu, C.L.; Hsu, C.Y.; Wu, C.S. The Use of Benzodiazepine Receptor Agonists and the Risk of Hospitalization for Pneumonia: A Nationwide Population-Based Nested Case-Control Study. Chest 2018, 153, 161–171. [Google Scholar] [CrossRef]
- Talic, S.; Shah, S.; Wild, H.; Gasevic, D.; Maharaj, A.; Ademi, Z.; Li, X.; Xu, W.; Mesa-Eguiagaray, I.; Rostron, J.; et al. Effectiveness of public health measures in reducing the incidence of COVID-19, SARS-CoV-2 transmission, and COVID-19 mortality: Systematic review and meta-analysis. BMJ 2021, 375, e068302. [Google Scholar] [CrossRef]
- Fonseca, L.; Diniz, E.; Mendonça, G.; Malinowski, F.; Mari, J.; Gadelha, A. Schizophrenia and COVID-19: Risks and recommendations. Rev. Bras. Psiquiatr. 2020, 42, 236–238. [Google Scholar] [CrossRef] [PubMed]


| Parameters | Pre-Pandemic | Post-Pandemic | p-Value |
|---|---|---|---|
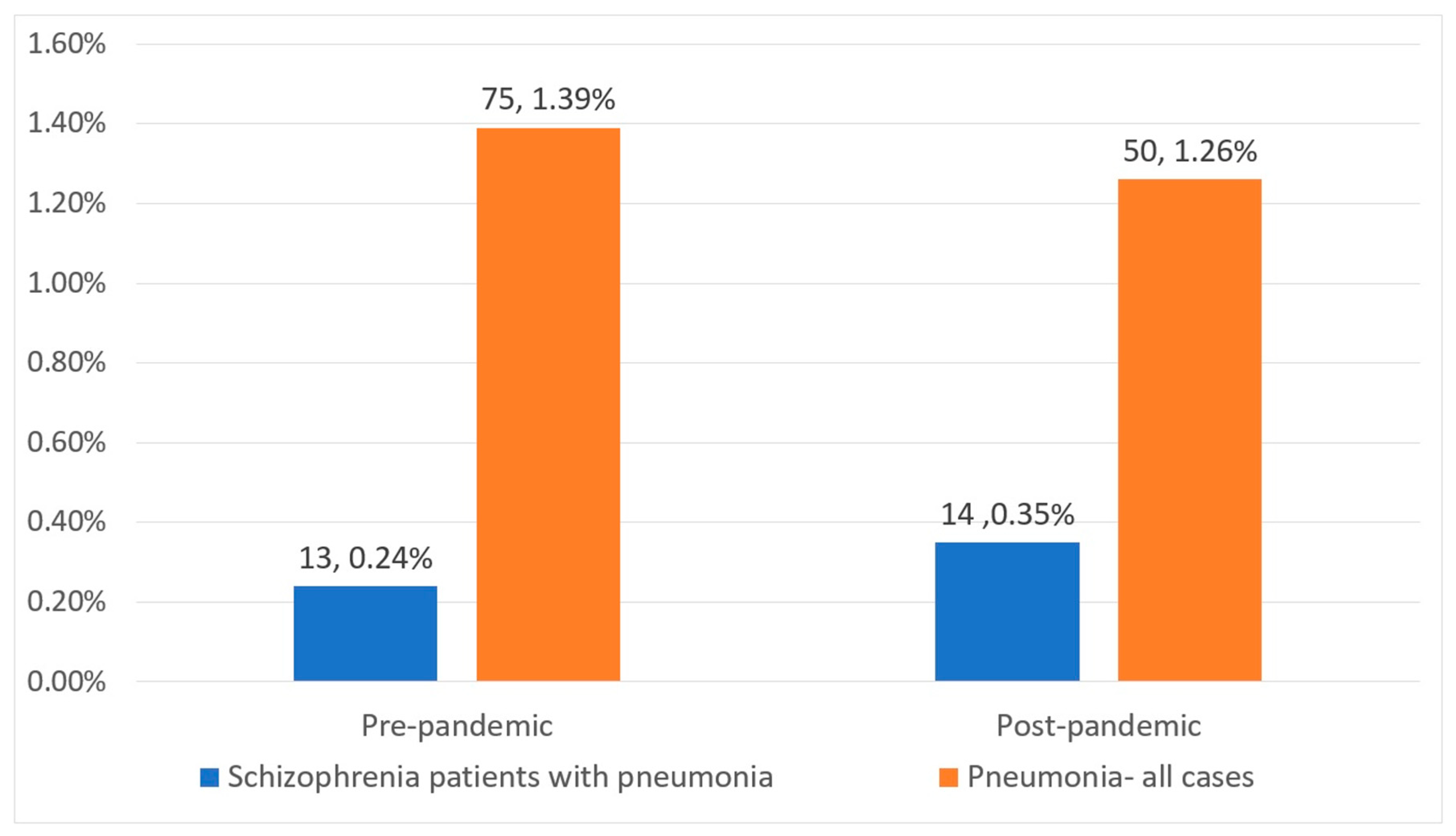
| Total patients (N, %) | 13 (48%) | 14 (52%) | 0.8 |
| Male gender (N, %) | 5 (38.46%) | 4 (28.57%) | 0.5 |
