Salivary Interleukins as Non-Invasive Biomarkers for Psoriasis: Advances and Challenges in Diagnosis and Monitoring
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. The Context of Psoriasis as a Chronic Inflammatory Disease
3.2. The Role of Biomarkers in Monitoring Psoriasis
Different Types of Biomarkers in Psoriasis
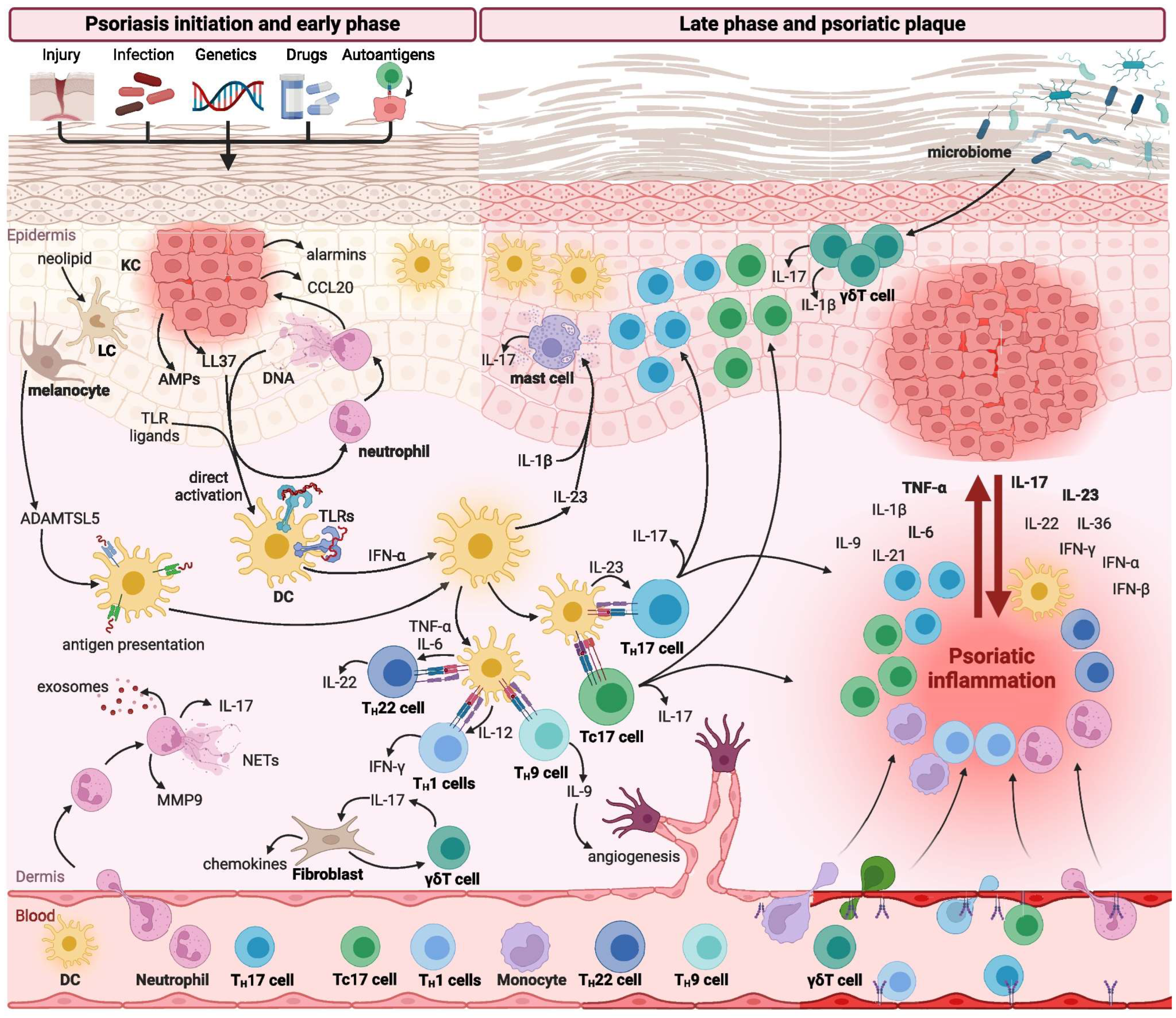
3.3. Interleukins in Psoriasis—General Overview
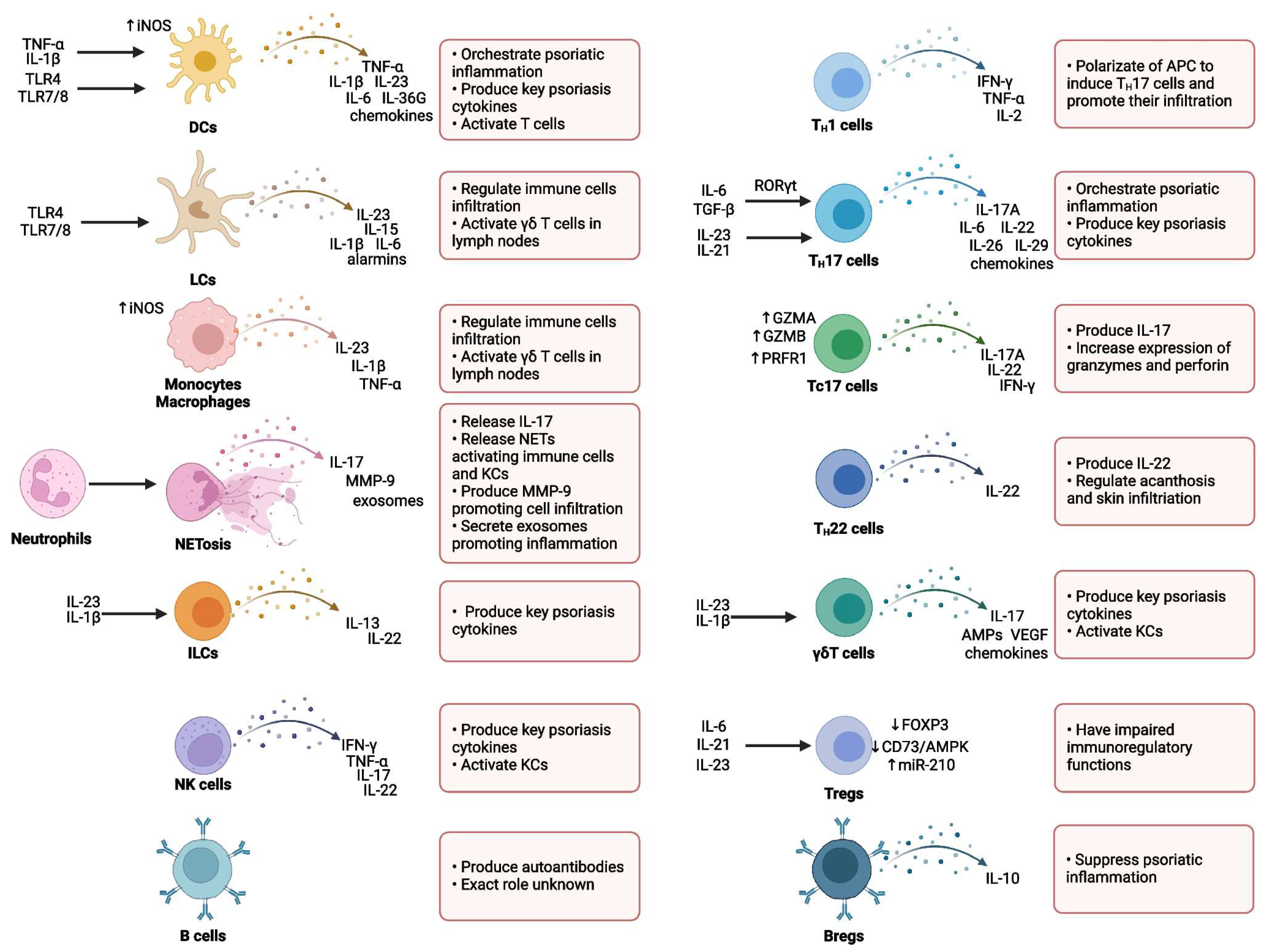
Immunological Mechanisms Involved in Psoriasis
3.4. Main Involved Interleukins
3.5. Correlation Between Disease Severity and Serum Interleukin Levels
Fluctuations in Cytokine Levels During Exacerbations and Remissions in Psoriasis
3.6. Salivary Interleukins—Potential Biomarkers in Psoriasis
3.7. Advantages of Saliva as an Analytical Medium (Non-Invasive, Repeatable, Cost-Effective)
Standardization of Saliva Collection
3.8. Rationale for Using Saliva as an Alternative Biological Fluid
3.9. Existing Studies on Salivary Interleukins in Psoriasis
3.10. Role of Interleukins in Monitoring Treatment Response
3.11. Future Research Directions—Standardization of Saliva Collection and Analysis Methods
3.12. Longitudinal Studies and Larger Cohorts
3.13. Possibilities of Integration into Clinical Practice
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lowes, M.A.; Lew, W.; Krueger, J.G. Current concepts in the immunopathogenesis of psoriasis. Dermatol. Clin. 2004, 22, 349–369. [Google Scholar] [CrossRef] [PubMed]
- Bos, J.D.; De Rie, M.A. The pathogenesis of psoriasis: Immunological facts and speculations. Immunol. Today 1999, 20, 40–46. [Google Scholar] [CrossRef]
- Griffiths, C.E. The immunological basis of psoriasis. J. Eur. Acad. Dermatol. Venereol. 2003, 17 (Suppl. 2), 1–5. [Google Scholar] [CrossRef] [PubMed]
- Chamian, F.; Krueger, J.G. Psoriasis vulgaris: An interplay of T lymphocytes, dendritic cells, and inflammatory cytokines in pathogenesis. Curr. Opin. Rheumatol. 2004, 16, 331–337. [Google Scholar] [CrossRef]
- Sugumaran, D.; Yong, A.C.H.; Stanslas, J. Advances in psoriasis research: From pathogenesis to therapeutics. Life Sci. 2024, 355, 122991. [Google Scholar] [CrossRef] [PubMed]
- Gudjonsson, J.E.; Johnston, A.; Sigmundsdottir, H.; Valdimarsson, H. Immunopathogenic mechanisms in psoriasis. Clin. Exp. Immunol. 2004, 135, 1–8. [Google Scholar] [CrossRef]
- Alsabbagh, M.M. Cytokines in psoriasis: From pathogenesis to targeted therapy. Hum. Immunol. 2024, 85, 110814. [Google Scholar] [CrossRef]
- Baliwag, J.; Barnes, D.H.; Johnston, A. Cytokines in psoriasis. Cytokine 2015, 73, 342–350. [Google Scholar] [CrossRef]
- Sawyer, L.M.; Malottki, K.; Sabry-Grant, C.; Yasmeen, N.; Wright, E.; Sohrt, A.; Borg, E.; Warren, R.B. Assessing the relative efficacy of interleukin-17 and interleukin-23 targeted treatments for moderate-to-severe plaque psoriasis: A systematic review and network meta-analysis of PASI response. PLoS ONE 2019, 14, e0220868. [Google Scholar] [CrossRef]
- Parisi, R.; Symmons, D.P.; Griffiths, C.E.; Ashcroft, D.M. Global epidemiology of psoriasis: A systematic review of incidence and prevalence. J. Investig. Dermatol. 2013, 133, 377–385. [Google Scholar] [CrossRef]
- Badri, T.; Kumar, P.; Oakley, A.M. Plaque Psoriasis. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Schadler, E.D.; Ortel, B.; Mehlis, S.L. Biologics for the primary care physician: Review and treatment of psoriasis. Dis. Mon. 2019, 65, 51–90. [Google Scholar] [CrossRef] [PubMed]
- Burfield, L.; Burden, A.D. Psoriasis. J. R. Coll. Physicians Edinb. 2013, 43, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef]
- Singh, R.; Koppu, S.; Perche, P.O.; Feldman, S.R. The Cytokine Mediated Molecular Pathophysiology of Psoriasis and Its Clinical Implications. Int. J. Mol. Sci. 2021, 22, 12793. [Google Scholar] [CrossRef]
- Campanati, A.; Marani, A.; Martina, E.; Diotallevi, F.; Radi, G.; Offidani, A. Psoriasis as an Immune-Mediated and Inflammatory Systemic Disease: From Pathophysiology to Novel Therapeutic Approaches. Biomedicines 2021, 9, 1511. [Google Scholar] [CrossRef] [PubMed]
- de Vlam, K.; Gottlieb, A.B.; Fitzgerald, O. Biological biomarkers in psoriatic disease. A review. J. Rheumatol. 2008, 35, 1443–1448. [Google Scholar]
- Group, F.-N.B.W. BEST (Biomarkers, EndpointS, and Other Tools) Resource; Food and Drug Administration (US): Silver Spring, MD, USA; National Institutes of Health (US): Bethesda, MD, USA, 2016.
