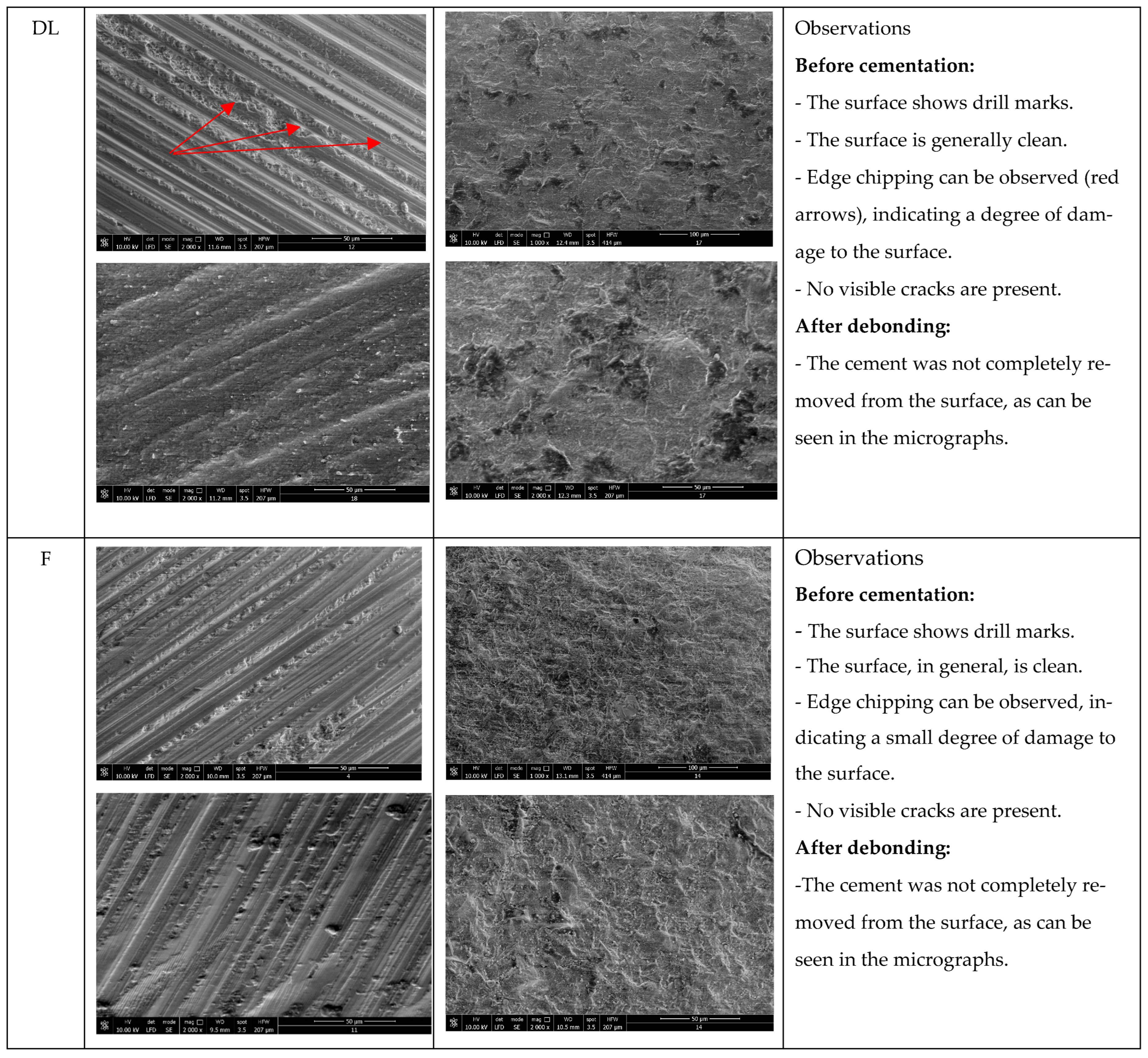
1. Introduction
Traditional techniques for removing permanently cemented fixed prosthetic restorations (FPRs) do not provide reliable and satisfactory results. At their best, they allow tooth preservation at the cost of a time-consuming procedure, causing wear of rotary instruments and handpieces or even abutment fractures. The restorations are seldom reusable, which is a disadvantage for both patient and physician in the event of incorrect cementation [
1].