| Mean age (mean, ± SD) | 54.85 (±10.05) | 57.07 (±12.5) | 0.6 |
| Age of onset (mean, ± SD) | 29.77 (±8.94) | 29.43 (±12.9) | 0.9 |
| Duration of hospitalization (mean, ± SD) | 30 (±18.54) | 49.36 (±49.44) | 0.1 |
| Duration of illness (mean, ± SD) | 25.08 (±12.38) | 27.64 (±14.64) | 0.6 |
| Urban residence (N, %) | 9 (69.23%) | 13 (92.86%) | 0.1 |
| Poor living conditions (N, %) | 1 (7.69%) | 1 (7.14%) | 0.9 |
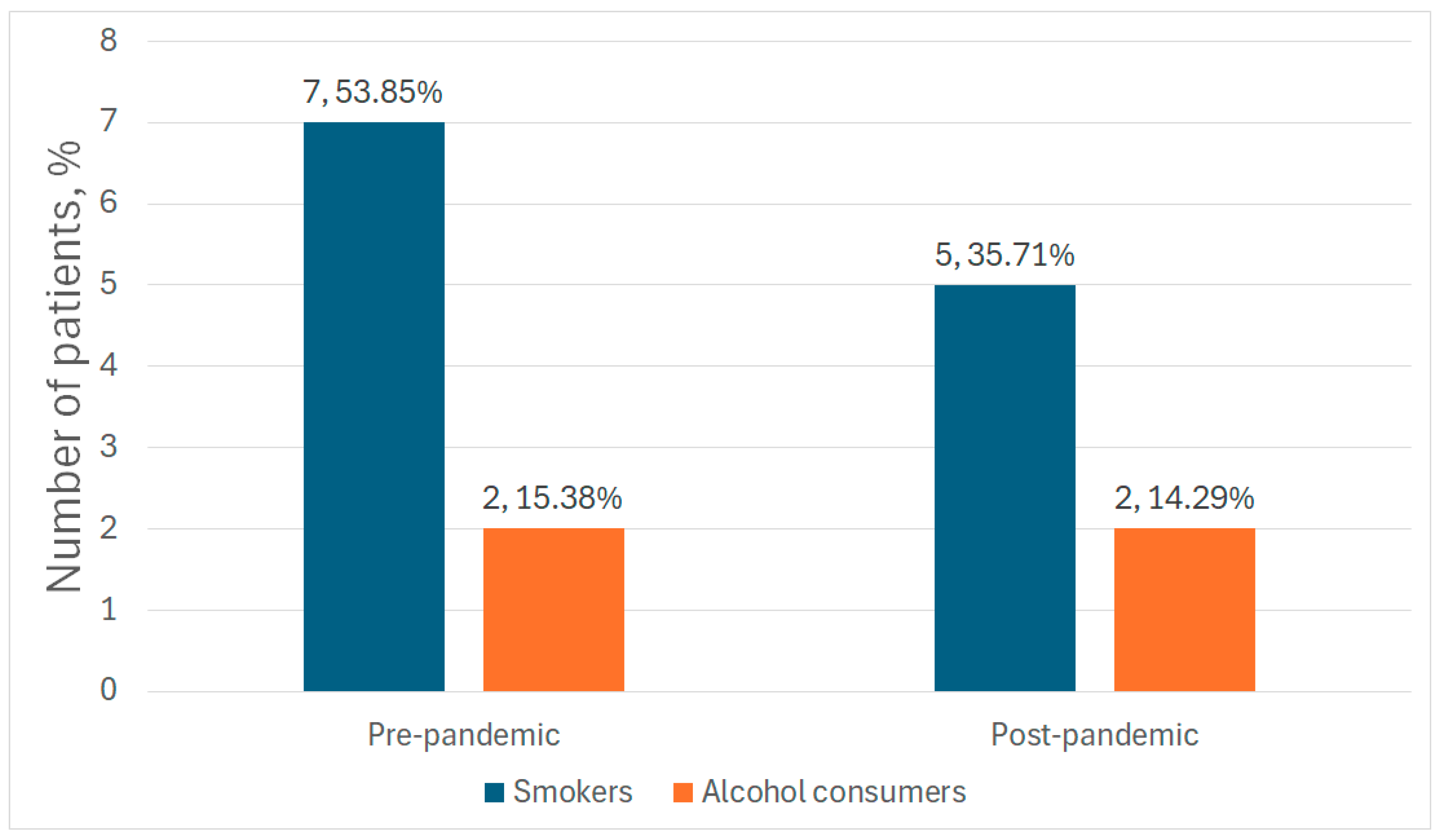
| Smokers (N, %) | 7 (53.85%) | 5 (35.71%) | 0.3 |
| Alcohol consumers (N, %) | 2 (15.38%) | 2 (14.29%) | 0.9 |
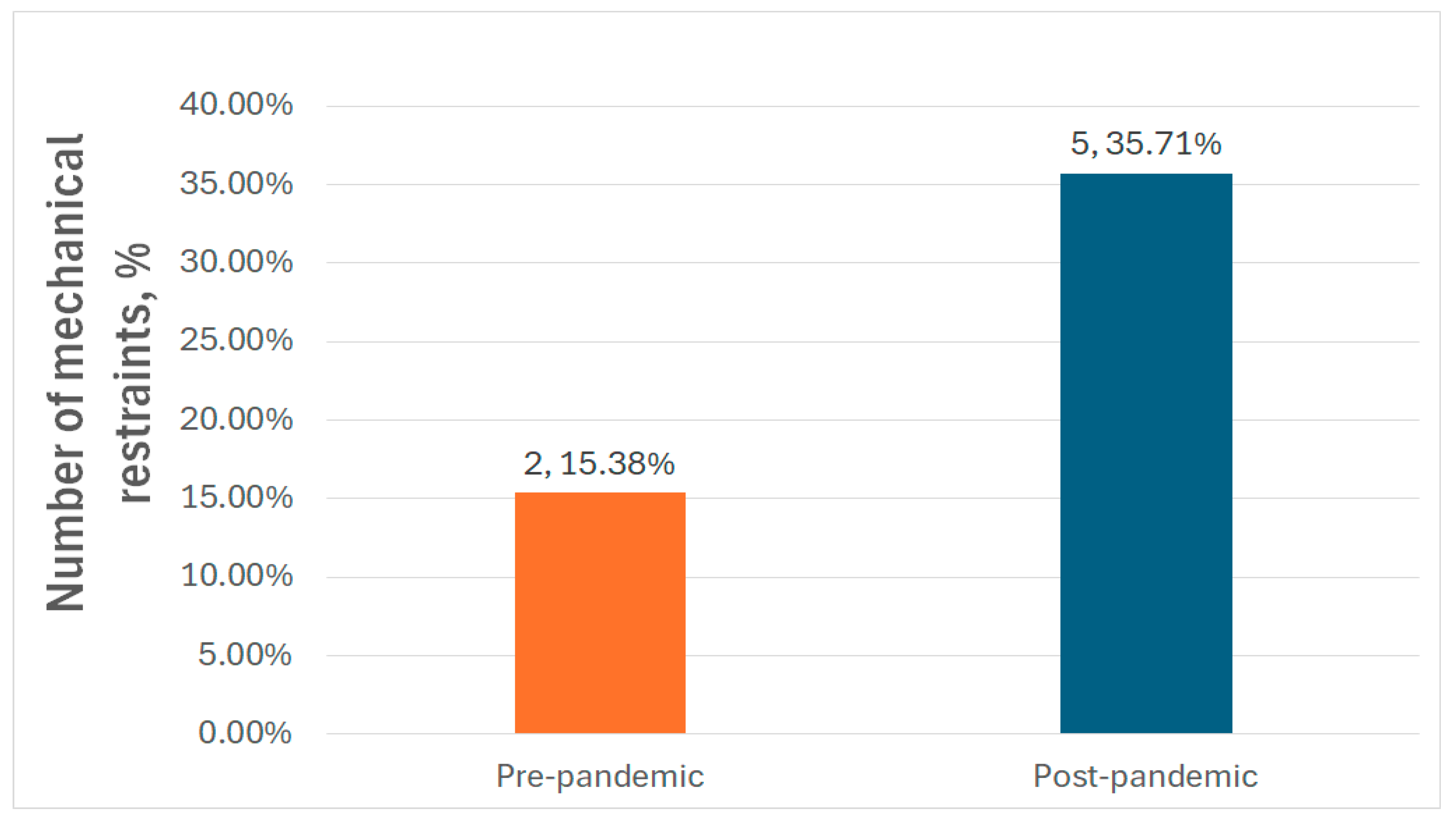
| Mechanical restraint (N, %) | 2 (15.38%) | 5 (35.71%) | 0.2 |
| AP (Type, Formulation) | No Cases (%) | Mean Dose (mg; ±SD) | CPZ Equivalent (mg) | p-Value | |
|---|---|---|---|---|---|
| Quetiapine | Pre-pandemic | 1 (7.69%) | 600 | 800 | 0.5 |
| Post-pandemic | 2 (14.29%) | 600 (±282.84) | 800 | ||
| Olanzapine | Pre-pandemic | 4 (30.77%) | 13.75 (±4.79) | 275 | 0.3 |
