Correlation Between Core Stability and Plantar Pressure Distribution During Double-Leg Stance, Single-Leg Stance, and Squat Positions in Healthy Male Athletes
Abstract
1. Introduction
2. Materials and Methods
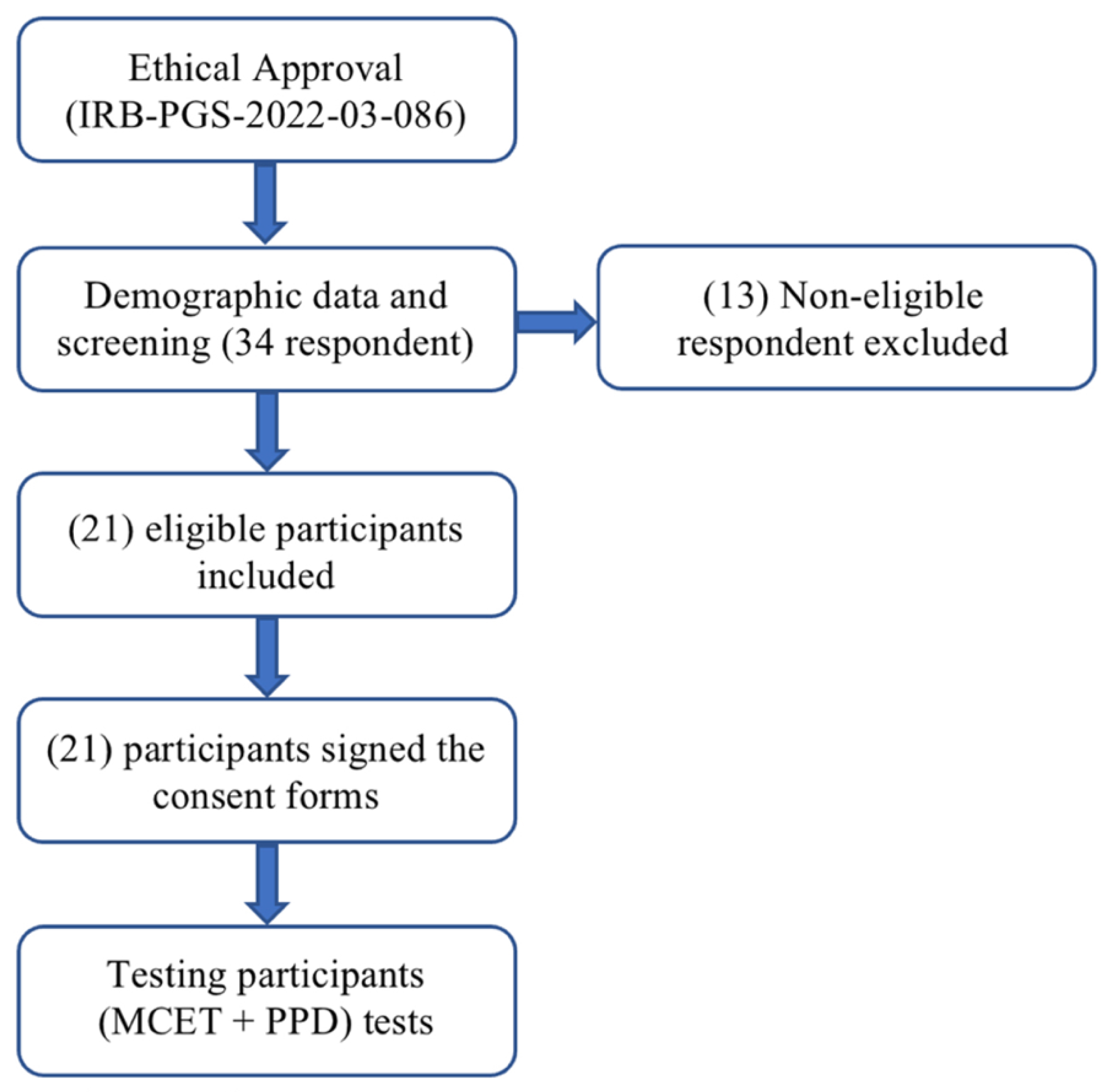
2.1. Participants
2.2. Procedure
2.3. Overview of Outcome Measures
2.4. Core Endurance Testing
2.4.1. Core Flexor Endurance Test
2.4.2. Core Extensor Endurance Test
2.4.3. Left and Right Lateral Plank Tests
2.5. Plantar Pressure Distribution Testing
Collecting Plantar Pressure Data
2.6. Data Analysis
3. Results
Additional Findings
4. Discussion
4.1. Additional Findings
4.2. Clinical Implications
4.3. Study Limitations
4.4. Recommendations for Future Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dong, K.; Yu, T.; Chun, B. Effects of core training on sport-specific performance of athletes: A meta-analysis of randomized controlled trials. Behav. Sci. 2023, 13, 148. [Google Scholar] [CrossRef] [PubMed]
- Hodges, P.W.; Richardson, C.A. Contraction of the abdominal muscles associated with movement of the lower limb. Phys. Ther. 1997, 77, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Butcher, S.J.; Craven, B.R.; Chilibeck, P.D.; Spink, K.S.; Grona, S.L.; Sprigings, E.J. The effect of trunk stability training on vertical takeoff velocity. J. Orthop. Sports Phys. Ther. 2007, 37, 223–231. [Google Scholar] [CrossRef] [PubMed]
- McGill, S.M.; Childs, A.; Liebenson, C. Endurance times for low back stabilization exercises: Clinical targets for testing and training from a normal database. Arch. Phys. Med. Rehabil. 1999, 80, 941–944. [Google Scholar] [CrossRef]
- Saeterbakken, A.H.; Fimland, M.; Navarsete, J.; Kroken, T.; van der Tillaar, R. Muscle activity, and the association between core strength, core endurance and core stability. J. Nov. Physiother. Phys. Rehabil. 2015, 2, 28–34. [Google Scholar] [CrossRef]
- de Bruin, M.; Coetzee, D.; Schall, R. The relationship between core stability and athletic performance in female university athletes. S. Afr. J. Sports Med. 2021, 33, v33i31a10825. [Google Scholar] [CrossRef]
- Sato, K.; Mokha, M. Does core strength training influence kinetic efficiency, lower extremity stability, and 5000m performance in runners? J. Strength Cond. Res. 2009, 23, 133–140. [Google Scholar] [CrossRef]
- Cobanoglu, G.; Keklik, S.S.; Zorlular, A.; Polat, E.A.; Akaras, E. The relationship between scapular and core muscle endurance in professional athletes. Ann. Med. Res. 2019, 26, 1295–1300. [Google Scholar]