The obstacles encountered are common to all conventional removal of prosthetic restorations procedures, and they include: the retention capacity, which is conditioned by the type and shape of the preparation, the contact surface between the abutment and the prosthetic device, the cement type, and the form and structure of the abutment. Regardless of the method used to remove FPRs, the dentist faces several significant challenges, including the following: some of the pressure exerted by the instrument is absorbed by the periodontal ligament, with the risk of pain and even luxation; in cases of massive reconstruction, there is an increased risk of fracturing the reconstruction [
1].
There is currently no ideal conventional removal system [
1,
2,
3,
4,
5], and its choice depends on the type of FPR, the cement used, the type of preparation, the abutment (natural or reconstructed tooth), and its position in the oral cavity [
2].
Depending on the method of operation, techniques for removing prosthetic works can be classified as follows [
2]:
very conservative: ultrasonic, FPR Removal Thermoplastic Adhesive Resin, lasers, and FPR forceps;
conservative: various manual, semi-automatic, automatic, or pneumatic removal instruments;
semi-conservative: Metalift, Kline, WAMkey, or Higa systems;
invasive: in which FPRs are sectioned with diamond or tungsten carbide burs [
2,
3], after which different forceps or removal instruments are applied.
No universal system can ensure intact and safe removal of classically cemented FPRs; therefore, combining several systems seems to be a better option, but with very little chance of reusing the prosthetic piece.
Since the introduction of the laser into prosthetic dentistry, a new era has opened, with an impact on the quality of treatment and the hope of overcoming the disadvantages of conventional methods. Laser technology has many advantages: it causes less or no pain; eliminates the noise of the traditional instruments that represents a source of anxiety for some patients; is minimally invasive and precise, offering rapid healing without vibrations; helps achieve immediate hemostasis and moisture control; and is being successfully used in patients with phobias or allergies to local anesthetics [
6]. Lasers have been introduced in every area of dentistry: oral surgery, general dentistry, pedodontics, endodontics, periodontology, implantology, and prosthetics [
6]. In fixed restorations, removing FPRs without damaging tooth structure is difficult and time-consuming [
2,
4,
7,
8]. Due to the disadvantages of conventional methods, other methods have been sought to remove FPRs safely, predictably, and quickly, Er:YAG and Er,Cr:YSGG being among them [
2,
8,
9].
According to numerous studies, the effectiveness of the Er:YAG laser in debonding FPRs is achieved without damaging the tooth structure, measuring the energy used and the time required [
6,
8,
9,
10,
11], but the parameters are contradictory, and the results are uneven in various studies.
The Er laser family exists between 2780 nm (Er,Cr:YSGG) and 2940 nm (Er:YAG), and these wavelengths are well absorbed in water and hydroxyapatite. This type of laser wavelength has been used experimentally since the early 1990s to remove the ceramic orthodontic brackets [
12]. As Er:YAG lasers demonstrated their effectiveness in the debonding of brackets, the studies were continued in the case of ceramic FPR debonding.
The detachment of ceramic FPRs is achieved by the H₂O/O₂ absorption band, which coincides with the wavelength of the Er:YAG laser. Not all ceramic FPRs can absorb the wavelength; therefore, the laser energy is transmitted through the FPR, displacing the bonding cement. Most FPRs can be removed intact, which is useful in the event of a cementation error. Flexural strength is key to maintaining the integrity of ceramic FPRs during laser debonding [
10,
11,
13].
