Comparing Frailty Status Among Clusters Identified Based on EQ-5D-5L Dimensions in Older Patients with Chronic Low Back Pain
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics and Consent
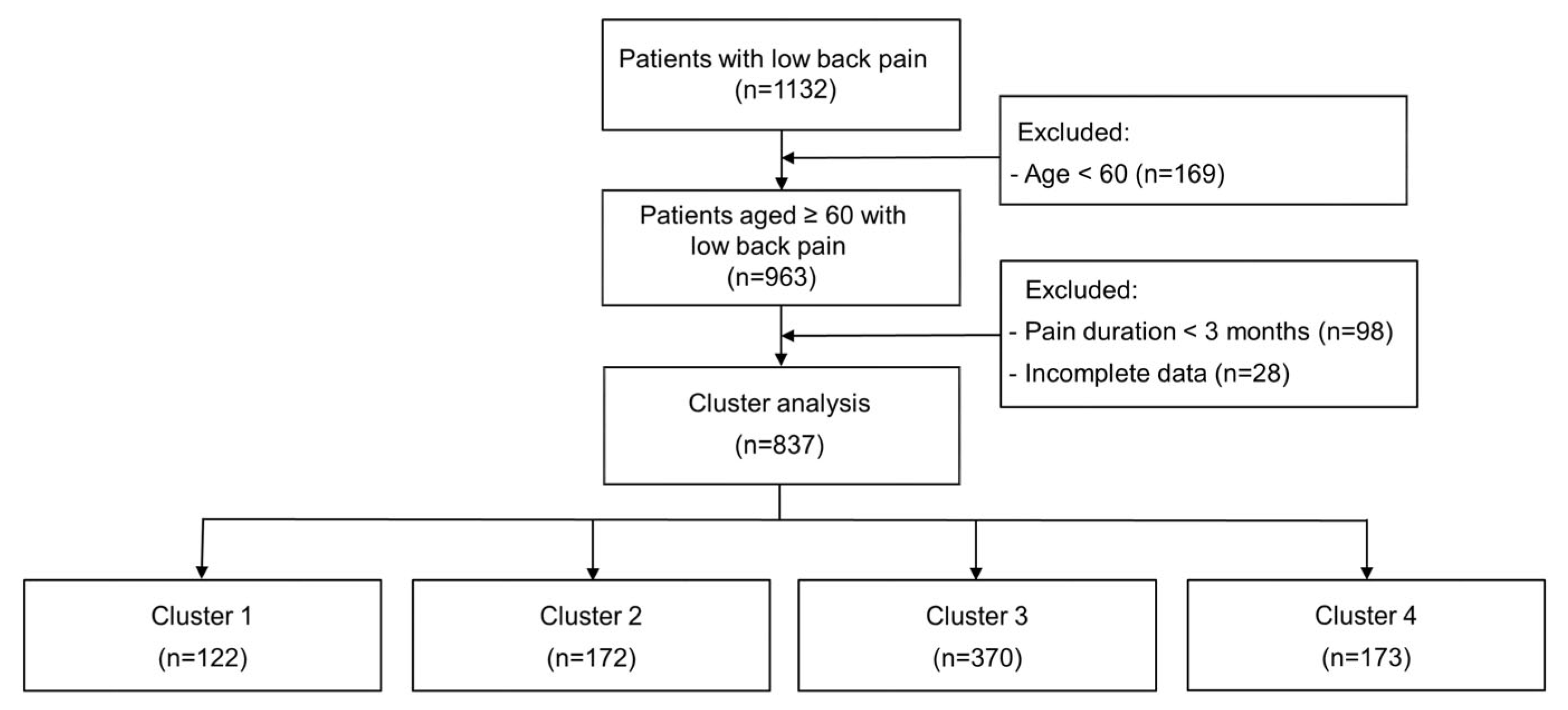
2.2. Study Population
2.3. Evaluation of HRQoL
2.4. Frailty Screening
- Fatigue: Participants receive 1 point if they report feeling that everything they did was a lot of effort for more than 3 days a week in the past week, otherwise 0 points.
- Resistance: Participants receive 1 point if they have difficulty climbing 10 stairs without assistance, otherwise 0 points.
- Ambulation: Participants receive 1 point if they report any difficulty walking around a 400 m playground track, otherwise 0 points.
- Inactivity: Participants receive 1 point if they do not engage in moderate or vigorous physical activity in the past 7 days, otherwise 0 points.
- Unintentional weight loss: Participants receive 1 point if they lost 4.5 kg or more unintentionally in the past year, otherwise 0 points.