- Santos, M.S.; Behm, D.G.; Barbado, D.; DeSantana, J.M.; Da Silva-Grigoletto, M.E. Core endurance relationships with athletic and functional performance in inactive people. Front. Physiol. 2019, 10, 1490. [Google Scholar] [CrossRef]
- Nesser, T.W.; Lee, W.L. The relationship between core strength and performance in Division I female soccer players. J. Exerc. Physiol. Online 2009, 12, 21–26. [Google Scholar]
- Mohammadi, H.; Fathi, J. The Relationship Between Core Endurance and Performance in National Female Badmin-ton Athletes. Phys. Treat. 2018, 8, 123–131. [Google Scholar]
- I Shaikh, A.; Nuhmani, S.; Kachanathu, S.J.; I Muaidi, Q. Relationship of Core Power and Endurance with Performance in Random Intermittent Dynamic Type Sports. Asian J. Sports Med. 2019, 10, e62843. [Google Scholar] [CrossRef]
- Wilkerson, G.B.; Giles, J.L.; Seibel, D.K. Prediction of core and lower extremity strains and sprains in collegiate football players: A preliminary study. J. Athl. Train. 2012, 47, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, A.A.; Mohamed, N.A.; Hegazy, M.A. A comparative study of core musculature endurance and strength between soccer players with and without lower extremity sprain and strain injury. Int. J. Sports Phys. Ther. 2019, 14, 525–536. [Google Scholar] [CrossRef]
- Cejudo, A.; Ginés-Díaz, A.; Rodríguez-Ferrán, O.; Santonja-Medina, F.; Sainz de Baranda, P. Trunk Lateral Flexor Endurance and Body Fat: Predictive Risk Factors for Low Back Pain in Child Equestrian Athletes. Children 2020, 7, 172. [Google Scholar] [CrossRef]
- Weist, R.; Eils, E.; Rosenbaum, D. The influence of muscle fatigue on electromyogram and plantar pressure patterns as an explanation for the incidence of metatarsal stress fractures. Am. J. Sports Med. 2004, 32, 1893–1898. [Google Scholar] [CrossRef]
- Lung, C.-W.; Liau, B.-Y.; Peters, J.A.; He, L.; Townsend, R.; Jan, Y.-K. Effects of various walking intensities on leg muscle fatigue and plantar pressure distributions. BMC Musculoskelet. Disord. 2021, 22, 831. [Google Scholar] [CrossRef]
- Zhao, C. Effect of sports fatigue on plantar pressure distribution of healthy male college students. Rev. Bras. Med. Esporte 2022, 28, 398–401. [Google Scholar] [CrossRef]
- Askari, Z.; Esmaeili, H. Effect of trunk muscles fatigue on plantar pressure distribution in novice runners. J. Biomech. 2021, 122, 110487. [Google Scholar] [CrossRef]