Laser debonding of ceramic FPRs has many advantages but can be affected by a variety of clinical factors, including the chemical composition and type of ceramic, restoration thickness, type and shade of resin cement, ceramic shade and opacity, material porosity, and laser parameters (power, pulse, irradiation time) [
8,
12]. Probably due to the high variability of all these factors that can influence the outcome, studies report different results. For example, according to Morford’s studies [
10], it has been demonstrated that FPRs can be debonded using the Er:YAG (2940 nm) and Er,Cr:YSGG (2780 nm) lasers, provided the FPRs are made of ceramic masses, but these lasers are not effective in the case of FPRs made of zirconia or with a metal component. On the other hand, the studies of Rechmann et al. [
11] concluded that Er:YAG can be effective in removing both ceramic and zirconium oxide FPRs. The use of laser energy in FPR debonding is based on the absorption of energy by the cement, causing its degradation through three mechanisms: thermal degradation, thermal ablation, and photoablation [
14]. The laser energy is transferred through the veneers and crowns, which then react with the cement, damaging it, followed by the detachment of the FPR from the tooth [
11].
The device parameters and the correct laser action technique applied to the cement–ceramic interface are essential in trying not to cause additional damage to the tooth structure. The parameters of the Er:YAG laser undergo changes dictated by the type of ceramic FPR and its thickness, as well as the properties of the adhesive material [
15].
The purpose of this study is to address the following areas of interest:
- (1)
Examine the influence of the Er:YAG laser used for the debonding of different types of ceramic FPRs.
- (2)
Perform a comparative analysis of the behavior of different types of ceramic prosthetic restorations and cements under the action of laser radiation.
- (3)
Analyze the integrity of prosthetic restorations and tooth surfaces subjected to the action of laser radiation.
4. Discussion
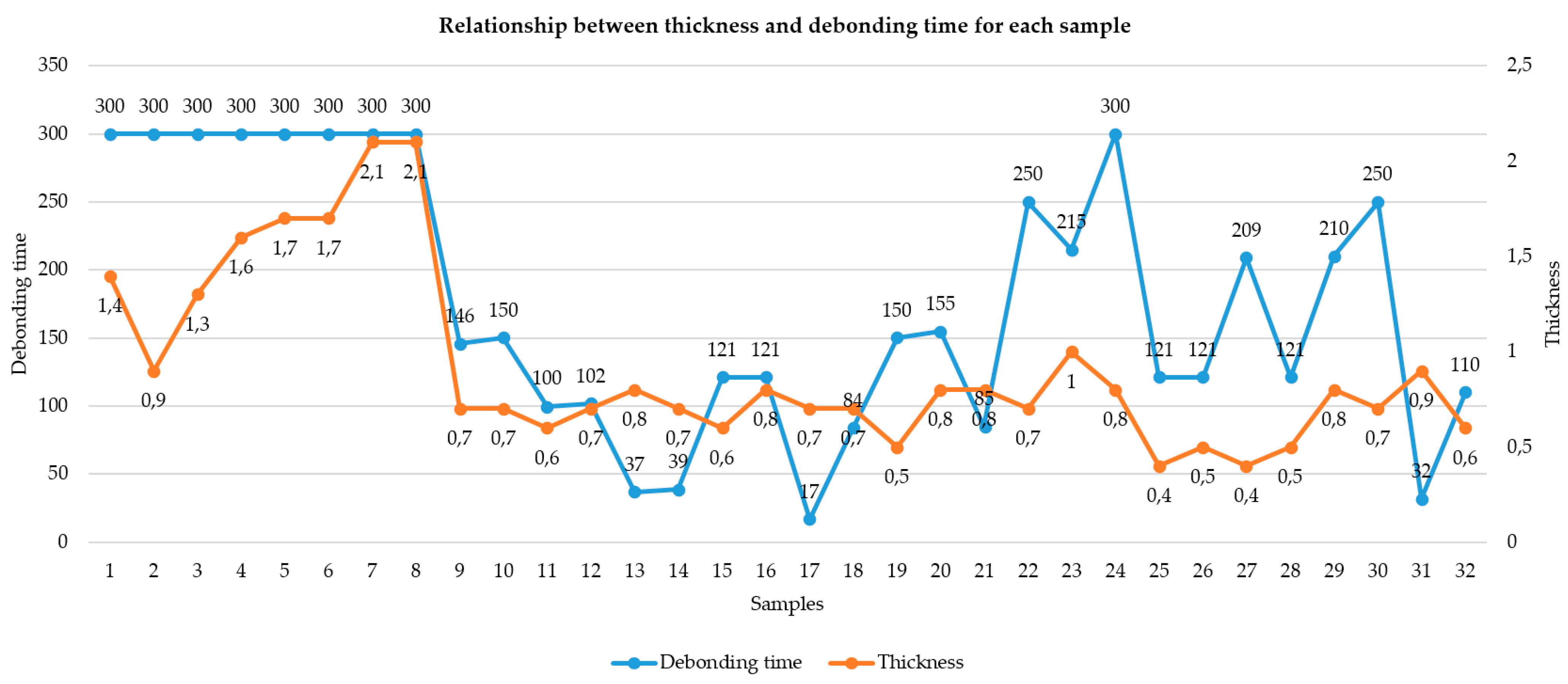
According to our study data, the factor that directly influences the debonding process by laser irradiation is the thickness of the ceramic FPR. Ceramic restorations with a thickness of over 1 mm could not be debonded at an irradiation of 5 min, 275 mJ, 20 Hz, and 5.5 W (
Figure 14). This binary outcome was confirmed by a significant difference in mean thickness between debonded and non-debonded groups (
p < 0.001, t = −5.30), establishing 1.0 mm as a critical clinical threshold.
Our results suggest that laser energy is both geometrically and optically dependent, with thicker and more crystalline structures attenuating energy transmission to a degree that renders debonding ineffective.
Furthermore, the finding that restorations near the 1.0 mm cutoff in lithium disilicate and layered zirconia showed occasional delays in debonding supports the hypothesis that even small increases in thickness can significantly reduce clinical efficiency, especially in materials with moderate opacity.