| Post-pandemic | 2 (14.29%) | 15 | 300 | ||
| Haloperidol | Pre-pandemic | 1 (7.69%) | 5 | 250 | 0.3 |
| Post-pandemic | 3 (21.43%) | 5.66 (±4.04) | 283 | ||
| Clozapine | Pre-pandemic | 2 (15.38%) | 325 (±318.2) | 165.5 | 0.5 |
| Post-pandemic | 1 (7.14%) | 400 | 200 | ||
| Risperidone | Pre-pandemic | 1 (7.69%) | 6 | 300 | 0.3 |
| Post-pandemic | 3 (21.43%) | 3.5 (±0.86 SD) | 175 | ||
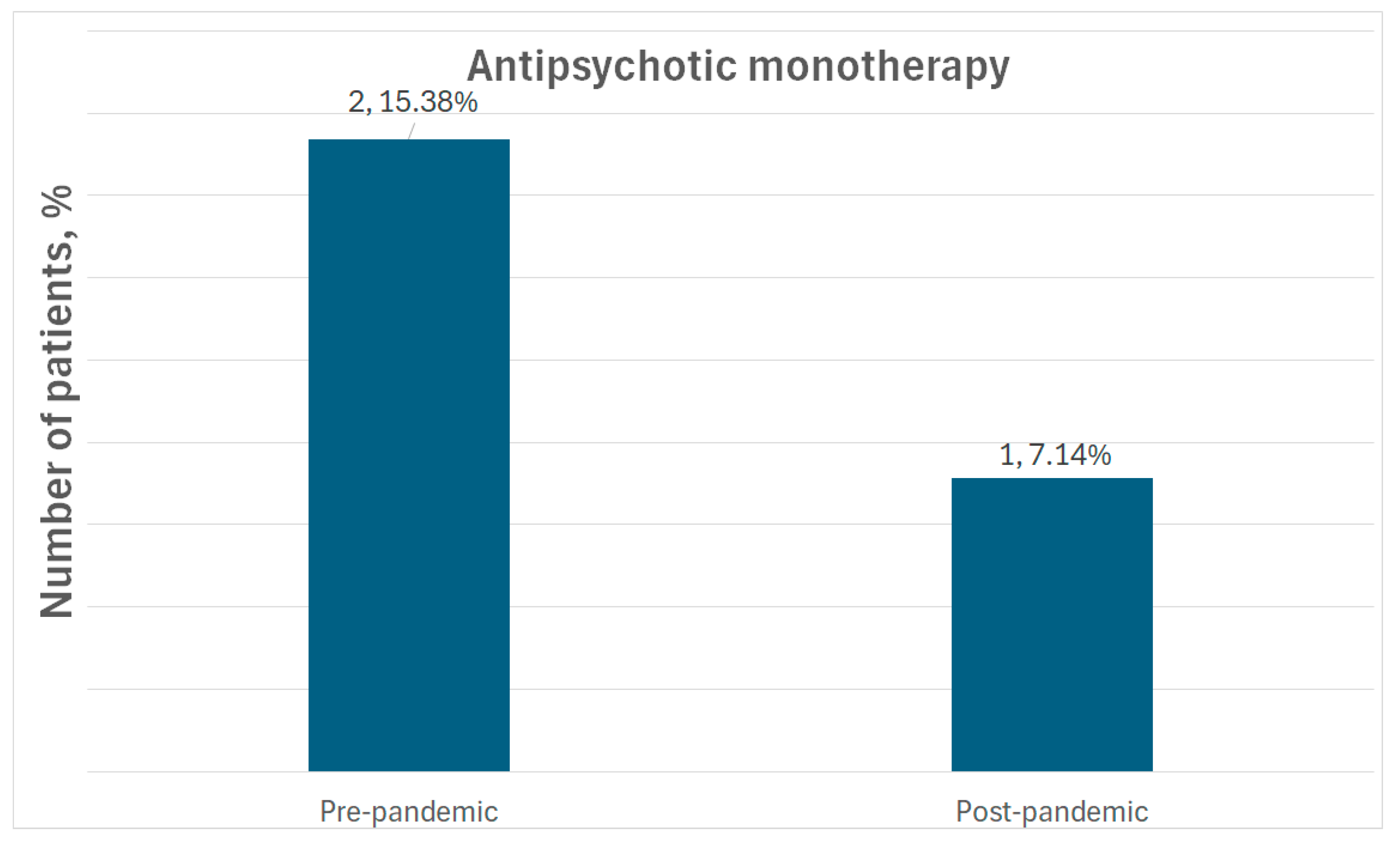
| Polypharmacy | Pre-pandemic | 4 (30.77%) | - | - | - |
| Post-pandemic | 3 (21.43%) | - | - | ||
| Antipsychotics Used | Pre-Pandemic (N, %) | Post-Pandemic (N, %) |
|---|---|---|
| HAL + ZUC + QUE | 1 (7.69%) | - |
| HAL + QUE | 1 (7.69%) | - |
| HAL + RIS | 1 (7.69%) | - |
| HAL + OLZ | 1 (7.69%) | - |
| CLZ + RIS | - | 1 (7.14%) |
| CLZ + FLX | - | 1 (7.14%) |
| CLZ + ARI | - | 1 (7.14%) |
| Medication Type | Number of Cases (%) | Mean Dose (mg; ±SD) | p-Value | ||
|---|---|---|---|---|---|