- Teyhen, D.S.; Stoltenberg, B.E.; Collinsworth, K.M.; Giesel, C.L.; Williams, D.G.; Kardouni, C.H.; Molloy, J.M.; Goffar, S.L.; Christie, D.S.; McPoil, T. Dynamic plantar pressure parameters associated with static arch height index during gait. Clin. Biomech. 2009, 24, 391–396. [Google Scholar] [CrossRef]
- Chappell, J.D.; Yu, B.; Kirkendall, D.T.; Garrett, W.E. A comparison of knee kinetics between male and female recreational athletes in stop-jump tasks. Am. J. Sports Med. 2002, 30, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Vaisman, A.; Guiloff, R.; Rojas, J.; Delgado, I.; Figueroa, D.; Calvo, R. Lower Limb Symmetry: Comparison of Muscular Power Between Dominant and Nondominant Legs in Healthy Young Adults Associated With Single-Leg-Dominant Sports. Orthop. J. Sports Med. 2017, 5, 2325967117744240. [Google Scholar] [CrossRef] [PubMed]
- Hammouda, O.; Chtourou, H.; Chaouachi, A.; Chahed, H.; Bellimem, H.; Chamari, K.; Souissi, N. Time-of-day effects on biochemical responses to soccer-specific endurance in elite Tunisian football players. J. Sports Sci. 2013, 31, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Racinais, S.; Blonc, S.; Jonville, S.; Hue, O. Time of day influences the environmental effects on muscle force and contractility. Med. Sci. Sports Exerc. 2005, 37, 256–261. [Google Scholar] [CrossRef]
- Okada, T.; Huxel, K.C.; Nesser, T.W. Relationship Between Core Stability, Functional Movement, and Performance. J. Strength Cond. Res. 2011, 25, 252–261. [Google Scholar] [CrossRef]
- Al-Magsoosi, S.K.; Chong, A.K. Foot Loading Pattern Variations between Normal Weight, Overweight, and Obese Adults Aged 24 to 50 Years. J. Biosci. Med. 2019, 7, 34–49. [Google Scholar] [CrossRef]
- Ambegaonkar, J.P.; Mettinger, L.M.; Caswell, S.V.; Burtt, A.; Cortes, N. Relationships between core endurance, hip strength, and balance in collegiate female athletes. Int. J. Sports Phys. Ther. 2014, 9, 604–616. [Google Scholar]
- Shamsi, M.; Mirzaei, M.; Khabiri, S.S. Universal goniometer and electro-goniometer intra-examiner reliability in measuring the knee range of motion during active knee extension test in patients with chronic low back pain with short hamstring muscle. BMC Sports Sci. Med. Rehabil. 2019, 11, 4. [Google Scholar] [CrossRef]
- Abhilash, P.; Sudeep, S.; Anjana, K. Relationship between core endurance and dynamic balance in professional basketball players: A pilot study. Int. J. Phys. Educ. Sports Health 2021, 8, 1–5. [Google Scholar]
- Evans, K.; Refshauge, K.M.; Adams, R. Trunk muscle endurance tests: Reliability, and gender differences in athletes. J. Sci. Med. Sport 2007, 10, 447–455. [Google Scholar] [CrossRef]