Conversely, restorations with a thickness of 1.0 mm or less consistently responded to laser irradiation, supporting the conclusion that ceramic thickness is a primary determinant of successful laser-assisted debonding under fixed energy parameters.
From a clinical standpoint, this evidence reinforces the need to limit ceramic thickness—particularly in the occlusal third—when planning restorations in situations where future retrievability may be required. Restoration design protocols may benefit from incorporating retrievability consideration during digital planning, especially in the case of adhesive cementation.
When analyzing the thickness in relation to different types of ceramics, several important conclusions can be drawn (
Figure 15). When observing the monolithic zirconia group (not debonded), the outcome supports the hypothesis that high material density and excessive thickness significantly attenuate laser energy before it can reach and soften the underlying resin cement. Regarding layered zirconia, all samples were successfully debonded, suggesting that the material’s layered structure and relatively lower opacity allowed for sufficient laser transmission and energy absorption at the cement interface, facilitating effective debonding even at slightly greater thicknesses. The lithium disilicate group showed increased occlusal measurements; these thicker occlusal zones could have limited laser penetration and contributed to resistance during the debonding process.
Feldspathic ceramic samples, the thinnest, were also successfully debonded. This finding is consistent with the high optical transmittance and low structural density of feldspathic ceramics, which allow efficient laser energy propagation to the adhesive interface with minimal attenuation.
Taken together, these data confirm that ceramic thickness is a critical variable in laser-assisted debonding. While composition and translucency play key roles, restorations exceeding 1.0 mm in occlusal thickness consistently showed reduced laser efficiency, particularly in dense materials such as monolithic zirconia. This reinforces the clinical recommendation to consider both material selection and design thickness when planning restorations intended for potential laser retrieval. This finding aligns with previous literature indicating that laser energy is significantly attenuated when passing through thicker and denser ceramics, especially in materials with low translucency, such as monolithic zirconia.
From a clinical perspective, these findings emphasize the importance of material selection in cases where retrievability is a concern. Highly dense and low-translucency materials such as monolithic zirconia may not be suitable for laser-assisted retrieval protocols, while more translucent ceramics demonstrate predictable removal and may support future reuse.