- Kar, B.R.; Sathishkumar, D.; Tahiliani, S.; Parthasarathi, A.; Neema, S.; Ganguly, S.; Venkatachalam, K.; Parasramani, S.G.; Komeravelli, H.; Thomas, J. Biomarkers in Psoriasis: The Future of Personalised Treatment. Indian J. Dermatol. 2024, 69, 256–263. [Google Scholar] [CrossRef]
- Rashmi, R.; Rao, K.S.; Basavaraj, K.H. A comprehensive review of biomarkers in psoriasis. Clin. Exp. Dermatol. 2009, 34, 658–663. [Google Scholar] [CrossRef]
- Solak, B.; Kara, R. Assessing systemic inflammatory markers in psoriasis: A retrospective study. Trop. Med. Int. Health 2024, 29, 971–978. [Google Scholar] [CrossRef]
- Leigh, I.M.; Navsaria, H.; Purkis, P.E.; McKay, I.A.; Bowden, P.E.; Riddle, P.N. Keratins (K16 and K17) as markers of keratinocyte hyperproliferation in psoriasis in vivo and in vitro. Br. J. Dermatol. 1995, 133, 501–511. [Google Scholar] [CrossRef]
- Zhang, P.; Su, Y.; Li, S.; Chen, H.; Wu, R.; Wu, H. The roles of T cells in psoriasis. Front. Immunol. 2023, 14, 1081256. [Google Scholar] [CrossRef] [PubMed]
- Chekanov, K.; Danko, D.; Tlyachev, T.; Kiselev, K.; Hagens, R.; Georgievskaya, A. State-of-the-Art in Skin Fluorescent Photography for Cosmetic and Skincare Research: From Molecular Spectra to AI Image Analysis. Life 2024, 14, 1271. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Y.; Chen, K.; Zhang, J.A. Mast cells as important regulators in the development of psoriasis. Front. Immunol. 2022, 13, 1022986. [Google Scholar] [CrossRef]
- Sieminska, I.; Pieniawska, M.; Grzywa, T.M. The Immunology of Psoriasis—Current Concepts in Pathogenesis. Clin. Rev. Allergy Immunol. 2024, 66, 164–191. [Google Scholar] [CrossRef]
- Kamiya, K.; Kishimoto, M.; Sugai, J.; Komine, M.; Ohtsuki, M. Risk Factors for the Development of Psoriasis. Int. J. Mol. Sci. 2019, 20, 4347. [Google Scholar] [CrossRef]
- Sun, L.; Liu, W.; Zhang, L.J. The Role of Toll-Like Receptors in Skin Host Defense, Psoriasis, and Atopic Dermatitis. J. Immunol. Res. 2019, 2019, 1824624. [Google Scholar] [CrossRef]
- Zhang, L.J.; Sen, G.L.; Ward, N.L.; Johnston, A.; Chun, K.; Chen, Y.; Adase, C.; Sanford, J.A.; Gao, N.; Chensee, M.; et al. Antimicrobial Peptide LL37 and MAVS Signaling Drive Interferon-β Production by Epidermal Keratinocytes during Skin Injury. Immunity 2016, 45, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Lande, R.; Gregorio, J.; Facchinetti, V.; Chatterjee, B.; Wang, Y.H.; Homey, B.; Cao, W.; Wang, Y.H.; Su, B.; Nestle, F.O.; et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 2007, 449, 564–569. [Google Scholar] [CrossRef]
- Ramirez-Carrozzi, V.; Sambandam, A.; Luis, E.; Lin, Z.; Jeet, S.; Lesch, J.; Hackney, J.; Kim, J.; Zhou, M.; Lai, J.; et al. IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat. Immunol. 2011, 12, 1159–1166. [Google Scholar] [CrossRef]
- Blanco, P.; Palucka, A.K.; Pascual, V.; Banchereau, J. Dendritic cells and cytokines in human inflammatory and autoimmune diseases. Cytokine Growth Factor Rev. 2008, 19, 41–52. [Google Scholar] [CrossRef]
- Christophers, E.; Metzler, G.; Röcken, M. Bimodal immune activation in psoriasis. Br. J. Dermatol. 2014, 170, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Turchin, I.; Bourcier, M. The Role of Interleukins in the Pathogenesis of Dermatological Immune-Mediated Diseases. Adv. Ther. 2022, 39, 4474–4508. [Google Scholar] [CrossRef]
- Nestle, F.O.; Kaplan, D.H.; Barker, J. Psoriasis. N. Engl. J. Med. 2009, 361, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.; Darji, K.; No, D.J.; Wu, J.J. Review of phase III trial data on IL-23 inhibitors tildrakizumab and guselkumab for psoriasis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1627–1632. [Google Scholar] [CrossRef]
- Sato, Y.; Ogawa, E.; Okuyama, R. Role of Innate Immune Cells in Psoriasis. Int. J. Mol. Sci. 2020, 21, 6604. [Google Scholar] [CrossRef]
- Yamanaka, K.; Yamamoto, O.; Honda, T. Pathophysiology of psoriasis: A review. J Dermatol 2021, 48, 722–731. [Google Scholar] [CrossRef]
- Grossman, R.M.; Krueger, J.; Yourish, D.; Granelli-Piperno, A.; Murphy, D.P.; May, L.T.; Kupper, T.S.; Sehgal, P.B.; Gottlieb, A.B. Interleukin 6 is expressed in high levels in psoriatic skin and stimulates proliferation of cultured human keratinocytes. Proc. Natl. Acad. Sci. USA 1989, 86, 6367–6371. [Google Scholar] [CrossRef] [PubMed]
- Takematsu, H.; Tagami, H. Quantification of chemotactic peptides (C5a anaphylatoxin and IL-8) in psoriatic lesional skin. Arch. Dermatol. 1993, 129, 74–80. [Google Scholar] [CrossRef]
- Duan, H.; Koga, T.; Kohda, F.; Hara, H.; Urabe, K.; Furue, M. Interleukin-8-positive neutrophils in psoriasis. J. Dermatol. Sci. 2001, 26, 119–124. [Google Scholar] [CrossRef]