- McGill, S. McGill’s torso muscular endurance test battery. Am. Counc. Exercise. Pristup. 2015, 3, 2022. [Google Scholar]
- Zammit, G.V.; Menz, H.B.; Munteanu, S.E. Reliability of the TekScan MatScan® system for the measurement of plantar forces and pressures during barefoot level walking in healthy adults. J. Foot Ankle Res. 2010, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Da, H.K.; Lee, J.D.; Kim, K. Plantar pressures in individuals with normal and pronated feet according to static squat depths. J. Phys. Ther. Sci. 2015, 27, 2833–2835. [Google Scholar]
- Bodkin, S.G.; Simpson, A.S.; Kirsch, A.N.; Hart, J.M. Postural Control in Patients with ACL Reconstruction Using Automated Error Detection from Instrumented Balance Measures. Athl. Train. Sports Health Care 2021, 13, e323–e328. [Google Scholar] [CrossRef]
- Gwynne, C.R. Alterations in center of pressure during single-limb loading in individuals with patellofemoral pain. J. Am. Podiatr. Med. Assoc. 2020, 110, 5. [Google Scholar] [CrossRef]
- Ugalde, V.; Brockman, C.; Bailowitz, Z.; Pollard, C.D. Single leg squat test and its relationship to dynamic knee valgus and injury risk screening. PMR 2015, 7, 229–235, quiz 235. [Google Scholar] [CrossRef]
- Portney, L.G.; Watkins, M.P. Foundations of Clinical Research: Applications to Practice; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2009; Volume 892. [Google Scholar]
- Riemann, B.L.; Schmitz, R. The relationship between various modes of single leg postural control assessment. Int. J. Sports Phys. Ther. 2012, 7, 257–266. [Google Scholar]
- Vecchio, L.; Daewoud, H.; Green, S. The health and performance benefits of the squat, deadlift, and bench press. MOJ Yoga Phys. Ther. 2018, 3, 40–47. [Google Scholar] [CrossRef]
- Ramirez-Campillo, R.; Andrade, D.C.; Nikolaidis, P.T.; Moran, J.; Clemente, F.M.; Chaabene, H.; Comfort, P. Effects of plyometric jump training on vertical jump height of volleyball players: A systematic review with meta-analysis of randomized-controlled trial. J. Sports Sci. Med. 2020, 19, 489–499. [Google Scholar]
- Onofrei, R.R.; Amaricai, E.; Petroman, R.; Suciu, O. Relative and absolute within-session reliability of the modified Star Excursion Balance Test in healthy elite athletes. PeerJ 2019, 7, e6999. [Google Scholar] [CrossRef]
