Use of Bone Bank Grafts in Revision Total Hip Arthroplasty: Patient Characteristics at a Referral Center
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Clinical and Demographic Profile of the Study Population
3.2. Comparative Analysis of Clinical Variables According to Graft Type
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CI | Confidence Interval |
| ICU | Intensive Care Unit |
| SD | Standard Deviation |
| THA | Total Hip Arthroplasty |
| THAR | Revision Total Hip Arthroplasty |
References
- Abdelaal, M.S.; Restrepo, C.; Sharkey, P.F. Global Perspectives on Arthroplasty of Hip and Knee Joints. Orthop. Clin. N. Am. 2020, 51, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.M.; Farley, K.X.; Guild, G.N.; Bradbury, T.L. Projections and Epidemiology of Revision Hip and Knee Arthroplasty in the United States to 2030. J. Arthroplast. 2020, 35, S79–S85. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Saleh, H.; Bolz, N.; Buza, J.; Iorio, R.; Rathod, P.; Schwarzkopf, R.; Deshmukh, A. Re-revision total hip arthroplasty: Epidemiology and factors associated with outcomes. J. Clin. Orthop. Trauma 2020, 11, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Postler, A.E.; Beyer, F.; Wegner, T.; Lützner, J.; Hartmann, A.; Ojodu, I.; Günther, K.-P. Patient-reported outcomes after revision surgery compared to primary total hip arthroplasty. HIP Int. 2017, 27, 180–186. [Google Scholar] [CrossRef]
- Nichols, C.I.; Vose, J.G. Clinical Outcomes and Costs Within 90 Days of Primary or Revision Total Joint Arthroplasty. J. Arthroplast. 2016, 31, 1400–1406.e3. [Google Scholar] [CrossRef]
- Kamaruzaman, H.; Kinghorn, P.; Oppong, R. Cost-effectiveness of surgical interventions for the management of osteoarthritis: A systematic review of the literature. BMC Musculoskelet. Disord. 2017, 18, 183. [Google Scholar] [CrossRef]
- Haynes, J.A.; Stambough, J.B.; Sassoon, A.A.; Johnson, S.R.; Clohisy, J.C.; Nunley, R.M. Contemporary Surgical Indications and Referral Trends in Revision Total Hip Arthroplasty: A 10-Year Review. J. Arthroplast. 2016, 31, 622–625. [Google Scholar] [CrossRef]
- Zagorodniy, N.; Nikolaev, I.; Nuzhdin, V.; Kagramanov, S. Prospective cohort study of six hundred and sixty four revisions of loose failed acetabular implants. Int. Orthop. 2014, 38, 2021–2025. [Google Scholar] [CrossRef]