- Trepicchio, W.L.; Ozawa, M.; Walters, I.B.; Kikuchi, T.; Gilleaudeau, P.; Bliss, J.L.; Schwertschlag, U.; Dorner, A.J.; Krueger, J.G. Interleukin-11 therapy selectively downregulates type I cytokine proinflammatory pathways in psoriasis lesions. J. Clin. Investig. 1999, 104, 1527–1537. [Google Scholar] [CrossRef]
- Tonel, G.; Conrad, C.; Laggner, U.; Di Meglio, P.; Grys, K.; McClanahan, T.K.; Blumenschein, W.M.; Qin, J.Z.; Xin, H.; Oldham, E.; et al. Cutting edge: A critical functional role for IL-23 in psoriasis. J. Immunol. 2010, 185, 5688–5691. [Google Scholar] [CrossRef] [PubMed]
- Kulig, P.; Musiol, S.; Freiberger, S.N.; Schreiner, B.; Gyülveszi, G.; Russo, G.; Pantelyushin, S.; Kishihara, K.; Alessandrini, F.; Kündig, T.; et al. IL-12 protects from psoriasiform skin inflammation. Nat. Commun. 2016, 7, 13466. [Google Scholar] [CrossRef] [PubMed]
- Diels, J.; Thilakarathne, P.; Cameron, C.; McElligott, S.; Schubert, A.; Puig, L. Adjusted treatment COMPArisons between guSelkumab and uStekinumab for treatment of moderate-to-severe plaque psoriasis: The COMPASS analysis. Br. J. Dermatol. 2020, 183, 276–284. [Google Scholar] [CrossRef]
- Papp, K.A.; Blauvelt, A.; Bukhalo, M.; Gooderham, M.; Krueger, J.G.; Lacour, J.P.; Menter, A.; Philipp, S.; Sofen, H.; Tyring, S.; et al. Risankizumab versus Ustekinumab for Moderate-to-Severe Plaque Psoriasis. N. Engl. J. Med. 2017, 376, 1551–1560. [Google Scholar] [CrossRef]
- Lowes, M.A.; Kikuchi, T.; Fuentes-Duculan, J.; Cardinale, I.; Zaba, L.C.; Haider, A.S.; Bowman, E.P.; Krueger, J.G. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J. Investig. Dermatol. 2008, 128, 1207–1211. [Google Scholar] [CrossRef] [PubMed]
- Blauvelt, A.; Chiricozzi, A. The Immunologic Role of IL-17 in Psoriasis and Psoriatic Arthritis Pathogenesis. Clin. Rev. Allergy Immunol. 2018, 55, 379–390. [Google Scholar] [CrossRef]
- Stritesky, G.L.; Yeh, N.; Kaplan, M.H. IL-23 promotes maintenance but not commitment to the Th17 lineage. J. Immunol. 2008, 181, 5948–5955. [Google Scholar] [CrossRef]
- Rizzo, H.L.; Kagami, S.; Phillips, K.G.; Kurtz, S.E.; Jacques, S.L.; Blauvelt, A. IL-23-mediated psoriasis-like epidermal hyperplasia is dependent on IL-17A. J. Immunol. 2011, 186, 1495–1502. [Google Scholar] [CrossRef]
- Commins, S.; Steinke, J.W.; Borish, L. The extended IL-10 superfamily: IL-10, IL-19, IL-20, IL-22, IL-24, IL-26, IL-28, and IL-29. J. Allergy Clin. Immunol. 2008, 121, 1108–1111. [Google Scholar] [CrossRef]
- Li, H.H.; Lin, Y.C.; Chen, P.J.; Hsiao, C.H.; Lee, J.Y.; Chen, W.C.; Tzung, T.Y.; Wu, J.C.; Chang, M.S. Interleukin-19 upregulates keratinocyte growth factor and is associated with psoriasis. Br. J. Dermatol. 2005, 153, 591–595. [Google Scholar] [CrossRef]
- Wei, C.C.; Chen, W.Y.; Wang, Y.C.; Chen, P.J.; Lee, J.Y.; Wong, T.W.; Chen, W.C.; Wu, J.C.; Chen, G.Y.; Chang, M.S.; et al. Detection of IL-20 and its receptors on psoriatic skin. Clin. Immunol. 2005, 117, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Kunz, S.; Wolk, K.; Witte, E.; Witte, K.; Doecke, W.D.; Volk, H.D.; Sterry, W.; Asadullah, K.; Sabat, R. Interleukin (IL)-19, IL-20 and IL-24 are produced by and act on keratinocytes and are distinct from classical ILs. Exp. Dermatol. 2006, 15, 991–1004. [Google Scholar] [CrossRef] [PubMed]
- Lebre, M.C.; Jonckheere, C.L.; Kraan, M.C.; van Kuijk, A.W.; Bos, J.D.; de Rie, M.; Gerlag, D.M.; Tak, P.P. Expression of IL-20 in synovium and lesional skin of patients with psoriatic arthritis: Differential response to alefacept treatment. Arthritis Res. Ther. 2012, 14, R200. [Google Scholar] [CrossRef]
- Boniface, K.; Guignouard, E.; Pedretti, N.; Garcia, M.; Delwail, A.; Bernard, F.X.; Nau, F.; Guillet, G.; Dagregorio, G.; Yssel, H.; et al. A role for T cell-derived interleukin 22 in psoriatic skin inflammation. Clin. Exp. Immunol. 2007, 150, 407–415. [Google Scholar] [CrossRef]
- Wittmann, M.; Zeitvogel, J.; Wang, D.; Werfel, T. IL-27 is expressed in chronic human eczematous skin lesions and stimulates human keratinocytes. J. Allergy Clin. Immunol. 2009, 124, 81–89. [Google Scholar] [CrossRef]
- Wolk, K.; Witte, E.; Wallace, E.; Döcke, W.D.; Kunz, S.; Asadullah, K.; Volk, H.D.; Sterry, W.; Sabat, R. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: A potential role in psoriasis. Eur. J. Immunol. 2006, 36, 1309–1323. [Google Scholar] [CrossRef]
- Study Evaluating the Safety and Tolerability of ILV-094 in Subjects with Psoriasis. Available online: https://ctv.veeva.com/study/study-evaluating-the-safety-and-tolerability-of-ilv-094-in-subjects-with-psoriasis (accessed on 18 April 2025).