- Scinicarelli, G.; Trofenik, M.; Froböse, I.; Wilke, C. The reliability of common functional performance tests within an experimental test battery for the lower extremities. Sports 2021, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Nevitt, M.C.; Cummings, S.R.; Hudes, E.S. Risk factors for injurious falls: A prospective study. J. Gerontol. 1991, 46, M164–M170. [Google Scholar] [CrossRef] [PubMed]
- Trojian, T.H.; McKeag, D.B. Single leg balance test to identify risk of ankle sprains. Br. J. Sports Med. 2006, 40, 610–613, discussion 613. [Google Scholar] [CrossRef] [PubMed]
- Stickler, L.; Finley, M.; Gulgin, H. Relationship between hip and core strength and frontal plane alignment during a single leg squat. Phys. Ther. Sport 2015, 16, 66–71. [Google Scholar] [CrossRef]
- Tyler, S. Physical Development in Early Adulthood. In Human Behavior and the Social Environment I; University of Arkansas: Fayetteville, AR, USA, 2020. [Google Scholar]
- Vie, B.; Gomez, N.; Brerro-Saby, C.; Weber, J.P.; Jammes, Y. Changes in stationary upright standing and proprioceptive reflex control of foot muscles after fatiguing static foot inversion. J. Biomech. 2013, 46, 1676–1682. [Google Scholar] [CrossRef]
- Lee, K.-S.; Wang, J.-W.; Lee, D.Y.; Yu, J.H.; Kim, J.S.; Kim, S.G.; heon Hong, J. Effects of progressive core and ankle muscle strengthening exercises using thera-band on body balance. J. Korean Phys. Ther. 2022, 34, 121–127. [Google Scholar] [CrossRef]
- Kim, S.M.; Hyun, G.J.; Jung, T.-W.; Son, Y.D.; Cho, I.-H.; Kee, B.S.; Han, D.H. Balance deficit and brain connectivity in children with attention-deficit/hyperactivity disorder. Psychiatry Investig. 2017, 14, 452. [Google Scholar] [CrossRef][Green Version]
- Eils, E.; Streyl, M.; Linnenbecker, S.; Thorwesten, L.; Völker, K.; Rosenbaum, D. Characteristic plantar pressure distribution patterns during soccer-specific movements. Am. J. Sports Med. 2004, 32, 140–145. [Google Scholar] [CrossRef]
- Knoll, M.G.; Davidge, M.; Wraspir, C.; Korak, J.A. Comparisons of single leg squat variations on lower limb muscle activation and center of pressure alterations. Int. J. Exerc. Sci. 2019, 12, 950–959. [Google Scholar] [CrossRef]
- Jeon, W.; Whitall, J.; Griffin, L.; Westlake, K.P. Trunk kinematics and muscle activation patterns during stand-to-sit movement and the relationship with postural stability in aging. Gait Posture 2021, 86, 292–298. [Google Scholar] [CrossRef]
- Chakravarty, K.; Chatterjee, D.; Das, R.K.; Tripathy, S.R.; Sinha, A. Analysis of muscle activation in lower extremity for static balance. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju Island, Republic of Korea, 11–15 July 2017; pp. 4118–4122. [Google Scholar] [CrossRef]