- Hartmann, E.S.; Köhler, M.I.; Huber, F.; Redeker, J.I.; Schmitt, B.; Schmitt-Sody, M.; Summer, B.; Fottner, A.; Jansson, V.; Mayer-Wagner, S. Factors regulating bone remodeling processes in aseptic implant loosening. J. Orthop. Res. 2017, 35, 248–257. [Google Scholar] [CrossRef]
- Kurcz, B.; Lyons, J.; Sayeed, Z.; Anoushiravani, A.A.; Iorio, R. Osteolysis as it Pertains to Total Hip Arthroplasty. Orthop. Clin. N. Am. 2018, 49, 419–435. [Google Scholar] [CrossRef]
- Brachet, A.; Bełżek, A.; Furtak, D.; Geworgjan, Z.; Tulej, D.; Kulczycka, K.; Karpiński, R.; Maciejewski, M.; Baj, J. Application of 3D Printing in Bone Grafts. Cells 2023, 12, 859. [Google Scholar] [CrossRef]
- Krakowski, P.; Jonak, J.; Karpinski, R.; Jaworski, Ł. Usefulness of rapid prototyping in planning complex trauma surgeries. Appl. Comput. Sci. 2019, 15, 65–72. [Google Scholar] [CrossRef]
- Karpiński, R.; Jaworski, Ł.; Zubrzycki, J. STRUCTURAL ANALYSIS OF ARTICULAR CARTILAGE OF THE HIP JOINT USING FINITE ELEMENT METHOD. Adv. Sci. Technol. Res. J. 2016, 10, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Göktaş, H.; Subaşi, E.; Uzkut, M.; Kara, M.; Biçici, H.; Shirazi, H.; Chethan, K.N.; Mihçin, Ş. Optimization of Hip Implant Designs Based on Its Mechanical Behaviour. In Lecture Notes in Networks and Systems; Springer: Cham, Switzerland, 2022. [Google Scholar]
- Beer, A.J.; Tauro, T.M.; Redondo, M.L.; Christian, D.R.; Cole, B.J.; Frank, R.M. Use of Allografts in Orthopaedic Surgery: Safety, Procurement, Storage, and Outcomes. Orthop. J. Sports Med. 2019, 7, 2325967119891435. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Witzmann, L.; Wieding, J.; Grifka, J.; Renkawitz, T.; Craiovan, B. Customized implants for acetabular Paprosky III defects may be positioned with high accuracy in revision hip arthroplasty. Int. Orthop. 2019, 43, 2235–2243. [Google Scholar] [CrossRef]
- Malchau, H.; Garellick, G.; Berry, D.; Harris, W.H.; Robertson, O.; Kärrlholm, J.; Lewallen, D.; Bragdon, C.R.; Lidgren, L.; Herberts, P. Arthroplasty implant registries over the past five decades: Development, current, and future impact. J. Orthop. Res. 2018, 36, 2319–2330. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data Data for Categorical of Observer Agreement The Measurement. Biometrics 2011, 33, 159–174. [Google Scholar] [CrossRef]
- Portney, L.; Watkins, M. Foundations of Clinical Research: Appications to Practice, 3rd ed.; Prentice Hall Health: Saddle River, NJ, USA, 2009. [Google Scholar]