| Anticholinergic (Trihexyphenidyl) | Pre-pandemic | 3 (23.08%) | 3.33 (±1.15) | 0.2 | |
| Post-pandemic | 3 (21.43%) | 4.66 (±1.15) | |||
| BZD | Lorazepam | Pre-pandemic | 7 (53.85%) | 3.2 (±1.78) | 0.7 |
| Post-pandemic | 4 (28.57%) | 3.5 (±1.73) | |||
| Diazepam | Pre-pandemic | 3 (23.08%) | 16.66 (±5.77) | 0.6 | |
| Post-pandemic | 9 (64.29%) | 19.44 (±10.14) | |||
| Hypnotic medication | Zopiclone | Pre-pandemic | 3 (23.08%) | 7.5 | - |
| Post-pandemic | 0 | 0 | |||
| Zolpidem | Pre-pandemic | 2 (15.38%) | 10 | 0.4 | |
| Post-pandemic | 4 (28.57%) | 10 | |||
| Mood stabilizers (Valproate) | Pre-pandemic | 8 (61.54%) | 837.5 (±333.57) | 0.1 | |
| Post-pandemic | 9 (64.29%) | 1055.55 (±283.33) | |||
| Medication Type | Number of Cases (N, %) | ||
|---|---|---|---|
| Antibiotics | Cefuroxime | Pre-pandemic | 1 (7.69%) |
| Post-pandemic | 1 (7.14%) | ||
| Ceftriaxone | Pre-pandemic | 4 (30.76%) | |
| Post-pandemic | 1 (7.14%) | ||
| Amoxicillin + Clavulanic Acid | Pre-pandemic | 1 (7.69%) | |
| Post-pandemic | 1 (7.14%) | ||