- Imamura, M.; Imamura, S.T.; Salomão, O.; Pereira, C.A.M.; De Carvalho Jr, A.E.; Neto, R.B. Pedobarometric evaluation of the normal adult male foot. Foot Ankle Int. 2002, 23, 804–810. [Google Scholar] [CrossRef]
- Woźniacka, R.; Oleksy, Ł.; Jankowicz-Szymańska, A.; Mika, A.; Kielnar, R.; Stolarczyk, A. The association between high-arched feet, plantar pressure distribution and body posture in young women. Sci. Rep. 2019, 9, 17187. [Google Scholar] [CrossRef]
| Outcome Measures | Testing Sequence |
|---|---|
| The sequence of the testing procedure was undertaken randomly for both McGill core endurance and PPD tests (participants chose from folded papers blindly) to rule out that one test might affect the result of the other. | |
| McGill core endurance tests: - Flexors endurance test. - Extensors endurance test. - Left lateral plank test. - Right lateral plank test. | Explanation and demonstration of the testing position and procedure was provided to each participant. - Each participant was allowed to practice the testing position for no longer than 5 s to avoid fatigue. - One official trial was completed only to avoid fatigue [25]. - Once participants assumed the desired position, the official trial started with the word “Start” and ended with “Stop” upon the trunk deviation from the midline noticed. - The holding time was recorded in seconds. - Participants were allowed to rest for five minutes between the testing trials to eliminate the effect of fatigue on the next test’s performance [4]. |
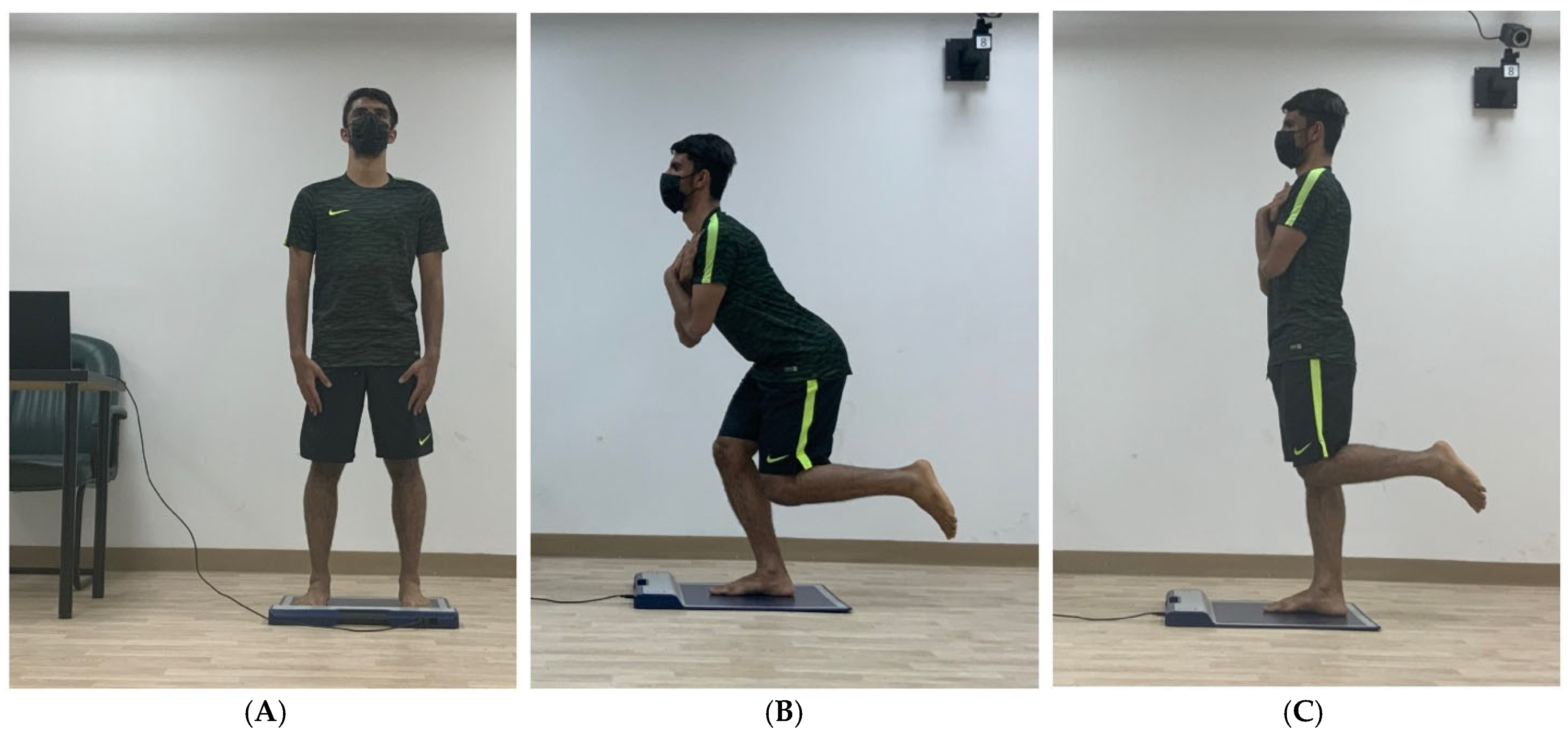
| Plantar pressure distribution measurement: Data were recorded during the following static positions: - Double-leg stance. - Single-leg stance. - single-leg squat. | For all the testing positions: - Explanation and demonstration of the testing position and procedure was provided to each participant. - Two practicing trials before the formal ones were allowed. - Two formal trials were conducted [26]. - Formal trial recording started (after assuming the testing position) with the word “Start” and ended with “Stop”. - Each formal trial lasted six seconds. - The average of the two formal trials was taken in data analysis. For PPD testing in single-leg stance and squat positions: - The selection of which foot to test first (dominant or non-dominant) was made randomly after a coin flip. - Before recording, the researcher measured (using a Goniometer) the non-supporting leg’s 90° knee flexion in the single-leg stance and single-leg squat positions. Besides measuring the supporting leg’s 30° knee flexion in the single-leg squat position. |