- Neumann, L.T.V.; Albert, S.M. Aging in Brazil. Gerontologist 2018, 58, 611–617. [Google Scholar] [CrossRef]
- American Joint Replacement Registry. 2019 Annual Report. American Academy of Orthopaedic Surgeons. 2019. Available online: https://connect.registryapps.net/2019-ajrr-annual-report (accessed on 9 July 2025).
- Vastel, L.; Lemoine, C.T.; Kerboull, M.; Courpied, J.P. Structural allograft and cemented long-stem prosthesis for complex revision hip arthroplasty: Use of a trochanteric claw plate improves final hip function. Int. Orthop. 2007, 31, 851–857. [Google Scholar] [CrossRef]
- Zazgyva, A.; Zuh, S.G.; Roman, C.O.; Gergely, I.; Pop, T.S. Acetabular reconstruction with a reinforcement device and bone grafting in revision arthroplasty—A mean five years of follow-up. Int. Orthop. 2016, 40, 1631–1638. [Google Scholar] [CrossRef]
- Walker, R.P.; Gee, M.; Wong, F.; Shah, Z.; George, M.; Bankes, M.J.K.; Ajuied, A. Functional outcomes of total hip arthroplasty in patients aged 30 years or less: A systematic review and meta-analysis. HIP Int. 2016, 26, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Halvorsen, V.; Fenstad, A.M.; Engesæter, L.B.; Nordsletten, L.; Overgaard, S.; Pedersen, A.B.; Kärrholm, J.; Mohaddes, M.; Eskelinen, A.; Mäkelä, K.T.; et al. Outcome of 881 total hip arthroplasties in 747 patients 21 years or younger: Data from the Nordic Arthroplasty Register Association (NARA) 1995–2016. Acta Orthop. 2019, 90, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Bayliss, L.E.; Culliford, D.; Monk, A.P.; Glyn-Jones, S.; Prieto-Alhambra, D.; Judge, A.; Cooper, C.; Carr, A.J.; Arden, N.K.; Beard, D.J.; et al. The effect of patient age at intervention on risk of implant revision after total replacement of the hip or knee: A population-based cohort study. Lancet 2017, 389, 1424–1430. [Google Scholar] [CrossRef]
- Schmitz, M.W.J.L.; Timmer, C.; Hannink, G.; Schreurs, B.W. Systematic review: Lack of evidence for the success of revision arthroplasty outcome in younger patients. HIP Int. 2018, 28, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Sheth, N.P.; Nelson, C.L.; Springer, B.D.; Fehring, T.K.; Paprosky, W.G. Acetabular bone loss in revision total hip arthroplasty: Evaluation and management. J. Am. Acad. Orthop. Surg. 2013, 21, 128–139. [Google Scholar] [CrossRef]
- Baauw, M.; Van Hooff, M.L.; Spruit, M. Current construct options for revision of large acetabular defects: A systematic review. JBJS Rev. 2016, 4, e2. [Google Scholar] [CrossRef]