- Itoh, T.; Hatano, R.; Komiya, E.; Otsuka, H.; Narita, Y.; Aune, T.M.; Dang, N.H.; Matsuoka, S.; Naito, H.; Tominaga, M.; et al. Biological Effects of IL-26 on T Cell-Mediated Skin Inflammation, Including Psoriasis. J. Investig. Dermatol. 2019, 139, 878–889. [Google Scholar] [CrossRef] [PubMed]
- Andrys, C.; Borska, L.; Pohl, D.; Fiala, Z.; Hamakova, K.; Krejsek, J. Angiogenic activity in patients with psoriasis is significantly decreased by Goeckerman’s therapy. Arch. Dermatol. Res. 2007, 298, 479–483. [Google Scholar] [CrossRef]
- Shibata, S.; Tada, Y.; Kanda, N.; Nashiro, K.; Kamata, M.; Karakawa, M.; Miyagaki, T.; Kai, H.; Saeki, H.; Shirakata, Y.; et al. Possible roles of IL-27 in the pathogenesis of psoriasis. J. Investig. Dermatol. 2010, 130, 1034–1039. [Google Scholar] [CrossRef]
- Mitsui, A.; Tada, Y.; Takahashi, T.; Shibata, S.; Kamata, M.; Miyagaki, T.; Fujita, H.; Sugaya, M.; Kadono, T.; Sato, S.; et al. Serum IL-33 levels are increased in patients with psoriasis. Clin. Exp. Dermatol. 2016, 41, 183–189. [Google Scholar] [CrossRef]
- Wolk, K.; Witte, K.; Witte, E.; Raftery, M.; Kokolakis, G.; Philipp, S.; Schönrich, G.; Warszawska, K.; Kirsch, S.; Prösch, S.; et al. IL-29 is produced by TH17 cells and mediates the cutaneous antiviral competence in psoriasis. Sci. Transl. Med. 2013, 5, 204ra129. [Google Scholar] [CrossRef]
- Kempuraj, D.; Conti, P.; Vasiadi, M.; Alysandratos, K.D.; Tagen, M.; Kalogeromitros, D.; Kourelis, T.; Gregoriou, S.; Makris, M.; Stavrianeas, N.G.; et al. IL-32 is increased along with tryptase in lesional psoriatic skin and is up-regulated by substance P in human mast cells. Eur. J. Dermatol. 2010, 20, 865–867. [Google Scholar] [CrossRef]
- Li, J.; Liu, L.; Rui, W.; Li, X.; Xuan, D.; Zheng, S.; Yu, Y.; Zhang, J.; Kong, N.; Zhu, X.; et al. New Interleukins in Psoriasis and Psoriatic Arthritis Patients: The Possible Roles of Interleukin-33 to Interleukin-38 in Disease Activities and Bone Erosions. Dermatology 2017, 233, 37–46. [Google Scholar] [CrossRef]
- Teng, X.; Hu, Z.; Wei, X.; Wang, Z.; Guan, T.; Liu, N.; Liu, X.; Ye, N.; Deng, G.; Luo, C.; et al. IL-37 ameliorates the inflammatory process in psoriasis by suppressing proinflammatory cytokine production. J. Immunol. 2014, 192, 1815–1823. [Google Scholar] [CrossRef] [PubMed]
- Arican, O.; Aral, M.; Sasmaz, S.; Ciragil, P. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediat. Inflamm. 2005, 2005, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Kyriakou, A.; Patsatsi, A.; Vyzantiadis, T.A.; Sotiriadis, D. Serum levels of TNF-α, IL-12/23p40, and IL-17 in plaque psoriasis and their correlation with disease severity. J. Immunol. Res. 2014, 2014, 467541. [Google Scholar] [CrossRef] [PubMed]
- Kyriakou, A.; Patsatsi, A.; Vyzantiadis, T.A.; Sotiriadis, D. Serum levels of TNF- α, IL-12/23 p40, and IL-17 in psoriatic patients with and without nail psoriasis: A cross-sectional study. Sci. World J. 2014, 2014, 508178. [Google Scholar] [CrossRef]
- Omar, N.S.; Long, X.; Xian, J.; Afewerky, H.K.; Hussain, S.G.; Peng, X. Serum interleukin-30 level in patients with psoriasis and its correlation with psoriasis severity: A case-control study. J. Int. Med. Res. 2021, 49, 3000605211004039. [Google Scholar] [CrossRef]
- Jacob, S.E.; Nassiri, M.; Kerdel, F.A.; Vincek, V. Simultaneous measurement of multiple Th1 and Th2 serum cytokines in psoriasis and correlation with disease severity. Mediat. Inflamm. 2003, 12, 309–313. [Google Scholar] [CrossRef]
- el Barnawi, N.Y.; Giasuddin, A.S.; Ziu, M.M.; Singh, M. Serum cytokine levels in psoriasis vulgaris. Br. J. Biomed. Sci. 2001, 58, 40–44. [Google Scholar]
- Gomi, T.; Shiohara, T.; Munakata, T.; Imanishi, K.; Nagashima, M. Interleukin 1 alpha, tumor necrosis factor alpha, and interferon gamma in psoriasis. Arch. Dermatol. 1991, 127, 827–830. [Google Scholar] [CrossRef] [PubMed]
- Mussi, A.; Bonifati, C.; Carducci, M.; D’Agosto, G.; Pimpinelli, F.; D’Urso, D.; D’Auria, L.; Fazio, M.; Ameglio, F. Serum TNF-alpha levels correlate with disease severity and are reduced by effective therapy in plaque-type psoriasis. J. Biol. Regul. Homeost. Agents 1997, 11, 115–118. [Google Scholar]
- Mizutani, H.; Ohmoto, Y.; Mizutani, T.; Murata, M.; Shimizu, M. Role of increased production of monocytes TNF-alpha, IL-1beta and IL-6 in psoriasis: Relation to focal infection, disease activity and responses to treatments. J. Dermatol. Sci. 1997, 14, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Abanmi, A.; Al Harthi, F.; Al Agla, R.; Khan, H.A.; Tariq, M. Serum levels of proinflammatory cytokines in psoriasis patients from Saudi Arabia. Int. J. Dermatol. 2005, 44, 82–83. [Google Scholar] [CrossRef]
- Okubo, Y.; Koga, M. Peripheral blood monocytes in psoriatic patients overproduce cytokines. J. Dermatol. Sci. 1998, 17, 223–232. [Google Scholar] [CrossRef]
- Biasi, D.; Carletto, A.; Caramaschi, P.; Bellavite, P.; Maleknia, T.; Scambi, C.; Favalli, N.; Bambara, L.M. Neutrophil functions and IL-8 in psoriatic arthritis and in cutaneous psoriasis. Inflammation 1998, 22, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, A.; Kozioł-Montewka, M.; Lecewicz-Toruń, B.; Krasowska, D. Is there any correlation between the total number of neutrophils in plasma and concentration of interleukin-8 in psoriatic patients? Med. Sci. Monit. 2000, 6, 867–870. [Google Scholar]
- Gangemi, S.; Merendino, R.A.; Guarneri, F.; Minciullo, P.L.; DiLorenzo, G.; Pacor, M.; Cannavò, S.P. Serum levels of interleukin-18 and s-ICAM-1 in patients affected by psoriasis: Preliminary considerations. J. Eur. Acad. Dermatol. Venereol. 2003, 17, 42–46. [Google Scholar] [CrossRef]
- Bonifati, C.; Ameglio, F. Cytokines in psoriasis. Int. J. Dermatol. 1999, 38, 241–251. [Google Scholar] [CrossRef]
- Pietrzak, A.; Chabros, P.; Grywalska, E.; Pietrzak, D.; Kandzierski, G.; Wawrzycki, B.O.; Roliñski, J.; Gawêda, K.; Krasowska, D. Serum concentration of interleukin 6 is related to inflammation and dyslipidemia in patients with psoriasis. Postep. Dermatol. Alergol. 2020, 37, 41–45. [Google Scholar] [CrossRef]
- Sehgal, P.B. Interleukin-6: Molecular pathophysiology. J. Investig. Dermatol. 1990, 94, 2S–6S. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Hirano, T. IL-6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine Growth Factor Rev. 2002, 13, 357–368. [Google Scholar] [CrossRef]
- Balato, A.; Schiattarella, M.; Di Caprio, R.; Lembo, S.; Mattii, M.; Balato, N.; Ayala, F. Effects of adalimumab therapy in adult subjects with moderate-to-severe psoriasis on Th17 pathway. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 1016–1024. [Google Scholar] [CrossRef]
- Koliadenko, V.H.; Chernyshov, P.V. Interleukin-6 as a marker of the activity of a pathological process in patients with psoriasis. Lik. Sprava 2005, 5–6, 80–82. [Google Scholar]
- Verghese, B.; Bhatnagar, S.; Tanwar, R.; Bhattacharjee, J. Serum cytokine profile in psoriasis-a case-control study in a tertiary care hospital from northern India. Indian J. Clin. Biochem. 2011, 26, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Dowlatshahi, E.A.; van der Voort, E.A.; Arends, L.R.; Nijsten, T. Markers of systemic inflammation in psoriasis: A systematic review and meta-analysis. Br. J. Dermatol. 2013, 169, 266–282. [Google Scholar] [CrossRef]