| Normally Distributed Variables | Mean ± Standard Deviation |
|---|---|
| McGill core endurance test | |
| Extension test (s) | 34.32 ± 13.88 |
| Left lateral plank test (s) | 30.76 ± 14.50 |
| Bilateral stance average values of plantar pressure distribution | |
| DPPA | 22.81 ± 10.32 |
| NDPP | 25.50 ± 9.97 |
| DPA | 16.61 ± 6.59 |
| NDPA | 18.40 ± 6.00 |
| Single-leg stance average values of plantar pressure distribution | |
| DPPA | 47.09 ± 7.15 |
| NDPPA | 50.42 ± 10.34 |
| DPA | 32.90 ± 6.34 |
| NDPA | 38.04 ± 7.51 |
| Single-leg squat average values of plantar pressure distribution | |
| DPPA | 53.85 ± 9.58 |
| NDPPA | 52.61 ± 8.52 |
| DPA | 35.52 ± 7.19 |
| NDPA | 34.57 ± 5.32 |
| Non-Normally Distributed Variables | Median (25–75 Percentiles) |
|---|---|
| McGill core endurance test | |
| Flexion test (s) | 74.38 (47.78–109.13) |
| Right lateral plank test (s) | 24.63 (15.76–35.02) |
| Plantar Pressure Data | McGill Core Endurance Tests | |||
|---|---|---|---|---|
| Flexion Endurance Test | Extension Endurance Test | Right Lateral Plank Test | Left Lateral Plank Test | |
| Double-leg stance position | ||||
| DPPA | rho = −0.117 p = 0.614 | r = 0.148 p = 0.523 | rho = 0.068 p = 0.769 | r = 0.030 p = 0.896 |
| NDPPA | rho = 0.114 p = 0.622 | r = 0.159 p = 0.490 | rho = 0.112 p = 0.630 | r = 0.034 p = 0.883 |
| DPA | rho = −0.149 p = 0.520 | r = 0.125 p = 0.590 | rho = 0.109 p = 0.640 | r = 0.061 p = 0.794 |
| NDPA | rho = 0.129 p = 0.578 | r = 0.215 p = 0.348 | rho = 0.050 p = 0.829 | r = 0.060 p = 0.796 |
| Single-leg stance position | ||||
| DPPA | rho = −0.079 p = 0.732 | r = −0.025 p = 0.915 | rho = 0.120 p = 0.605 | r = 0.175 p = 0.449 |
| NDPPA | rho = −0.040 p = 0.865 | r = −0.299 p = 0.188 | rho = 0.136 p = 0.555 | r = −0.104 p = 0.653 |
| DPA | rho = −0.024 p = 0.918 | r = −0.331 p = 0.142 | rho = 0.112 p = 0.630 | r = 0.011 p = 0.963 |
| NDPA | rho = 0.025 p = 0.913 | r = −0.297 p = 0.191 | rho = 0.279 p = 0.220 | r = 0.078 p = 0.737 |
| Single-leg squat position | ||||
| DPPA | rho = 0.364 p = 0.105 | r = 0.341 p = 0.131 | rho = 0.068 p = 0.769 | r = −0.051 p = 0.826 |
| NDPPA | rho = 0.182 p = 0.430 | r = 0.147 p = 0.526 | rho = −0.178 p = 0.440 | r = −0.146 p = 0.527 |
| DPA | rho = 0.331 p = 0.143 | r = 0.307 p = 0.176 | rho = 0.003 p = 0.991 | r = 0.111 p = 0.631 |
| NDPA | rho = 0.337 p = 0.135 | r = 0.257 p = 0.261 | rho = −0.071 p = 0.760 | r = 0.164 p = 0.476 |