2.5. Patient Demographics and Clinical Data Measurements
2.6. Statistical Analysis
2.7. Cluster Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LBP | Low back pain |
| HRQoL | Health-related quality of life |
| EQ-VAS | EQ Visual Analogue Scale |
| BMI | Body mass index |
| NRS | Numeric Rating Scale |
| SD | Standard deviation |
References
- Hartvigsen, J.; Hancock, M.J.; Kongsted, A.; Louw, Q.; Ferreira, M.L.; Genevay, S.; Hoy, D.; Karppinen, J.; Pransky, G.; Sieper, J.; et al. What low back pain is and why we need to pay attention. Lancet 2018, 391, 2356–2367. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, N.N.; Candido, K.D.; Vlaeyen, J.W.S.; Van Zundert, J.; Cohen, S.P. Low back pain. Lancet 2021, 398, 78–92. [Google Scholar] [CrossRef]
- Semeru, G.M.; Halim, M.S. Acceptance versus catastrophizing in predicting quality of life in patients with chronic low back pain. Korean J. Pain. 2019, 32, 22–29. [Google Scholar] [CrossRef]
- Henschke, N.; Maher, C.G.; Refshauge, K.M.; Herbert, R.D.; Cumming, R.G.; Bleasel, J.; York, J.; Das, A.; McAuley, J.H. Prognosis in patients with recent onset low back pain in Australian primary care: Inception cohort study. BMJ 2008, 337, a171. [Google Scholar] [CrossRef]
- Rudy, T.E.; Weiner, D.K.; Lieber, S.J.; Slaboda, J.; Boston, R.J. The impact of chronic low back pain on older adults: A comparative study of patients and controls. Pain 2007, 131, 293–301. [Google Scholar] [CrossRef]
- Geigl, C.; Loss, J.; Leitzmann, M.; Janssen, C. Social factors of health-related quality of life in older adults: A multivariable analysis. Qual. Life Res. 2023, 32, 3257–3268. [Google Scholar] [CrossRef]
- Etxeberria, I.; Urdaneta, E.; Galdona, N. Factors associated with health-related quality of life (HRQoL): Differential patterns depending on age. Qual. Life Res. 2019, 28, 2221–2231. [Google Scholar] [CrossRef]
- Herdman, M.; Gudex, C.; Lloyd, A.; Janssen, M.; Kind, P.; Parkin, D.; Bonsel, G.; Badia, X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual. Life Res. 2011, 20, 1727–1736. [Google Scholar] [CrossRef]
- Staartjes, V.E.; Klukowska, A.M.; Stumpo, V.; Vandertop, W.P.; Schröder, M.L. Identifying clusters of objective functional impairment in patients with degenerative lumbar spinal disease using unsupervised learning. Eur. Spine J. 2024, 33, 1320–1331. [Google Scholar] [CrossRef]
- Hoogendijk, E.O.; Afilalo, J.; Ensrud, K.E.; Kowal, P.; Onder, G.; Fried, L.P. Frailty: Implications for clinical practice and public health. Lancet 2019, 394, 1365–1375. [Google Scholar] [CrossRef]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Masel, M.C.; Graham, J.E.; Reistetter, T.A.; Markides, K.S.; Ottenbacher, K.J. Frailty and health related quality of life in older Mexican Americans. Health Qual. Life Outcomes 2009, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.W.; Chen, W.L.; Lin, F.G.; Fang, W.H.; Yen, M.Y.; Hsieh, C.C.; Kao, T.W. Frailty and its impact on health-related quality of life: A cross-sectional study on elder community-dwelling preventive health service users. PLoS ONE 2012, 7, e38079. [Google Scholar] [CrossRef]
- Kim, H.J.; Jun, B.; Lee, H.W.; Kim, S.H. Influence of frailty status on the health-related quality of life in older patients with chronic low back pain: A retrospective observational study. Qual. Life Res. 2024, 33, 1905–1913. [Google Scholar] [CrossRef]