- Sticherling, M.; Sautier, W.; Schröder, J.M.; Christophers, E. Interleukin-8 plays its role at local level in psoriasis vulgaris. Acta Derm. Venereol. 1999, 79, 4–8. [Google Scholar] [CrossRef]
- Teranishi, Y.; Mizutani, H.; Murata, M.; Shimizu, M.; Matsushima, K. Increased spontaneous production of IL-8 in peripheral blood monocytes from the psoriatic patient: Relation to focal infection and response to treatments. J. Dermatol. Sci. 1995, 10, 8–15. [Google Scholar] [CrossRef]
- Pietrzak, A.; Lecewicz-Toruń, B.; Kozioł-Montewka, M. Plasma level of IL-8 in patients with psoriasis and its correlation with psoriasis area and severity index and the clinical type of the disease. Ann. Univ. Mariae Curie Sklodowska Med. 2000, 55, 261–267. [Google Scholar]
- McInnes, I.B.; Illei, G.G.; Danning, C.L.; Yarboro, C.H.; Crane, M.; Kuroiwa, T.; Schlimgen, R.; Lee, E.; Foster, B.; Flemming, D.; et al. IL-10 improves skin disease and modulates endothelial activation and leukocyte effector function in patients with psoriatic arthritis. J. Immunol. 2001, 167, 4075–4082. [Google Scholar] [CrossRef]
- Yawalkar, N.; Karlen, S.; Hunger, R.; Brand, C.U.; Braathen, L.R. Expression of interleukin-12 is increased in psoriatic skin. J. Investig. Dermatol. 1998, 111, 1053–1057. [Google Scholar] [CrossRef]
- Purzycka-Bohdan, D.; Szczerkowska-Dobosz, A.; Zablotna, M.; Wierzbicka, J.; Piotrowska, A.; Zmijewski, M.A.; Nedoszytko, B.; Nowicki, R. Assessment of Interleukin 16 Serum Levels and Skin Expression in Psoriasis Patients in Correlation with Clinical Severity of the Disease. PLoS ONE 2016, 11, e0165577. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, S.B.; Cicek, N.; Coskun, M.; Yegin, O.; Alpsoy, E. Serum and tissue levels of IL-17 in different clinical subtypes of psoriasis. Arch. Dermatol. Res. 2012, 304, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Michalak-Stoma, A.; Bartosińska, J.; Kowal, M.; Juszkiewicz-Borowiec, M.; Gerkowicz, A.; Chodorowska, G. Serum levels of selected Th17 and Th22 cytokines in psoriatic patients. Dis. Markers 2013, 35, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Tsuji, H.; Hashimoto, Y.; Ishida-Yamamoto, A.; Iizuka, H. Serum cytokines and growth factor levels in Japanese patients with psoriasis. Clin. Exp. Dermatol. 2010, 35, 645–649. [Google Scholar] [CrossRef]
- El-Moaty Zaher, H.A.; El-Komy, M.H.M.; Hegazy, R.A.; Mohamed El Khashab, H.A.; Ahmed, H.H. Assessment of interleukin-17 and vitamin D serum levels in psoriatic patients. J. Am. Acad. Dermatol. 2013, 69, 840–842. [Google Scholar] [CrossRef]
- Teunissen, M.B.; Koomen, C.W.; de Waal Malefyt, R.; Wierenga, E.A.; Bos, J.D. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J. Investig. Dermatol. 1998, 111, 645–649. [Google Scholar] [CrossRef]
- Spriggs, M.K. Interleukin-17 and its receptor. J. Clin. Immunol. 1997, 17, 366–369. [Google Scholar] [CrossRef]
- Pietrzak, A.; Lecewicz-Torun, B.; Chodorowska, G.; Rolinski, J. Interleukin-18 levels in the plasma of psoriatic patients correlate with the extent of skin lesions and the PASI score. Acta Derm. Venereol. 2003, 83, 262–265. [Google Scholar] [CrossRef]
- Kathleen Newton, E.L.S.; Sawyer, J.R.; McCluskey, A.M.; Beck, E.; Phipps, W. Medical Surgical Nursing; C.V. Mosby Company: St. Louis, MO, USA, 1975. [Google Scholar]
- Casu, B.; Dondero, A.; Regis, S.; Caliendo, F.; Petretto, A.; Bartolucci, M.; Bellora, F.; Bottino, C.; Castriconi, R. Novel Immunoregulatory Functions of IL-18, an Accomplice of TGF-β1. Cancers 2019, 11, 75. [Google Scholar] [CrossRef]
- Shafer, K.N. Medical-Surgical Nursing; Mosby: St. Louis, MO, USA, 1975. [Google Scholar]
- Nicoletti, F.; Conget, I.; Di Marco, R.; Speciale, A.M.; Morìnigo, R.; Bendtzen, K.; Gomis, R. Serum levels of the interferon-gamma-inducing cytokine interleukin-18 are increased in individuals at high risk of developing type I diabetes. Diabetologia 2001, 44, 309–311. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.; Ho, C.Y.; Li, E.K.; Lam, C.W. Elevation of proinflammatory cytokine (IL-18, IL-17, IL-12) and Th2 cytokine (IL-4) concentrations in patients with systemic lupus erythematosus. Lupus 2000, 9, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Teramoto, S.; Oashi, K.; Saikai, T.; Tanaka, S.; Suzuki, K.; Hashimoto, M.; Abe, S. Effects of candesartan on cough and bronchial hyperresponsiveness in mildly to moderately hypertensive patients with symptomatic asthma. Circulation 2001, 104, 281–285. [Google Scholar] [CrossRef]
- Tanaka, T.; Tsutsui, H.; Yoshimoto, T.; Kotani, M.; Matsumoto, M.; Fujita, A.; Wang, W.; Higa, S.; Koshimoto, T.; Nakanishi, K.; et al. Interleukin-18 is elevated in the sera from patients with atopic dermatitis and from atopic dermatitis model mice, NC/Nga. Int. Arch. Allergy Immunol. 2001, 125, 236–240. [Google Scholar] [CrossRef]
- Rau, B.; Baumgart, K.; Paszkowski, A.S.; Mayer, J.M.; Beger, H.G. Clinical relevance of caspase-1 activated cytokines in acute pancreatitis: High correlation of serum interleukin-18 with pancreatic necrosis and systemic complications. Crit. Care Med. 2001, 29, 1556–1562. [Google Scholar] [CrossRef] [PubMed]
- Takada, H.; Nomura, A.; Ohga, S.; Hara, T. Interleukin-18 in hemophagocytic lymphohistiocytosis. Leuk. Lymphoma 2001, 42, 21–28. [Google Scholar] [CrossRef]
- Naik, S.M.; Cannon, G.; Burbach, G.J.; Singh, S.R.; Swerlick, R.A.; Wilcox, J.N.; Ansel, J.C.; Caughman, S.W. Human keratinocytes constitutively express interleukin-18 and secrete biologically active interleukin-18 after treatment with pro-inflammatory mediators and dinitrochlorobenzene. J. Investig. Dermatol. 1999, 113, 766–772. [Google Scholar] [CrossRef]
- McKenzie, R.C.; Boyce, F.; Forsey, R.; Gracie, A.; Szepietowski, J.; Weller, R.; Sabin, E.; Howie, S. Psoriatic skin expresses high levels of interleukin-18 (IL-18) and IL-18 receptor (IL-18R). Br. J. Dermatol. 2000, 142, 618. [Google Scholar]
- Torre, D.; Giola, M.; Speranza, F.; Matteelli, A.; Basilico, C.; Biondi, G. Serum levels of interleukin-18 in patients with uncomplicated Plasmodium falciparum malaria. Eur. Cytokine Netw. 2001, 12, 361–364. [Google Scholar]
- Ohta, Y.; Hamada, Y.; Katsuoka, K. Expression of IL-18 in psoriasis. Arch. Dermatol. Res. 2001, 293, 334–342. [Google Scholar] [CrossRef]
- Sabat, R.; Wolk, K. Research in practice: IL-22 and IL-20: Significance for epithelial homeostasis and psoriasis pathogenesis. J. Dtsch. Dermatol. Ges. 2011, 9, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Wolk, K.; Witte, E.; Warszawska, K.; Schulze-Tanzil, G.; Witte, K.; Philipp, S.; Kunz, S.; Döcke, W.D.; Asadullah, K.; Volk, H.D.; et al. The Th17 cytokine IL-22 induces IL-20 production in keratinocytes: A novel immunological cascade with potential relevance in psoriasis. Eur. J. Immunol. 2009, 39, 3570–3581. [Google Scholar] [CrossRef] [PubMed]
- Wolk, K.; Kunz, S.; Asadullah, K.; Sabat, R. Cutting edge: Immune cells as sources and targets of the IL-10 family members? J. Immunol. 2002, 168, 5397–5402. [Google Scholar] [CrossRef] [PubMed]
- Wolk, K.; Haugen, H.S.; Xu, W.; Witte, E.; Waggie, K.; Anderson, M.; Vom Baur, E.; Witte, K.; Warszawska, K.; Philipp, S.; et al. IL-22 and IL-20 are key mediators of the epidermal alterations in psoriasis while IL-17 and IFN-gamma are not. J. Mol. Med. 2009, 87, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Wawrzycki, B.; Pietrzak, A.; Grywalska, E.; Krasowska, D.; Chodorowska, G.; Roliński, J. Interleukin-22 and Its Correlation with Disease Activity in Plaque Psoriasis. Arch. Immunol. Ther. Exp. 2019, 67, 103–108. [Google Scholar] [CrossRef]