| Double-Leg Stance DPPA | Double-Leg Stance DPA | Single-Leg Stance DPPA | Single-Leg Stance DPA | Single-Leg Squat DPPA | Single-Leg Squat DPA | |
|---|---|---|---|---|---|---|
| Double-leg stance DPPA | ||||||
| Double-leg stance DPA | ||||||
| Single-leg stance DPPA | r = 0.570 p = 0.007 * | r = 0.527 p = 0.014 * | ||||
| Single-leg stance DPA | r = 0.335 p = 0.137 | r = 0.320 p = 0.157 | ||||
| Single-leg squat DPPA | r = 0.088 p = 0.706 | r = 0.031 p = 0.894 | r = 0.086 p = 0.711 | r = −0.052 p = 0.823 | ||
| Single-leg squat DPA | r = 0.283 p = 0.213 | r = 0.208 p = 0.367 | r = 0.310 p = 0.172 | r = 0.236 p = 0.302 |
| Double-Leg Stance NDPPA | Double-Leg Stance NDPA | Single-Leg Stance NDPPA | Single-Leg Stance NDPA | Single-Leg Squat NDPPA | Single-Leg Squat NDPA | |
|---|---|---|---|---|---|---|
| Double-leg stance NDPPA | ||||||
| Double-leg stance NDPA | ||||||
| Single-leg stance NDPPA | r = 0.265 p = 0.245 | r = 0.210 p = 0.362 | ||||
| Single-leg stance NDPA | r = 0.110 p = 0.634 | r = 0.108 p = 0.640 | ||||
| Single-leg squat NDPPA | r = 0.091 p = 0.695 | r = 0.051 p = 0.827 | r = 0.247 p = 0.280 | r = 0.210 p = 0.362 | ||
| Single-leg squat NDPA | r = 0.284 p = 0.212 | r = 0.247 p = 0.281 | r= 0.006 p = 0.978 | r = −0.137 p = 0.554 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babkair, R.A.; Nuhmani, S.; Abualait, T.; Muaidi, Q. Correlation Between Core Stability and Plantar Pressure Distribution During Double-Leg Stance, Single-Leg Stance, and Squat Positions in Healthy Male Athletes. Medicina 2025, 61, 1188. https://doi.org/10.3390/medicina61071188
Babkair RA, Nuhmani S, Abualait T, Muaidi Q. Correlation Between Core Stability and Plantar Pressure Distribution During Double-Leg Stance, Single-Leg Stance, and Squat Positions in Healthy Male Athletes. Medicina. 2025; 61(7):1188. https://doi.org/10.3390/medicina61071188
Chicago/Turabian StyleBabkair, Reem Abdullah, Shibili Nuhmani, Turki Abualait, and Qassim Muaidi. 2025. "Correlation Between Core Stability and Plantar Pressure Distribution During Double-Leg Stance, Single-Leg Stance, and Squat Positions in Healthy Male Athletes" Medicina 61, no. 7: 1188. https://doi.org/10.3390/medicina61071188
APA StyleBabkair, R. A., Nuhmani, S., Abualait, T., & Muaidi, Q. (2025). Correlation Between Core Stability and Plantar Pressure Distribution During Double-Leg Stance, Single-Leg Stance, and Squat Positions in Healthy Male Athletes. Medicina, 61(7), 1188. https://doi.org/10.3390/medicina61071188