Regarding the choice of power, we previously explained that, in our study, this was achieved after repeated trials, with different values, on ceramic blocks of different thicknesses. The literature does not offer a consensus in this direction, although the settings are comparable. For example, a study to which we can refer [
16] comparatively investigated powers of 4, 5, and 6 W used to debond novel zirconia restorations; in fact, the restorations were not cemented, but only laser radiation was applied to them, in order to investigate possible structural changes in the prosthetic restorations. The conclusion of the study was that Er:YAG laser debonding did not affect the optical or mechanical properties of dental zirconia ceramics and did not cause any microcracks of the zirconia ceramic surfaces. Clinical studies have also shown the lack of side effects on abutment teeth, especially when the laser is used with appropriate cooling parameters [
10,
11,
17]. From a clinical point of view, this could be of real help, especially considering the discomfort reported by patients regarding conventional removal techniques. Studies were conducted in this regard, aiming to determine the most effective methods in controlling pain during debonding procedures [
18].
Another very interesting study measured the rebonding strengths of 1 mm thickness leucite and lithium disilicate veneers after the debonding process using the Er:YAG laser and rebonding, respectively. The conclusions of the study showed statistically significant differences between the control group, in which no laser was used, and the one in which the debonding was performed using the laser. The Er:YAG laser demonstrated the ability to debond restorations with a power of 3 W, 10 Hz, and 300 mJ, with 100 μs pulse duration for 9 s, and rebonding did not influence the shear bond strength [
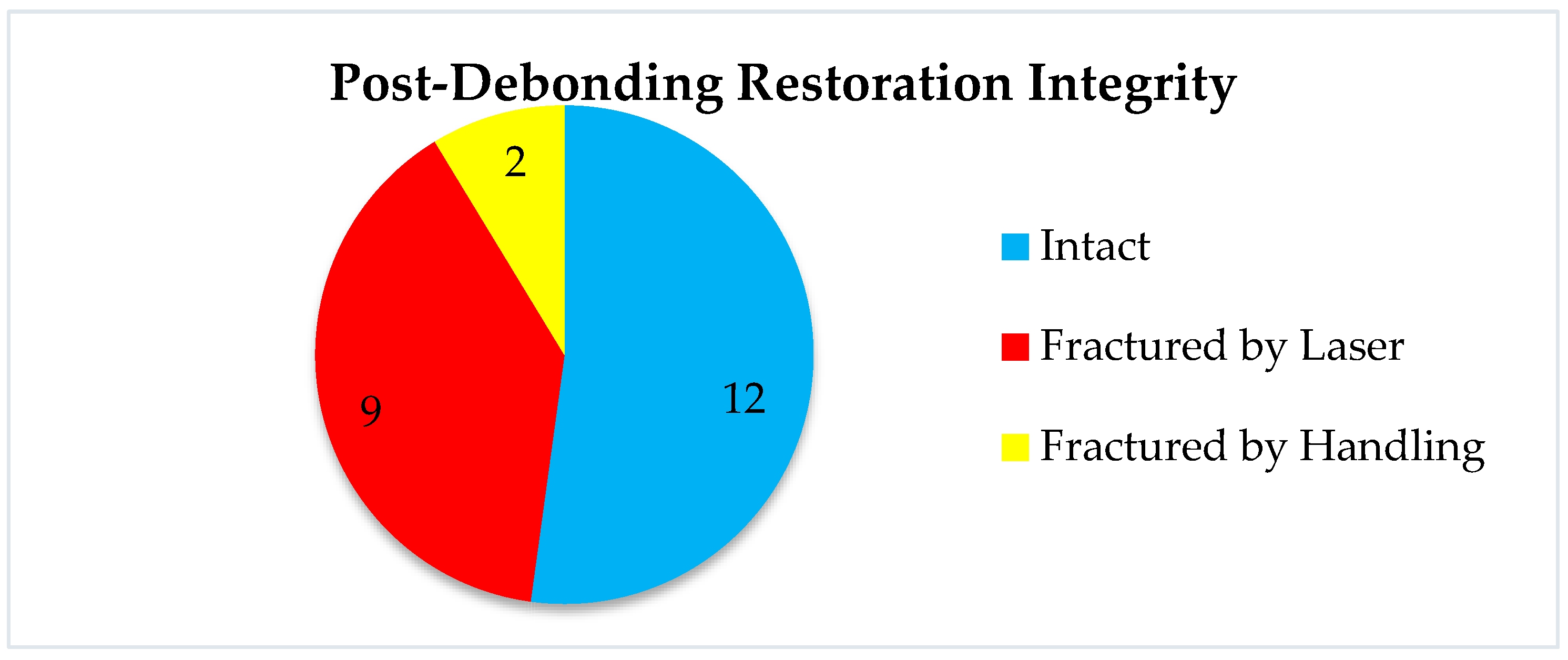
19]. This information is very important in all cases when retrievability as a concern. Our study aimed not only at the laser debonding efficacy but also at the reusability of the prosthetic restorations, i.e., the condition of the prosthetic piece after debonding. Following laser debonding, 52% of prosthetic restorations were intact, (which helps when we consider retrievability), 48% were fractured/cracked, of which 39% were fractured/cracked following irradiation, and 9% were fractured due to improper handling during cementation (