- Malahias, M.A.; Mancino, F.; Gu, A.; Adriani, M.; De Martino, I.; Boettner, F.; Sculco, P.K. Acetabular impaction grafting with mesh for acetabular bone defects: A systematic review. HIP Int. 2022, 32, 185–196. [Google Scholar] [CrossRef]
- Gee, M.J.; Ajuied, A.; Shah, Z.; George, M.; Bankes, M.J.K. Systematic review of total hip arthroplasty in patients under 30 years old. HIP Int. 2013, 23, 345–351. [Google Scholar] [CrossRef]
- Schmalzried, T.P.; Shepherd, E.F.; Dorey, F.J.; Jackson, W.O.; Dela Rosa, M.; Fa’vae, F.; A McKellop, H.; McClung, C.D.; Martell, J.; Moreland, J.R.; et al. Wear is a function of use, not time. Clin. Orthop. Relat. Res. 2000, 381, 36–46. [Google Scholar] [CrossRef]
- Langlois, J.; Zaoui, A.; Bichara, D.A.; Nich, C.; Bensidhoum, M.; Petite, H.; Muratoglu, O.K.; Hamadouche, M. Biological reaction to polyethylene particles in a murine calvarial model is highly influenced by age. J. Orthop. Res. 2016, 34, 574–580. [Google Scholar] [CrossRef]
- Hayashi, S.; Hashimoto, S.; Takayama, K.; Matsumoto, T.; Nishida, K.; Kuroda, R. Multiple Revision Surgeries and Acetabular Bone Defect Size May Predict Daily Activity After Revision Total Hip Arthroplasty. J. Arthroplast. 2017, 32, 1606–1611. [Google Scholar] [CrossRef] [PubMed]
- Paprosky, W.G.; Perona, P.G.; Lawrence, J.M. Acetabular defect classification and surgical reconstruction in revision arthroplasty. A 6-year follow-up evaluation. J. Arthroplast. 1994, 9, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Telleria, J.J.M.; Gee, A.O. Classifications in brief: Paprosky classification of acetabular bone loss. Clin. Orthop. Relat. Res. 2013, 471, 3725–3730. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Hofstaetter, J.G.; Sullivan, T.; Costi, K.; Howie, D.W.; Solomon, L.B. Validity and reliability of the paprosky acetabular defect classification hip. Clin. Orthop. Relat. Res. 2013, 471, 2259–2265. [Google Scholar] [CrossRef]
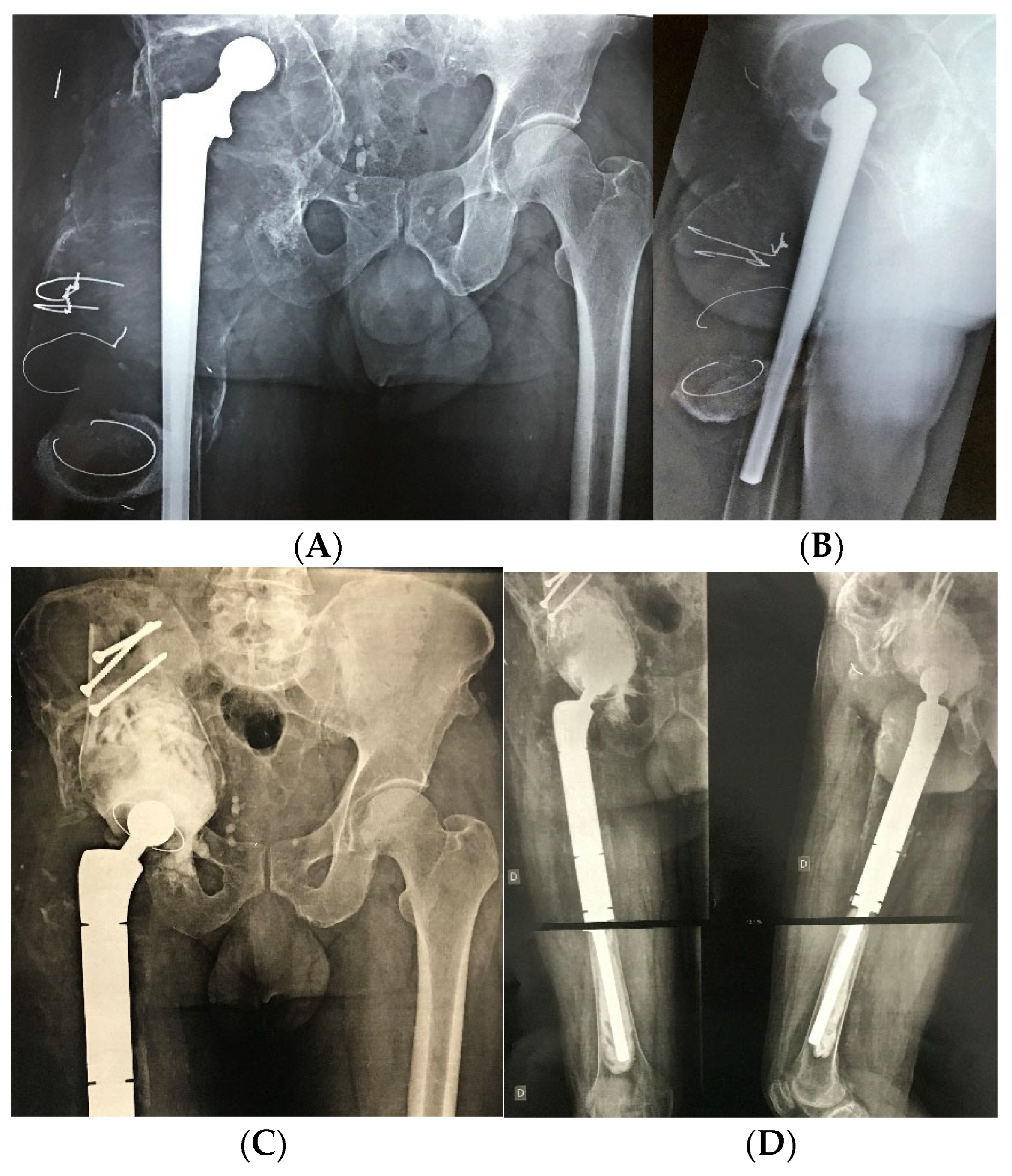
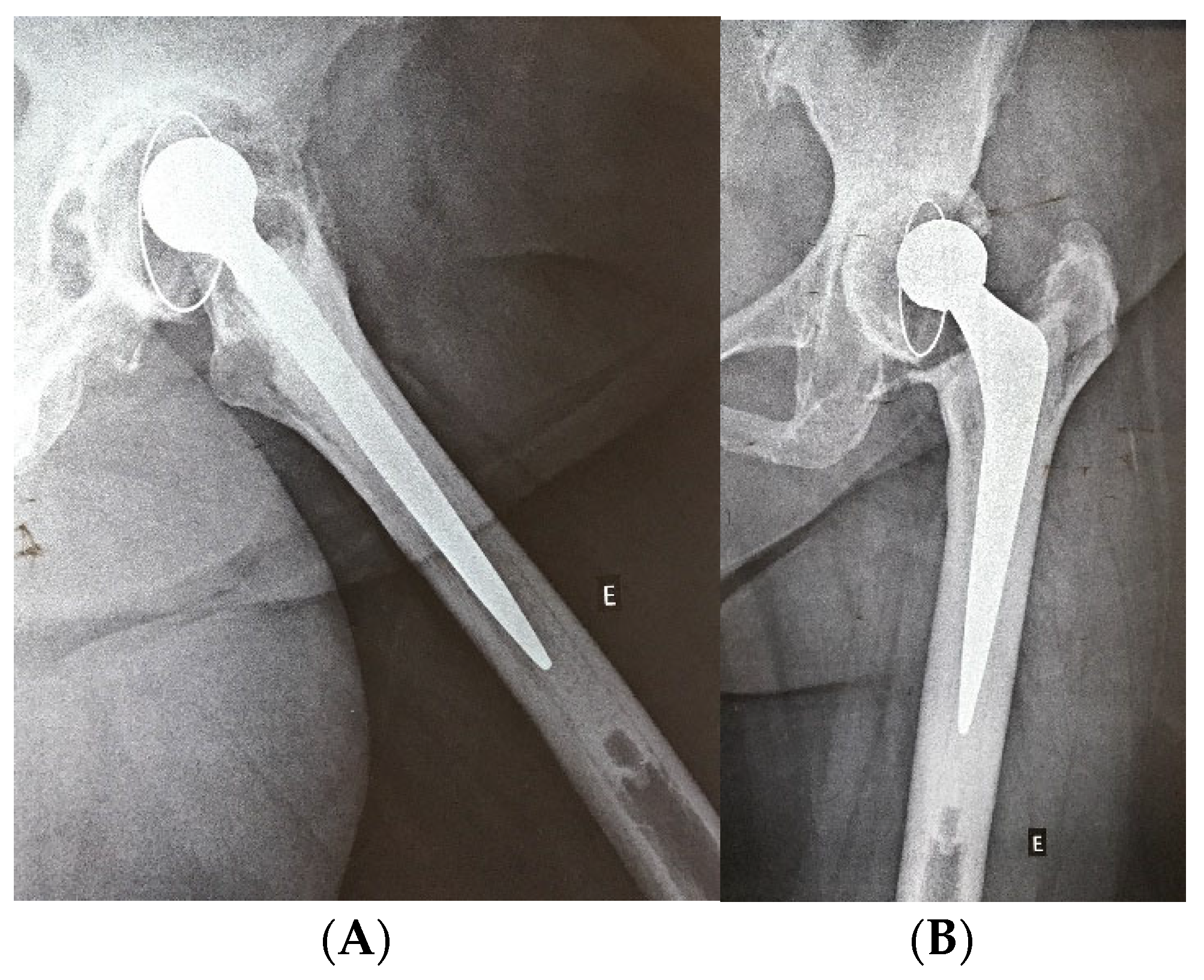

| Characteristic | n = 67 |
|---|---|
| Age, mean SD | 63.2 (10.9) |
| Sex, male—n (%) | 36 (53.7) |
| Region, metropolitan—n (%) | 35 (52.2) |
| Bone allograft, impacted bone graft—n (%) | 42 (66.7) |
| Basic pathology, arthrosis—n (%) | 50 (74.6) |
| Previous surgeries, only primary total hip arthroplasty—n (%) | 32 (47.8) |
| Previous complications—loosening—n (%) | 60 (89.6) |