- Herman, P.M.; Coulter, I.D.; Hays, R.D.; Rodriguez, A.; Edelen, M.O. A Scoping Review of Chronic Low Back Pain Classification Schemes Based on Patient-Reported Outcomes. Pain. Physician 2022, 25, 471–482. [Google Scholar]
- Kim, S.H.; Ahn, J.; Ock, M.; Shin, S.; Park, J.; Luo, N.; Jo, M.W. The EQ-5D-5L valuation study in Korea. Qual. Life Res. 2016, 25, 1845–1852. [Google Scholar] [CrossRef]
- Kim, S.; Kim, M.; Jung, H.W.; Won, C.W. Development of a Frailty Phenotype Questionnaire for Use in Screening Community-Dwelling Older Adults. J. Am. Med. Dir. Assoc. 2020, 21, 660–664. [Google Scholar] [CrossRef]
- Krebs, E.E.; Carey, T.S.; Weinberger, M. Accuracy of the pain numeric rating scale as a screening test in primary care. J. Gen. Intern. Med. 2007, 22, 1453–1458. [Google Scholar] [CrossRef]
- Langenmaier, A.M.; Amelung, V.E.; Karst, M.; Krauth, C.; Püschner, F.; Urbanski, D.; Schiessl, C.; Thoma, R.; Klasen, B. Subgroups in chronic low back pain patients—A step toward cluster-based, tailored treatment in inpatient standard care: On the need for precise targeting of treatment for chronic low back pain. Ger. Med. Sci. 2019, 17, Doc09. [Google Scholar] [CrossRef]
- Viniol, A.; Jegan, N.; Hirsch, O.; Leonhardt, C.; Brugger, M.; Strauch, K.; Barth, J.; Baum, E.; Becker, A. Chronic low back pain patient groups in primary care—A cross sectional cluster analysis. BMC Musculoskelet. Disord. 2013, 14, 294. [Google Scholar] [CrossRef]
- Fritz, J.M.; Cleland, J.A.; Childs, J.D. Subgrouping patients with low back pain: Evolution of a classification approach to physical therapy. J. Orthop. Sports Phys. Ther. 2007, 37, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.C.; Whitehurst, D.G.; Lewis, M.; Bryan, S.; Dunn, K.M.; Foster, N.E.; Konstantinou, K.; Main, C.J.; Mason, E.; Somerville, S.; et al. Comparison of stratified primary care management for low back pain with current best practice (STarT Back): A randomised controlled trial. Lancet 2011, 378, 1560–1571. [Google Scholar] [CrossRef] [PubMed]
- Gill, T.M.; Gahbauer, E.A.; Allore, H.G.; Han, L. Transitions between frailty states among community-living older persons. Arch. Intern. Med. 2006, 166, 418–423. [Google Scholar] [CrossRef]
- Dent, E.; Martin, F.C.; Bergman, H.; Woo, J.; Romero-Ortuno, R.; Walston, J.D. Management of frailty: Opportunities, challenges, and future directions. Lancet 2019, 394, 1376–1386. [Google Scholar] [CrossRef]
- Feldman, D.E.; Nahin, R.L. Disability Among Persons With Chronic Severe Back Pain: Results From a Nationally Representative Population-based Sample. J. Pain. 2022, 23, 2144–2154. [Google Scholar] [CrossRef]
- Coyle, P.C.; Sions, J.M.; Velasco, T.; Hicks, G.E. Older Adults with Chronic Low Back Pain: A Clinical Population Vulnerable to Frailty? J. Frailty Aging 2015, 4, 188–190. [Google Scholar] [CrossRef]
- Otones, P.; García, E.; Sanz, T.; Pedraz, A. A physical activity program versus usual care in the management of quality of life for pre-frail older adults with chronic pain: Randomized controlled trial. BMC Geriatr. 2020, 20, 396. [Google Scholar] [CrossRef]
- Haider, S.; Grabovac, I.; Dorner, T.E. Effects of physical activity interventions in frail and prefrail community-dwelling people on frailty status, muscle strength, physical performance and muscle mass-a narrative review. Wien. Klin. Wochenschr. 2019, 131, 244–254. [Google Scholar] [CrossRef]