Figure 16). El-Sheikh et. al. [
20] evaluated three ceramic types of 0.5 mm thickness debonded using Er;Cr:YSGG. In terms of debonded veneer damage, the Emax and zirconia samples revealed none. However, 40% of the LiSi samples fractured during debonding and 20% had cracks. Zhang et. al. [
21] investigated how different types of ceramics were affected by Er:YAG laser irradiation, in terms of optical and mechanical properties. They concluded that while mechanical properties remain unchanged, the optical ones are affected by laser radiation of greater power, causing perceptible color changes. The selection of appropriate laser parameters is therefore very important, especially in cases of retrievability. Another aspect investigated in a recent experimental study was that of roughness modifications [
22]. The authors concluded that lasers are a noninvasive way to retrieve ceramic veneers, although surface roughness modifications were found in both types of investigated ceramics.
Several studies extend the applicability from the tooth level to the similar situation of the implant-supported prosthetic restorations debonding [
23,
24,
25,
26]. Studies revealed that both the Er:YAG and Er,Cr:YSGG lasers work similarly to remove a zirconia crown from a zirconia implant abutment with identical parameters, even though they operate at different wavelengths. The authors concluded that pulsed erbium laser irradiation does not cause any damage to the optical or elemental composition.
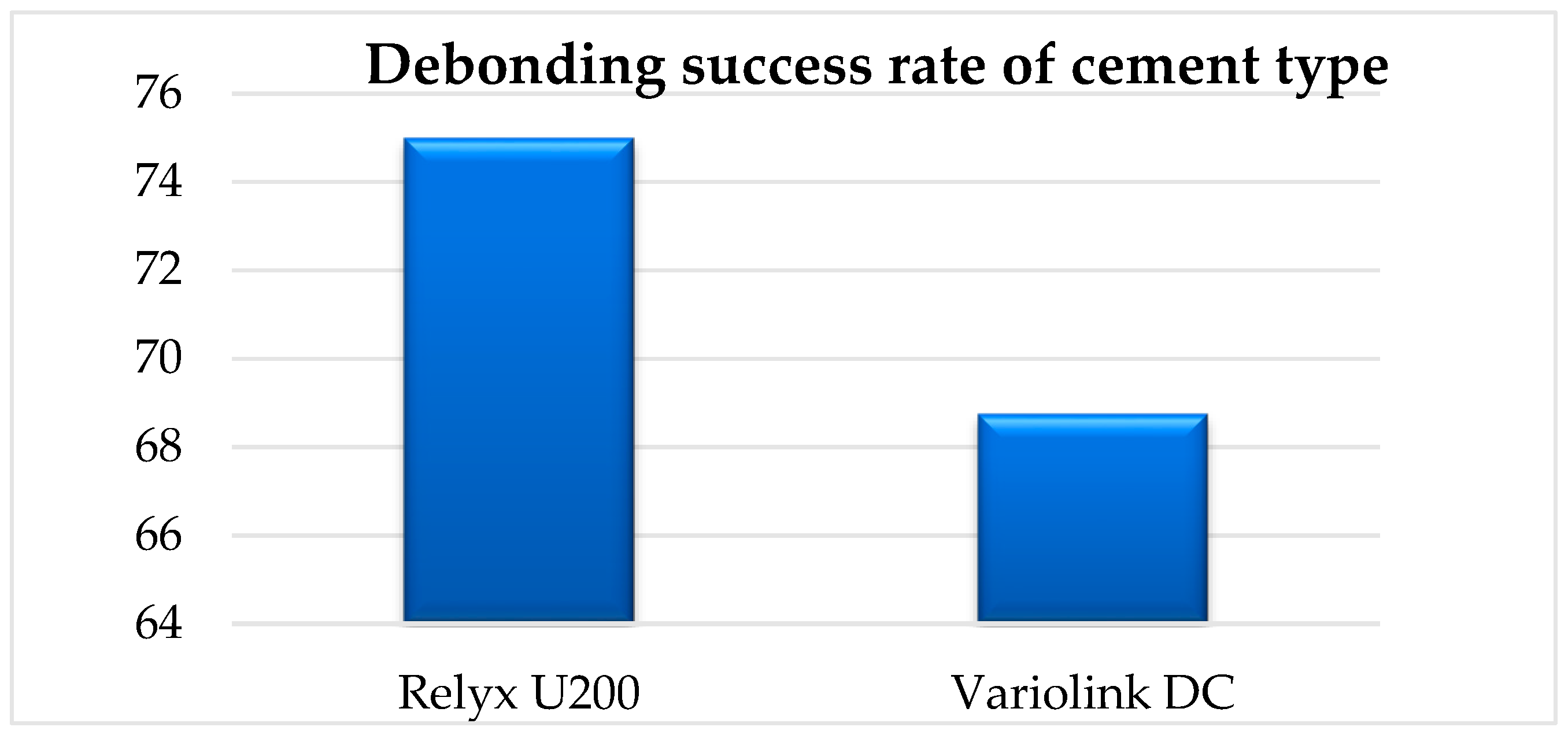
Referring to other parameters that could influence the effectiveness of the debonding treatment, it can be observed that a large surface area requires a longer laser irradiation time for debonding, but it does not represent a direct factor that influences the outcome of the treatment. According to our results, we can observe that the FPR surface area influences the irradiation time. Another important variable is the type of cement used. In our study, ceramic restorations cemented with RELYX U200 were debonded in 75% of the cases; meanwhile, the same type of ceramic restorations that were cemented with Variolink DC were debonded in a proportion of 69% (