- Lo, Y.H.; Torii, K.; Saito, C.; Furuhashi, T.; Maeda, A.; Morita, A. Serum IL-22 correlates with psoriatic severity and serum IL-6 correlates with susceptibility to phototherapy. J. Dermatol. Sci. 2010, 58, 225–227. [Google Scholar] [CrossRef]
- Shimauchi, T.; Hirakawa, S.; Suzuki, T.; Yasuma, A.; Majima, Y.; Tatsuno, K.; Yagi, H.; Ito, T.; Tokura, Y. Serum interleukin-22 and vascular endothelial growth factor serve as sensitive biomarkers but not as predictors of therapeutic response to biologics in patients with psoriasis. J. Dermatol. 2013, 40, 805–812. [Google Scholar] [CrossRef]
- Nikamo, P.; Cheuk, S.; Lysell, J.; Enerbäck, C.; Bergh, K.; Xu Landén, N.; Eidsmo, L.; Ståhle, M. Genetic variants of the IL22 promoter associate to onset of psoriasis before puberty and increased IL-22 production in T cells. J. Investig. Dermatol. 2014, 134, 1535–1541. [Google Scholar] [CrossRef]
- Prans, E.; Kingo, K.; Traks, T.; Silm, H.; Vasar, E.; Kõks, S. Copy number variations in IL22 gene are associated with Psoriasis vulgaris. Hum. Immunol. 2013, 74, 792–795. [Google Scholar] [CrossRef]
- Catalan-Dibene, J.; McIntyre, L.L.; Zlotnik, A. Interleukin 30 to Interleukin 40. J. Interferon Cytokine Res. 2018, 38, 423–439. [Google Scholar] [CrossRef]
- Petes, C.; Mariani, M.K.; Yang, Y.; Grandvaux, N.; Gee, K. Interleukin (IL)-6 Inhibits IL-27- and IL-30-Mediated Inflammatory Responses in Human Monocytes. Front. Immunol. 2018, 9, 256. [Google Scholar] [CrossRef]
- Di Carlo, E. Decoding the Role of Interleukin-30 in the Crosstalk Between Cancer and Myeloid Cells. Cells 2020, 9, 615. [Google Scholar] [CrossRef]
- Venerito, V.; Natuzzi, D.; Bizzoca, R.; Lacarpia, N.; Cacciapaglia, F.; Lopalco, G.; Iannone, F. Serum sCD40L levels are increased in patients with psoriatic arthritis and are associated with clinical response to apremilast. Clin. Exp. Immunol. 2020, 201, 200–204. [Google Scholar] [CrossRef]
- Baran, P.; Nitz, R.; Grötzinger, J.; Scheller, J.; Garbers, C. Minimal interleukin 6 (IL-6) receptor stalk composition for IL-6 receptor shedding and IL-6 classic signaling. J. Biol. Chem. 2013, 288, 14756–14768. [Google Scholar] [CrossRef] [PubMed]
- Stumhofer, J.S.; Tait, E.D.; Quinn, W.J., 3rd; Hosken, N.; Spudy, B.; Goenka, R.; Fielding, C.A.; O’Hara, A.C.; Chen, Y.; Jones, M.L.; et al. A role for IL-27p28 as an antagonist of gp130-mediated signaling. Nat. Immunol. 2010, 11, 1119–1126. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Huang, N.; Hu, Z.; Wu, W.; Teng, X.; Wang, Z.; Wei, X.; Tang, H.; Wu, X.; et al. Soluble expression and purification of the functional interleukin-30 protein in Escherichia coli. Prep. Biochem. Biotechnol. 2016, 46, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamid, M.F.; Aly, D.G.; Saad, N.E.; Emam, H.M.; Ayoub, D.F. Serum levels of interleukin-8, tumor necrosis factor-α and γ-interferon in Egyptian psoriatic patients and correlation with disease severity. J. Dermatol. 2011, 38, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Carlin, C.S.; Feldman, S.R.; Krueger, J.G.; Menter, A.; Krueger, G.G. A 50% reduction in the Psoriasis Area and Severity Index (PASI 50) is a clinically significant endpoint in the assessment of psoriasis. J. Am. Acad. Dermatol. 2004, 50, 859–866. [Google Scholar] [CrossRef]
- Spuls, P.I.; Lecluse, L.L.; Poulsen, M.L.; Bos, J.D.; Stern, R.S.; Nijsten, T. How good are clinical severity and outcome measures for psoriasis?: Quantitative evaluation in a systematic review. J. Investig. Dermatol. 2010, 130, 933–943. [Google Scholar] [CrossRef]
- Puzenat, E.; Bronsard, V.; Prey, S.; Gourraud, P.A.; Aractingi, S.; Bagot, M.; Cribier, B.; Joly, P.; Jullien, D.; Le Maitre, M.; et al. What are the best outcome measures for assessing plaque psoriasis severity? A systematic review of the literature. J. Eur. Acad. Dermatol. Venereol. 2010, 24 (Suppl. 2), 10–16. [Google Scholar] [CrossRef]
- Roussaki-Schulze, A.V.; Kouskoukis, C.; Petinaki, E.; Klimi, E.; Zafiriou, E.; Galanos, A.; Rallis, E. Evaluation of cytokine serum levels in patients with plaque-type psoriasis. Int. J. Clin. Pharmacol. Res. 2005, 25, 169–173. [Google Scholar] [PubMed]
- Tigalonova, M.; Bjerke, J.R.; Gallati, H.; Degré, M.; Jablonska, S.; Majewski, S.; Matre, R. Serum levels of interferons and TNF-alpha are not correlated to psoriasis activity and therapy. Acta Derm. Venereol. Suppl. 1994, 186, 25–27. [Google Scholar] [CrossRef]
- Anderson, K.S.; Petersson, S.; Wong, J.; Shubbar, E.; Lokko, N.N.; Carlström, M.; Enerbäck, C. Elevation of serum epidermal growth factor and interleukin 1 receptor antagonist in active psoriasis vulgaris. Br. J. Dermatol. 2010, 163, 1085–1089. [Google Scholar] [CrossRef] [PubMed]
- Bonifati, C.; Carducci, M.; Cordiali Fei, P.; Trento, E.; Sacerdoti, G.; Fazio, M.; Ameglio, F. Correlated increases of tumour necrosis factor-alpha, interleukin-6 and granulocyte monocyte-colony stimulating factor levels in suction blister fluids and sera of psoriatic patients--relationships with disease severity. Clin. Exp. Dermatol. 1994, 19, 383–387. [Google Scholar] [CrossRef]
- Piskin, G.; Tursen, U.; Sylva-Steenland, R.M.; Bos, J.D.; Teunissen, M.B. Clinical improvement in chronic plaque-type psoriasis lesions after narrow-band UVB therapy is accompanied by a decrease in the expression of IFN-gamma inducers—IL-12, IL-18 and IL-23. Exp. Dermatol. 2004, 13, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Szegedi, A.; Aleksza, M.; Gonda, A.; Irinyi, B.; Sipka, S.; Hunyadi, J.; Antal-Szalmás, P. Elevated rate of Thelper1 (TH1) lymphocytes and serum IFN-gamma levels in psoriatic patients. Immunol. Lett. 2003, 86, 277–280. [Google Scholar] [CrossRef]
- Williams, J.D.; Griffiths, C.E. Cytokine blocking agents in dermatology. Clin. Exp. Dermatol. 2002, 27, 585–590. [Google Scholar] [CrossRef]
- da Silva, L.C.; Guimarães, L.C.A.; Santos, D.B.d.N.; Jural, L.A.; de Oliveira, S.P.; de Andrade, B.A.B.; Tenório, J.R. Assessment of salivary biomarkers in psoriasis: A comprehensive scoping review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2025, 140, 186–197. [Google Scholar] [CrossRef]
- Lowes, M.A.; Suárez-Fariñas, M.; Krueger, J.G. Immunology of psoriasis. Annu. Rev. Immunol. 2014, 32, 227–255. [Google Scholar] [CrossRef]
- Di Cesare, A.; Di Meglio, P.; Nestle, F.O. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J. Investig. Dermatol. 2009, 129, 1339–1350. [Google Scholar] [CrossRef]
- Kaczor-Urbanowicz, K.E.; Martin Carreras-Presas, C.; Aro, K.; Tu, M.; Garcia-Godoy, F.; Wong, D.T. Saliva diagnostics—Current views and directions. Exp. Biol. Med. 2017, 242, 459–472. [Google Scholar] [CrossRef]
- Yu, K.; Syed, M.N.; Bernardis, E.; Gelfand, J.M. Machine Learning Applications in the Evaluation and Management of Psoriasis: A Systematic Review. J. Psoriasis Psoriatic Arthritis 2020, 5, 147–159. [Google Scholar] [CrossRef]
- BiologyInsights Team. Saliva Test: A Noninvasive Approach to Health Analysis. Available online: https://biologyinsights.com/saliva-test-a-noninvasive-approach-to-health-analysis/ (accessed on 7 May 2025).
- Why Researchers Are Looking at Saliva Instead of Blood for Disease Detection. Available online: https://www.salignostics.com/blog/saliva-vs-blood-disease-detection/ (accessed on 7 May 2025).