- Ní Mhaoláin, A.M.; Fan, C.W.; Romero-Ortuno, R.; Cogan, L.; Cunningham, C.; Kenny, R.A.; Lawlor, B. Frailty, depression, and anxiety in later life. Int. Psychogeriatr. 2012, 24, 1265–1274. [Google Scholar] [CrossRef]
- Woods, N.F.; LaCroix, A.Z.; Gray, S.L.; Aragaki, A.; Cochrane, B.B.; Brunner, R.L.; Masaki, K.; Murray, A.; Newman, A.B. Women’s Health Initiative Frailty: Emergence consequences in women aged 65, older in the Women’s Health Initiative Observational Study. J. Am. Med. Dir. Assoc. 2005, 53, 1321–1330. [Google Scholar] [CrossRef]
- Zhao, D.; Chai, S.; Gao, T.; Li, J.; Zhou, C. Physical Mobility, Social Isolation and Cognitive Function: Are There Really Gender Differences? Am. J. Geriatr. Psychiatry 2023, 31, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Paluska, S.A.; Schwenk, T.L. Physical activity and mental health: Current concepts. Sports Med. 2000, 29, 167–180. [Google Scholar] [CrossRef]
- Rebar, A.L.; Duncan, M.J.; Short, C.; Vandelanotte, C. Differences in health-related quality of life between three clusters of physical activity, sitting time, depression, anxiety, and stress. BMC Public Health 2014, 14, 1088. [Google Scholar] [CrossRef]
- Suh, J.H.; Kim, H.; Jung, G.P.; Ko, J.Y.; Ryu, J.S. The effect of lumbar stabilization and walking exercises on chronic low back pain: A randomized controlled trial. Medicine 2019, 98, e16173. [Google Scholar] [CrossRef]
- Geneen, L.J.; Moore, R.A.; Clarke, C.; Martin, D.; Colvin, L.A.; Smith, B.H. Physical activity and exercise for chronic pain in adults: An overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2017, 1, Cd011279. [Google Scholar] [CrossRef]
- Failla, M.D.; Beach, P.A.; Atalla, S.; Dietrich, M.S.; Bruehl, S.; Cowan, R.L.; Monroe, T.B. Gender Differences in Pain Threshold, Unpleasantness, and Descending Pain Modulatory Activation Across the Adult Life Span: A Cross Sectional Study. J. Pain. 2024, 25, 1059–1069. [Google Scholar] [CrossRef]
- Kaufman, J.M.; Lapauw, B. Role of testosterone in cognition and mobility of aging men. Andrology 2020, 8, 1567–1579. [Google Scholar] [CrossRef]
- Andrasinova, T.; Adamova, B.; Buskova, J.; Kerkovsky, M.; Jarkovsky, J.; Bednarik, J. Is there a Correlation Between Degree of Radiologic Lumbar Spinal Stenosis and its Clinical Manifestation? Clin. Spine Surg. 2018, 31, E403–E408. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, H.J. Frailty across chronic low back pain clusters: Insights from EQ-5D-5L. In Proceedings of the 78th Korean Pain Society Meeting, Seoul, Republic of Korea, 16 November 2024. [Google Scholar]


| Variables | Total (n = 837) |
|---|---|
| Patient characteristics | |
| Age, years | 72.01 ± 7.14 (60–96) |
| Female sex, n | 562 (67.1) |
| BMI, kg/m2 | 22.89 (20.81; 25.18) |
| Comorbid medical disease, n | |
| Hypertension | 251 (30.0) |
| Diabetes mellitus | 146 (17.4) |
| Cancer | 130 (15.5) |
| Mental health problems | 107 (12.8) |
| Osteopenia/osteoporosis | 104 (12.4) |
| Spine surgery history, n | 197 (23.5) |
| Pain-related data | |
| Baseline pain score | |
| Mild (NRS 1–3), n | 46 (5.5) |
| Moderate (NRS 4–6), n | 329 (39.3) |
| Severe (NRS 7–10), n | 462 (55.2) |
| Pain duration, months | 12.00 (6.00; 49.00) |
| Opioid usage, n | 284 (33.9) |
| Sleep disturbance, n | 273 (32.6) |
| EQ-5D-5L | |
| Mobility, >2, n | 433 (51.8) |
| Self-care, >2, n | 144 (17.2) |
| Usual activities, >2, n | 248 (29.6) |
| Pain/discomfort, >2, n | 587 (70.1) |
| Anxiety/depression, >2, n | 214 (25.6) |
| EQ-VAS, 0–100 | 61.87 ± 17.89 (10–100) |