Figure 17).
Regarding the material of the prosthetic restorations, the results obtained in our study are the following: ceramic restorations made of layered zirconia were 100% debonded, lithium disilicate ceramic restorations were 87% debonded, feldspathic ceramic restorations were 100% debonded, and ceramic restorations of monolithic zirconia could not be removed using our determined parameters (
Figure 15). Pairwise Fisher’s exact tests demonstrated that monolithic zirconia restorations were significantly more resistant to laser debonding than all other materials (
p < 0.001). In contrast, layered zirconia and feldspathic ceramics exhibited complete success (100%), and lithium disilicate showed a high but slightly reduced success rate (87.5%). Our results are also confirmed by the studies of Morford and Oztoprak [
10]. A similar study [
27] investigated the debonding efficiency of two types of lasers for bonded lithium disilicate and leucite-based veneers of different thicknesses. Both Er:YAG (600 μm tip, 300 mJ, 6 W, 20 Hz, pulse duration of 100 μs, energy density of 107 J/cm
2, and 80% water, 60% air) and Er,Cr:YSGG (600 μm tip, at a distance of 2 mm in a non-contact mode for 15 s, 300 mJ, 6 W, 20 Hz, pulse duration of 60 μs, energy density of 107 J/cm
2, 80% water, 60% air) worked well for glass ceramic debonding, the required time being impacted by ceramic thickness. The debonding time for lithium disilicate was less than that of leucite-based specimens, and the shear bond strength of the retrieved specimens was much lower than that of the control group (no laser group). Overall, Er:YAG worked faster and more efficiently in debonding the prosthetic restorations. Another study [
28] compared the ability of Er,Cr:YSGG to debond two types of ceramics, feldspathic and lithium disilicate reinforced glass ceramic veneers, and to measure debonding time and pulpal temperature increase during veneer removal. The authors concluded that lithium disilicate and feldspathic reinforced glass ceramic veneers can be effectively debonded with Er,Cr:YSGG with no uncomfortable temperature increase. The debonding times of these ceramic materials did not differ significantly.