- Bellagambi, F.G.; Lomonaco, T.; Salvo, P.; Vivaldi, F.; Hangouët, M.; Ghimenti, S.; Biagini, D.; Di Francesco, F.; Fuoco, R.; Errachid, A. Saliva sampling: Methods and devices. An overview. TrAC Trends Anal. Chem. 2020, 124, 115781. [Google Scholar] [CrossRef]
- Sun, F.; Reichenberger, E.J. Saliva as a source of genomic DNA for genetic studies: Review of current methods and applications. Oral Health Dent. Manag. 2014, 13, 217–222. [Google Scholar]
- Nunes, L.A.; Mussavira, S.; Bindhu, O.S. Clinical and diagnostic utility of saliva as a non-invasive diagnostic fluid: A systematic review. Biochem. Med. 2015, 25, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Nunes, L.A.; Brenzikofer, R.; Macedo, D.V. Reference intervals for saliva analytes collected by a standardized method in a physically active population. Clin. Biochem. 2011, 44, 1440–1444. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Duan, Y. Saliva: A potential media for disease diagnostics and monitoring. Oral Oncol. 2012, 48, 569–577. [Google Scholar] [CrossRef]
- Marley, G.; Kang, D.; Wilson, E.C.; Huang, T.; Qian, Y.; Li, X.; Tao, X.; Wang, G.; Xun, H.; Ma, W. Introducing rapid oral-fluid HIV testing among high risk populations in Shandong, China: Feasibility and challenges. BMC Public Health 2014, 14, 422. [Google Scholar] [CrossRef]
- Cardoso, E.M.; Arregger, A.L.; Tumilasci, O.R.; Elbert, A.; Contreras, L.N. Assessment of salivary urea as a less invasive alternative to serum determinations. Scand. J. Clin. Lab. Investig. 2009, 69, 330–334. [Google Scholar] [CrossRef]
- Soukup, M.; Biesiada, I.; Henderson, A.; Idowu, B.; Rodeback, D.; Ridpath, L.; Bridges, E.G.; Nazar, A.M.; Bridges, K.G. Salivary uric acid as a noninvasive biomarker of metabolic syndrome. Diabetol. Metab. Syndr. 2012, 4, 14. [Google Scholar] [CrossRef]
- Raggam, R.B.; Wagner, J.; Michelin, B.D.; Putz-Bankuti, C.; Lackner, A.; Bozic, M.; Stauber, R.E.; Santner, B.I.; Marth, E.; Kessler, H.H. Reliable detection and quantitation of viral nucleic acids in oral fluid: Liquid phase-based sample collection in conjunction with automated and standardized molecular assays. J. Med. Virol. 2008, 80, 1684–1688. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, E.; Lamster, I.B. The diagnostic applications of saliva—A review. Crit. Rev. Oral Biol. Med. 2002, 13, 197–212. [Google Scholar] [CrossRef] [PubMed]
- De La Peña, V.A.; Diz Dios, P.; Tojo Sierra, R. Relationship between lactate dehydrogenase activity in saliva and oral health status. Arch. Oral Biol. 2007, 52, 911–915. [Google Scholar] [CrossRef]
- Kibayashi, M.; Tanaka, M.; Nishida, N.; Kuboniwa, M.; Kataoka, K.; Nagata, H.; Nakayama, K.; Morimoto, K.; Shizukuishi, S. Longitudinal study of the association between smoking as a periodontitis risk and salivary biomarkers related to periodontitis. J. Periodontol. 2007, 78, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Surdu, A.; Foia, L.G.; Luchian, I.; Trifan, D.; Tatarciuc, M.S.; Scutariu, M.M.; Ciupilan, C.; Budala, D.G. Saliva as a Diagnostic Tool for Systemic Diseases—A Narrative Review. Medicina 2025, 61, 243. [Google Scholar] [CrossRef]
- Chiappin, S.; Antonelli, G.; Gatti, R.; De Palo, E.F. Saliva specimen: A new laboratory tool for diagnostic and basic investigation. Clin. Chim. Acta 2007, 383, 30–40. [Google Scholar] [CrossRef]
- Ilyin, S.E.; Belkowski, S.M.; Plata-Salamán, C.R. Biomarker discovery and validation: Technologies and integrative approaches. Trends Biotechnol. 2004, 22, 411–416. [Google Scholar] [CrossRef]
- Kumar, P.; Gupta, S.; Das, B.C. Saliva as a potential non-invasive liquid biopsy for early and easy diagnosis/prognosis of head and neck cancer. Transl. Oncol. 2024, 40, 101827. [Google Scholar] [CrossRef]
- Slots, J.; Slots, H. Bacterial and viral pathogens in saliva: Disease relationship and infectious risk. Periodontol. 2000 2011, 55, 48–69. [Google Scholar] [CrossRef]
- Riis, J.L.; Ahmadi, H.; Hamilton, K.R.; Hand, T.; Granger, D.A. Best practice recommendations for the measurement and interpretation of salivary proinflammatory cytokines in biobehavioral research. Brain Behav. Immun. 2021, 91, 105–116. [Google Scholar] [CrossRef]
- Ng, T.K.S.; Udeh-Momoh, C.; Lim, M.-A.; Gleerup, H.S.; Leifert, W.; Ajalo, C.; Ashton, N.; Zetterberg, H.; Rissman, R.A.; Winston, C.N.; et al. Guidelines for the standardization of pre-analytical variables for salivary biomarker studies in Alzheimer’s disease research: An updated review and consensus of the Salivary Biomarkers for Dementia Research Working Group. Alzheimer’s Dement. 2025, 21, e14420. [Google Scholar] [CrossRef] [PubMed]
- Granger, D.A.; Kivlighan, K.T.; Fortunato, C.; Harmon, A.G.; Hibel, L.C.; Schwartz, E.B.; Whembolua, G.L. Integration of salivary biomarkers into developmental and behaviorally-oriented research: Problems and solutions for collecting specimens. Physiol. Behav. 2007, 92, 583–590. [Google Scholar] [CrossRef]
- Sarkar, A.; Kuehl, M.N.; Alman, A.C.; Burkhardt, B.R. Linking the oral microbiome and salivary cytokine abundance to circadian oscillations. Sci. Rep. 2021, 11, 2658. [Google Scholar] [CrossRef]
- Navazesh, M.; Kumar, S.K. Measuring salivary flow: Challenges and opportunities. J. Am. Dent. Assoc. 2008, 139, 35S–40S. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Wong, D.T. Saliva: An emerging biofluid for early detection of diseases. Am. J. Dent. 2009, 22, 241–248. [Google Scholar]
- Samaranayake, L. Saliva as a diagnostic fluid *. Int. Dent. J. 2007, 57, 295–299. [Google Scholar] [CrossRef]
- Lopez-Jornet, P.; Martinez-Canovas, A.; Pons-Fuster, A. Salivary biomarkers of oxidative stress and quality of life in patients with oral lichen planus. Geriatr. Gerontol. Int. 2014, 14, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Sculley, D.V.; Langley-Evans, S.C. Salivary antioxidants and periodontal disease status. Proc. Nutr. Soc. 2002, 61, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Diesch, T.; Filippi, C.; Fritschi, N.; Filippi, A.; Ritz, N. Cytokines in saliva as biomarkers of oral and systemic oncological or infectious diseases: A systematic review. Cytokine 2021, 143, 155506. [Google Scholar] [CrossRef]
- Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar] [CrossRef]
- Villanova, F.; Di Meglio, P.; Nestle, F.O. Biomarkers in psoriasis and psoriatic arthritis. Ann. Rheum. Dis. 2013, 72 (Suppl. 2), ii104–ii110. [Google Scholar] [CrossRef]
- Turner, R.J.; Sugiya, H. Understanding salivary fluid and protein secretion. Oral Dis. 2002, 8, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Melvin, J.E.; Yule, D.; Shuttleworth, T.; Begenisich, T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu. Rev. Physiol. 2005, 67, 445–469. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, T.; Srivastava, A.; Romanenko, V.G.; Ovitt, C.E.; Perez-Cornejo, P.; Arreola, J.; Begenisich, T.; Melvin, J.E. Functional and molecular characterization of the fluid secretion mechanism in human parotid acinar cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R2380–R2390. [Google Scholar] [CrossRef]
- Gutiérrez-Corrales, A.; Campano-Cuevas, E.; Castillo-Dalí, G.; Torres-Lagares, D.; Gutiérrez-Pérez, J.L. Ability of salivary biomarkers in the prognostic of systemic and buccal inflammation. J. Clin. Exp. Dent. 2017, 9, e716–e722. [Google Scholar] [CrossRef] [PubMed]
- Asa’ad, F.; Fiore, M.; Alfieri, A.; Pigatto, P.D.M.; Franchi, C.; Berti, E.; Maiorana, C.; Damiani, G. Saliva as a Future Field in Psoriasis Research. Biomed Res. Int. 2018, 2018, 7290913. [Google Scholar] [CrossRef]
- Olejnik, M.; Adamski, Z.; Osmola-Mankowska, A.; Nijakowski, K.; Dorocka-Bobkowska, B. Oral health status and dental treatment needs of psoriatic patients with different therapy regimes. Aust. Dent. J. 2021, 66 (Suppl. 1), S42–S47. [Google Scholar] [CrossRef]
- Krasteva, A.; Grozdev, I.; Ivanova, A.; Altankova, I.; Bocheva, S.; Kisselova, A.; Tsankov, N. Psoriatic patients and salivary components. Oral Health Dent. Manag. Black Sea Ctries. 2009, 8, 12–15. [Google Scholar]
- Malamud, D. Saliva as a diagnostic fluid. Dent. Clin. North Am. 2011, 55, 159–178. [Google Scholar] [CrossRef]
- Farnaud, S.J.; Kosti, O.; Getting, S.J.; Renshaw, D. Saliva: Physiology and diagnostic potential in health and disease. Sci. World J. 2010, 10, 434–456. [Google Scholar] [CrossRef]