| EQ-5D-5L index | 0.63 ± 0.18 (−0.08–1.00) |
| Frailty status, n | |
| Robust | 244 (29.2) |
| Pre-frail | 409 (48.9) |
| Frail | 184 (22.0) |
| Cluster Label | Cluster 1 (n = 122) | Cluster 2 (n = 172) | Cluster 3 (n = 370) | Cluster 4 (n = 173) | p-Value |
|---|---|---|---|---|---|
| Patient characteristics | |||||
| Age, years | 71.90 ± 6.69 (60–88) | 71.86 ± 7.37 (60–91) | 72.13 ± 7.21 (60–96) | 71.97 ± 7.12 (60–91) | 0.975 |
| Female sex, n ** | 86 (70.5) 2 | 98 (57.0) 1,3,4 | 258 (69.7) 2 | 120 (69.4) 2 | 0.017 |
| BMI, kg/m2 | 22.62 (20.75; 24.44) | 23.14 (21.64; 25.35) | 22.89 (20.77; 25.58) | 22.83 (20.52; 24.85) | 0.141 |
| Comorbid medical disease, n | |||||
| Hypertension | 37 (30.3) | 57 (33.1) | 106 (28.6) | 51 (29.5) | 0.763 |
| Diabetes mellitus | 15 (12.3) | 35 (20.3) | 64 (17.3) | 32 (18.5) | 0.335 |
| Cancer | 19 (15.6) | 29 (16.9) | 54 (14.6) | 28 (16.2) | 0.911 |
| Mental health problems | 11 (9.0) | 22 (12.8) | 49 (13.2) | 25 (14.5) | 0.561 |
| Osteopenia/osteoporosis | 13 (10.7) | 23 (13.4) | 42 (11.4) | 26 (15.0) | 0.580 |
| Spine surgery history | 21 (17.2) | 38 (22.1) | 96 (25.9) | 42 (24.3) | 0.245 |
| Pain-related data | |||||
| Baseline pain score | |||||
| Mild (NRS 1–3), n ** | 5 (4.1) 3,4 | 4 (2.3) 3.4 | 26 (7.0) 1,2 | 11 (6.4) 1,2 | <0.001 |
| Moderate (NRS 4–6), n | 34 (27.9) 3,4 | 54 (31.4) 3,4 | 162 (43.8) 1,2 | 79 (45.7) 1,2 | |
| Severe (NRS 7–10), n | 83 (68.0) 3,4 | 114 (66.3) 3,4 | 182 (49.2) 1,2 | 83 (48.0) 1,2 | |
| Pain duration, months | 13.50 (6.00; 60.00) | 12.00 (5.00; 36.00) | 24.00 (6.00; 60.00) | 12.00 (6.00; 38.00) | 0.050 |
| Opioid usage, n | 38 (31.1) | 63 (36.6) | 123 (33.2) | 60 (34.7) | 0.777 |
| Sleep disturbance, n | 51 (41.8) | 61 (35.5) | 112 (30.3) | 49 (28.3) | 0.053 |
| EQ-5D-5L | |||||
| EQ-VAS, 0–100 * | 56.80 ± 18.53 (10–90) 2,3,4 | 63.84 ± 16.16 (20–100) 1 | 62.01 ± 18.10 (10–100) 1 | 63.21 ± 18.11 (10–100) 1 | 0.005 |
| EQ-5D-5L index * | 0.55 ± 0.18 (0.16–0.83) 3,4 | 0.55 ± 0.17 (0.08–0.80) 3,4 | 0.66 ± 0.17 (−0.08–1.00) 1,2 | 0.69 ± 0.15 (0.19–0.86) 1,2 | <0.001 |
| Frailty status, n ** | |||||
| Robust | 29 (23.8) 2,3,4 | 19 (11.0) 1,3,4 | 135 (36.5) 1,2,4 | 61 (35.3) 1,2,3 | <0.001 |
| Pre-frail | 64 (52.5) 2,3,4 | 104 (60.5) 1,3,4 | 152 (41.1) 1,2,4 | 89 (51.4) 1,2,3 | |
| Frail | 29 (23.8) 2,3,4 | 49 (28.5) 1,3,4 | 83 (22.4) 1,2,4 | 23 (13.3) 1,2,3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.J.; Kim, H.C.; Hwang, J.; Kim, S.H. Comparing Frailty Status Among Clusters Identified Based on EQ-5D-5L Dimensions in Older Patients with Chronic Low Back Pain. Medicina 2025, 61, 1217. https://doi.org/10.3390/medicina61071217
Kim HJ, Kim HC, Hwang J, Kim SH. Comparing Frailty Status Among Clusters Identified Based on EQ-5D-5L Dimensions in Older Patients with Chronic Low Back Pain. Medicina. 2025; 61(7):1217. https://doi.org/10.3390/medicina61071217
Chicago/Turabian StyleKim, Hee Jung, Hyeon Chang Kim, Jisung Hwang, and Shin Hyung Kim. 2025. "Comparing Frailty Status Among Clusters Identified Based on EQ-5D-5L Dimensions in Older Patients with Chronic Low Back Pain" Medicina 61, no. 7: 1217. https://doi.org/10.3390/medicina61071217
APA StyleKim, H. J., Kim, H. C., Hwang, J., & Kim, S. H. (2025). Comparing Frailty Status Among Clusters Identified Based on EQ-5D-5L Dimensions in Older Patients with Chronic Low Back Pain. Medicina, 61(7), 1217. https://doi.org/10.3390/medicina61071217