| Systemic arterial hypertension, yes—n (%) | 36 (53.7) |
| Diabetes mellitus, no—n (%) | 60 (89.6) |
| Other comorbidities, metabolic diseases—n (%) | 11 (42.3) |
| Total hip arthroplasty opposite member, no—n (%) | 45 (67.2) |
| Smoking, no—n (%) | 55 (84.6) |
| Alcoholism, no—n (%) | 50 (76.9) |
| Hospital complications, no—n (%) | 41 (61.2) |
| Operative infection, no—n (%) | 59 (88.1) |
| Late complications, no—n (%) | 57 (86.4) |
| Late operative infection, no—n (%) | 61 (92.4) |
| Growth intraoperative cultures, no—n (%) | 54 (87.1) |
| Blood transfusion, no—n (%) | 49 (73.1) |
| Variable | Mean (SD) | Mean Difference (SD) [CI 95%] | p-Value | Cohen’s d | |
|---|---|---|---|---|---|
| Previous surgery (n) | Structured | 2.14 (0.91) | 0.55 (0.21) [0.11 to 0.98] | 0.02 | 0.65 |
| Impacted bone graft | 1.60 (0.78) | ||||
| Last surgery (months) | Structured | 126.63 (87.99) | −3.61 (20.77) [−45.16 to 37.95] | 0.86 | −0.05 |
| Impacted bone graft | 129.24 (68.71) | ||||
| Date of primary total hip arthroplasty | Structured | 214.53 (119.16) | 53.40 (24.76) [3.85 to 102.96] | 0.04 | 0.59 |
| Impacted bone graft | 161.12 (71.76) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gontijo, T.d.C.; Xavier, L.O.P.; Morais, L.C.; Silva, G.W.; Polese, J.C.; da Silva, R.B.; Leopoldino, A.A.O. Use of Bone Bank Grafts in Revision Total Hip Arthroplasty: Patient Characteristics at a Referral Center. Medicina 2025, 61, 1246. https://doi.org/10.3390/medicina61071246
Gontijo TdC, Xavier LOP, Morais LC, Silva GW, Polese JC, da Silva RB, Leopoldino AAO. Use of Bone Bank Grafts in Revision Total Hip Arthroplasty: Patient Characteristics at a Referral Center. Medicina. 2025; 61(7):1246. https://doi.org/10.3390/medicina61071246
Chicago/Turabian StyleGontijo, Thiago de Carvalho, Luiz Octávio Pereira Xavier, Lucas Carneiro Morais, Gustavo Waldolato Silva, Janaíne Cunha Polese, Raquel Bandeira da Silva, and Amanda Aparecida Oliveira Leopoldino. 2025. "Use of Bone Bank Grafts in Revision Total Hip Arthroplasty: Patient Characteristics at a Referral Center" Medicina 61, no. 7: 1246. https://doi.org/10.3390/medicina61071246
APA StyleGontijo, T. d. C., Xavier, L. O. P., Morais, L. C., Silva, G. W., Polese, J. C., da Silva, R. B., & Leopoldino, A. A. O. (2025). Use of Bone Bank Grafts in Revision Total Hip Arthroplasty: Patient Characteristics at a Referral Center. Medicina, 61(7), 1246. https://doi.org/10.3390/medicina61071246