- Ganzetti, G.; Campanati, A.; Santarelli, A.; Pozzi, V.; Molinelli, E.; Minnetti, I.; Brisigotti, V.; Procaccini, M.; Emanuelli, M.; Offidani, A. Involvement of the oral cavity in psoriasis: Results of a clinical study. Br. J. Dermatol. 2015, 172, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Fadel, H.T.; Flytström, I.; Calander, A.M.; Bergbrant, I.M.; Heijl, L.; Birkhed, D. Profiles of dental caries and periodontal disease in individuals with or without psoriasis. J. Periodontol. 2013, 84, 477–485. [Google Scholar] [CrossRef]
- Sawada, S.; Chosa, N.; Ishisaki, A.; Naruishi, K. Enhancement of gingival inflammation induced by synergism of IL-1β and IL-6. Biomed Res. 2013, 34, 31–40. [Google Scholar] [CrossRef]
- Mastrolonardo, M.; Alicino, D.; Zefferino, R.; Pasquini, P.; Picardi, A. Effect of psychological stress on salivary interleukin-1β in psoriasis. Arch. Med. Res. 2007, 38, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Zaher, H.; Shaker, O.G.; MH, E.L.-K.; El-Tawdi, A.; Fawzi, M.; Kadry, D. Serum and tissue expression of transforming growth factor beta 1 in psoriasis. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Lembo, S.; Capasso, R.; Balato, A.; Cirillo, T.; Flora, F.; Zappia, V.; Balato, N.; Ingrosso, D.; Ayala, F. MCP-1 in psoriatic patients: Effect of biological therapy. J. Dermatolog. Treat. 2014, 25, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Ganzetti, G.; Campanati, A.; Santarelli, A.; Sartini, D.; Molinelli, E.; Brisigotti, V.; Di Ruscio, G.; Bobyr, I.; Emanuelli, M.; Offidani, A. Salivary interleukin-1β: Oral inflammatory biomarker in patients with psoriasis. J. Int. Med. Res. 2016, 44, 10–14. [Google Scholar] [CrossRef]
- Solberg, S.M.; Sandvik, L.F.; Eidsheim, M.; Jonsson, R.; Bryceson, Y.T.; Appel, S. Serum cytokine measurements and biological therapy of psoriasis—Prospects for personalized treatment? Scand. J. Immunol. 2018, 88, e12725. [Google Scholar] [CrossRef]
- Choe, Y.B.; Hwang, Y.J.; Hahn, H.J.; Jung, J.W.; Jung, H.J.; Lee, Y.W.; Ahn, K.J.; Youn, J.I. A comparison of serum inflammatory cytokines according to phenotype in patients with psoriasis. Br. J. Dermatol. 2012, 167, 762–767. [Google Scholar] [CrossRef]
- Bilgiç, Ö.; Sivrikaya, A.; Toker, A.; Ünlü, A.; Altınyazar, C. Serum levels of TWEAK in patients with psoriasis vulgaris. Cytokine 2016, 77, 10–13. [Google Scholar] [CrossRef]
- Cataldi, C.; Mari, N.L.; Lozovoy, M.A.B.; Martins, L.M.M.; Reiche, E.M.V.; Maes, M.; Dichi, I.; Simão, A.N.C. Proinflammatory and anti-inflammatory cytokine profiles in psoriasis: Use as laboratory biomarkers and disease predictors. Inflamm. Res. 2019, 68, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Kutwin, M.; Migdalska-Sęk, M.; Brzeziańska-Lasota, E.; Zelga, P.; Woźniacka, A. An Analysis of IL-10, IL-17A, IL-17RA, IL-23A and IL-23R Expression and Their Correlation with Clinical Course in Patients with Psoriasis. J. Clin. Med. 2021, 10, 5834. [Google Scholar] [CrossRef]
- Philipp, S.; Menter, A.; Nikkels, A.F.; Barber, K.; Landells, I.; Eichenfield, L.F.; Song, M.; Randazzo, B.; Li, S.; Hsu, M.C.; et al. Ustekinumab for the treatment of moderate-to-severe plaque psoriasis in paediatric patients (≥6 to <12 years of age): Efficacy, safety, pharmacokinetic and biomarker results from the open-label CADMUS Jr study. Br. J. Dermatol. 2020, 183, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Olejniczak-Staruch, I.; Narbutt, J.; Bednarski, I.; Woźniacka, A.; Sieniawska, J.; Kraska-Gacka, M.; Śmigielski, J.; Lesiak, A. Interleukin 22 and 6 serum concentrations decrease under long-term biologic therapy in psoriasis. Postep. Dermatol. Alergol. 2020, 37, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.S.B.; Kvist-Hansen, A.; Siewertsen, M.; Enevold, C.; Hansen, P.R.; Kaur-Knudsen, D.; Zachariae, C.; Nielsen, C.H.; Loft, N.; Skov, L. Blood Cell Biomarkers of Inflammation and Cytokine Levels as Predictors of Response to Biologics in Patients with Psoriasis. Int. J. Mol. Sci. 2023, 24, 6111. [Google Scholar] [CrossRef]
- Koschitzky, M.; Navrazhina, K.; Garshick, M.S.; Gonzalez, J.; Han, J.; Garcet, S.; Krueger, J.G. Ustekinumab reduces serum protein levels associated with cardiovascular risk in psoriasis vulgaris. Exp. Dermatol. 2022, 31, 1341–1351. [Google Scholar] [CrossRef]
- Florian, T.-L.; Florian, I.-A.; Vesa, S.C.; Beni, L.; Orăsan, M. Inflammatory Cytokines and Clinical Outcome Following Biological Therapy in Adult Bio-Naïve Psoriasis Patients. Curr. Issues Mol. Biol. 2024, 46, 7719–7729. [Google Scholar] [CrossRef]
- Krueger, J.G.; Fretzin, S.; Suárez-Fariñas, M.; Haslett, P.A.; Phipps, K.M.; Cameron, G.S.; McColm, J.; Katcherian, A.; Cueto, I.; White, T.; et al. IL-17A is essential for cell activation and inflammatory gene circuits in subjects with psoriasis. J. Allergy Clin. Immunol. 2012, 130, 145–154.e149. [Google Scholar] [CrossRef]
- Carrier, Y.; Ma, H.L.; Ramon, H.E.; Napierata, L.; Small, C.; O’Toole, M.; Young, D.A.; Fouser, L.A.; Nickerson-Nutter, C.; Collins, M.; et al. Inter-regulation of Th17 cytokines and the IL-36 cytokines in vitro and in vivo: Implications in psoriasis pathogenesis. J. Investig. Dermatol. 2011, 131, 2428–2437. [Google Scholar] [CrossRef]
- Johnston, A.; Xing, X.; Wolterink, L.; Barnes, D.H.; Yin, Z.; Reingold, L.; Kahlenberg, J.M.; Harms, P.W.; Gudjonsson, J.E. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J. Allergy Clin. Immunol. 2017, 140, 109–120. [Google Scholar] [CrossRef]
- Pondeljak, N.; Lugović-Mihić, L.; Davidović, B.L.; Karlović, D.; Hanžek, M.; Neuberg, M. Serum Levels of IL-6 and TNF-α, Salivary Morning Cortisol and Intensity of Psychological Stress in Patients with Allergic Contact Hand Dermatitis and Healthy Subjects. Life 2025, 15, 351. [Google Scholar] [CrossRef] [PubMed]
| Cytokine | Serum Level in Psoriasis | Correlation with PASI (Disease Severity) | Key Functional Roles |
|---|---|---|---|
| TNF-α | Elevated (in most studies) | Mixed findings; some positive correlation | Major pro-inflammatory cytokine; therapeutic target |
| IFN-γ | Elevated | Positive correlation | Induces IL-6, IL-8, IL-12, IL-18; lesion formation |
| IL-6 | Elevated | Positive correlation | T cell activation; keratinocyte proliferation; biomarker |
| IL-8 | Elevated | Conflicting results | Neutrophil chemoattractant; erythema promotion |
| IL-10 | Decreased | Not correlated with severity | Anti-inflammatory; immunoregulatory |
| IL-12 | Decreased | Mixed data | Th1 differentiation; IFN-γ production |
| IL-16 | Elevated | Positive correlation | CD4+ lymphocyte recruitment; few studies |
| IL-17 | Mixed results | Inconsistent correlation | Synergizes with IFN-γ; pro-inflammatory role |
| IL-18 | Elevated | Positive correlation | Angiogenesis; immunoregulation; lesion spread |
| IL-20 | Elevated | Positive correlation | Late effector cytokine; keratinocyte dysregulation |
| IL-22 | Elevated | Positive correlation | Barrier defense; disease onset; keratinocyte effects |
| IL-30 | Elevated | Trend toward positive correlation | Modulates Th1/Th17 responses; autoimmune impact |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sora, A.; Hangan, T.; Chirila, S.I.; Gurgas, L.; Botnarciuc, M.; Daba, L.C.; Cretu, A.M.; Burlacu, I.; Zamfirescu, M.; Petcu, A.; et al. Salivary Interleukins as Non-Invasive Biomarkers for Psoriasis: Advances and Challenges in Diagnosis and Monitoring. Medicina 2025, 61, 1180. https://doi.org/10.3390/medicina61071180
Sora A, Hangan T, Chirila SI, Gurgas L, Botnarciuc M, Daba LC, Cretu AM, Burlacu I, Zamfirescu M, Petcu A, et al. Salivary Interleukins as Non-Invasive Biomarkers for Psoriasis: Advances and Challenges in Diagnosis and Monitoring. Medicina. 2025; 61(7):1180. https://doi.org/10.3390/medicina61071180
Chicago/Turabian StyleSora, Anna, Tony Hangan, Sergiu Ioachim Chirila, Leonard Gurgas, Mihaela Botnarciuc, Lavinia Carmen Daba, Ana Maria Cretu, Ionut Burlacu, Mihaela Zamfirescu, Adina Petcu, and et al. 2025. "Salivary Interleukins as Non-Invasive Biomarkers for Psoriasis: Advances and Challenges in Diagnosis and Monitoring" Medicina 61, no. 7: 1180. https://doi.org/10.3390/medicina61071180
APA StyleSora, A., Hangan, T., Chirila, S. I., Gurgas, L., Botnarciuc, M., Daba, L. C., Cretu, A. M., Burlacu, I., Zamfirescu, M., Petcu, A., Rosca, A. C., Stoicescu, R. M., & Petcu, L. C. (2025). Salivary Interleukins as Non-Invasive Biomarkers for Psoriasis: Advances and Challenges in Diagnosis and Monitoring. Medicina, 61(7), 1180. https://doi.org/10.3390/medicina61071180