| Ampicillin + Sulbactam | Pre-pandemic | 0 | |
| Post-pandemic | 1 (7.14%) | ||
| Levofloxacin | Pre-pandemic | 1 (7.69%) | |
| Post-pandemic | 3 (21.42%) | ||
| Sultamicillin | Pre-pandemic | 1 (7.69%) | |
| Post-pandemic | 0 | ||
| Ceftriaxone + Levofloxacin | Pre-pandemic | 2 (15.38%) | |
| Post-pandemic | 0 | ||
| Cefalexin + Sultamicillin | Pre-pandemic | 1 (7.69%) | |
| Post-pandemic | 0 | ||
| Azithromycin | Pre-pandemic | 0 | |
| Post-pandemic | 2 (14.28%) | ||
| Meropenem + Vancomycin | Pre-pandemic | 0 | |
| Post-pandemic | 1 (7.14%) | ||
| Meropenem | Pre-pandemic | 0 | |
| Post-pandemic | 2 (14.28%) | ||
| Ceftazidime | Pre-pandemic | 0 | |
| Post-pandemic | 1 (7.14%) | ||
| Antiviral medication | Favipiravir | Pre-pandemic | 0 |
| Post-pandemic | 1 (7.14%) | ||
| Favipiravir + Remdesivir | Pre-pandemic | 0 | |