Another comprehensive study [
29] was conducted on 200 zirconia specimens, and Er:YAG energies of 80 mJ, 100 mJ, 120 mJ, 140 mJ, 160 mJ, 180 mJ, 200 mJ, 220 mJ, 240 mJ, and 260 mJ (with 10 Hz and 20 Hz) were tested for debonding after cementation with Rely X. Different parameters were observed, such as a change in dentin temperature, surface modifications, and flexural strength. Following laser treatment, neither the dentin nor the zirconia materials showed any surface alterations. The zirconia materials’ flexural strength and surface roughness did not change. The authors concluded that zirconia restorations can be safely and successfully removed using Er:YAG laser treatment, which has a safe temperature change of less than 5.6 °C and does not affect the restorations’ mechanical characteristics. For debonding 3Y-TZP and 5Y-TZP restorations, the ideal frequency and energy settings were determined to be 10/20 Hz and 220 mJ and 10/20 Hz and 200 mJ, respectively. Suliman [
30] was also interested in comparing the time required for an Er:YAG laser to remove different types of zirconia and lithium disilicate crowns and reported that the yttria content of the zirconia affected the retrieval time of the crown using the Er:YAG laser; the retrieval time decreased as the yttria content increased. Er:YAG laser debonding of zirconia crowns is a quick, easy, and noninvasive method of crown removal that may be used in clinical settings. A recent study [
31] added new light to the problem, comparing recent versus aged bonded ceramic restorations and concluding that the aging approach shortened the removal time for all polymerization procedures. Therefore, at least for the studied lithium disilicate veneers, the laser debonding after a period of intraoral usage is faster than immediate debonding.
The experimental results reported by both our group and other similar studies are also confirmed in clinical trials. El-Damanhoury et al. [
32] showed in his clinical study, in which he compared the effects of different Er:YAG laser powers on the pulp temperature and the time required to perform the debonding of lithium disilicate laminate veneers with various thicknesses, that laser power and veneer thickness are crucial; thinner veneers are removed more quickly. The authors concluded that 5.4 W laser power is more effective and results in less pulp temperature variations while debonding thick veneers. Laser applications in dentistry, and in dental prosthetics par excellence, are based on the optical properties of dental materials and the interaction between them and laser radiation. In prosthetics, extensive studies are still needed to establish safe and repeatable clinical protocols. In 2015, Olena Pich and co-authors [
33] described the behavior of different dental ceramics (Emax Ceram, Emax ZirCAD and Emax Press) under the action of an Er:YAG laser and a 810 nm diode, concluding that as the thickness of the irradiated sample increases, the laser energy transmitted through the ceramic material drops to 30–40% of its initial levels. Compared to ceramic samples without pigment, those with pigment exhibit a greater loss of laser energy.
These results reinforce the conclusion that ceramic material exerts a strong, categorical influence on the likelihood of successful laser-assisted debonding, with monolithic zirconia being predictably resistant and all other materials demonstrating favorable outcomes.
While Pearson correlation and regression analyses showed no significant association between either thickness (r = −0.067, p = 0.76) or surface area (r = −0.045, p = 0.84) and debonding time, these results reinforce that geometry alone does not predict procedure duration once debonding is feasible.
Among the limitations of our study, we can mention the relatively small number of samples, the lack of temperature measurement, and the possible reduction in shear bond strength. A newer and important direction of research is represented by the extension of these indications to the level of restorations on implants or implant prosthetic parts.