| Post-pandemic | 1 (7.14%) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miron, A.-A.; Ifteni, P.I.; Lungu, A.-E.; Dragomirescu, E.-L.; Dima, L.; Teodorescu, A. Pre- and Post- COVID-19 Pandemic Pneumonia Rates in Hospitalized Schizophrenia Patients. Medicina 2025, 61, 1251. https://doi.org/10.3390/medicina61071251
Miron A-A, Ifteni PI, Lungu A-E, Dragomirescu E-L, Dima L, Teodorescu A. Pre- and Post- COVID-19 Pandemic Pneumonia Rates in Hospitalized Schizophrenia Patients. Medicina. 2025; 61(7):1251. https://doi.org/10.3390/medicina61071251
Chicago/Turabian StyleMiron, Ana-Aliana, Petru Iulian Ifteni, Alexandra-Elena Lungu, Elena-Luiza Dragomirescu, Lorena Dima, and Andreea Teodorescu. 2025. "Pre- and Post- COVID-19 Pandemic Pneumonia Rates in Hospitalized Schizophrenia Patients" Medicina 61, no. 7: 1251. https://doi.org/10.3390/medicina61071251
APA StyleMiron, A.-A., Ifteni, P. I., Lungu, A.-E., Dragomirescu, E.-L., Dima, L., & Teodorescu, A. (2025). Pre- and Post- COVID-19 Pandemic Pneumonia Rates in Hospitalized Schizophrenia Patients. Medicina, 61(7), 1251. https://doi.org/10.3390/medicina61071251






