Long-Term Outcomes of Pediatric Cerebral Arteriovenous Malformations: A Ten-Year Single-Center Retrospective Study
Abstract
1. Introduction
2. Methods
2.1. Study Groups
2.2. Statistical Analysis
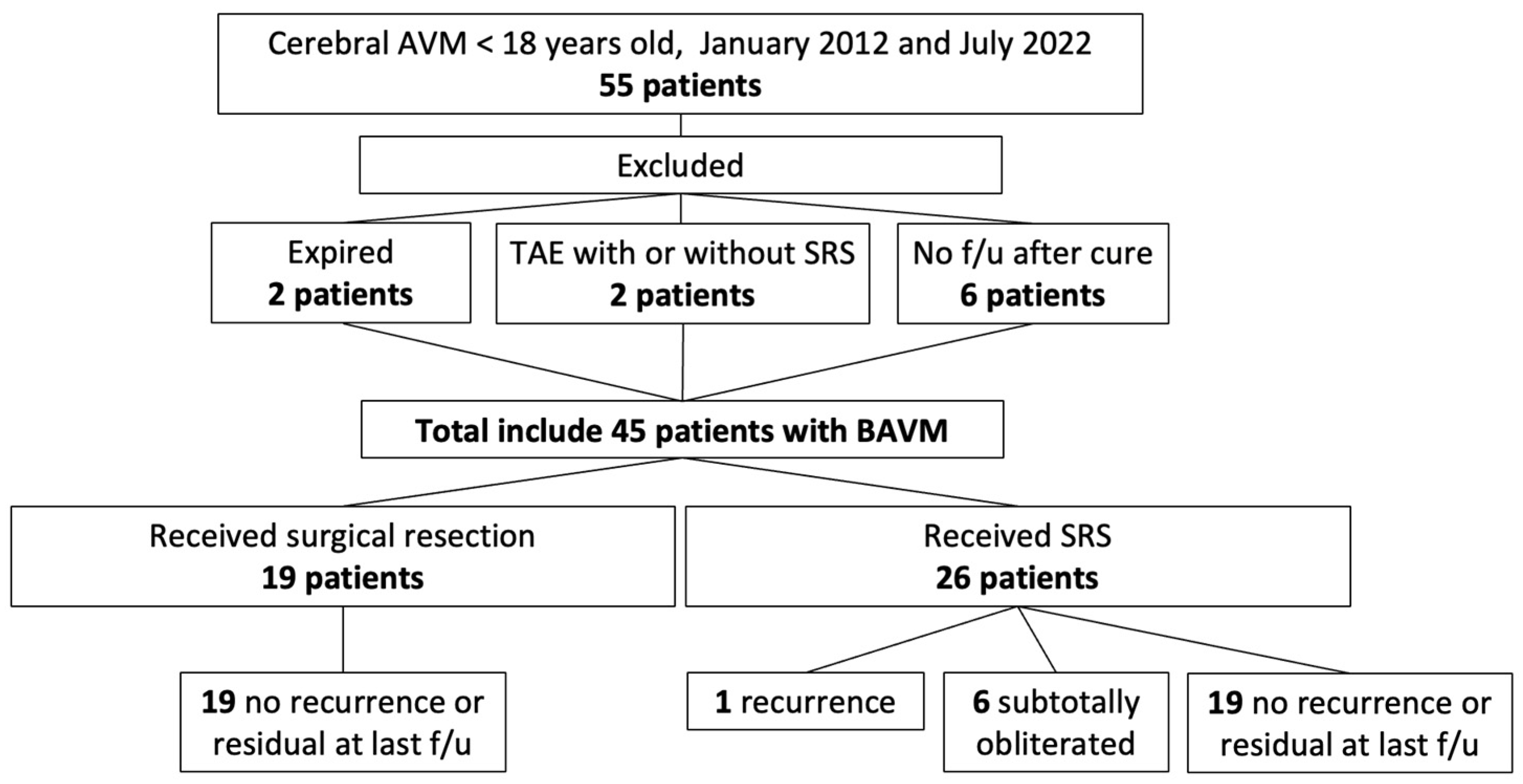
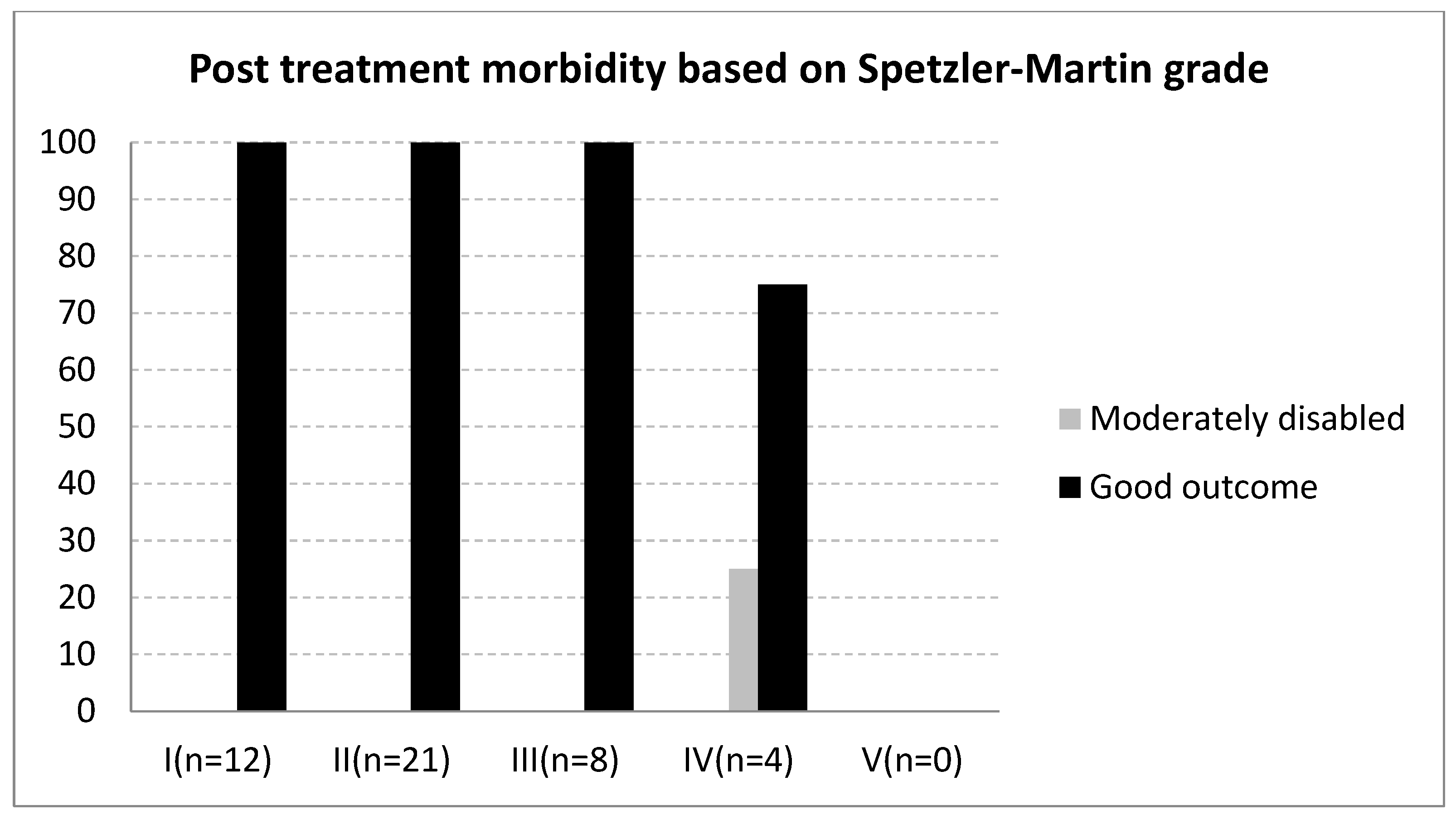
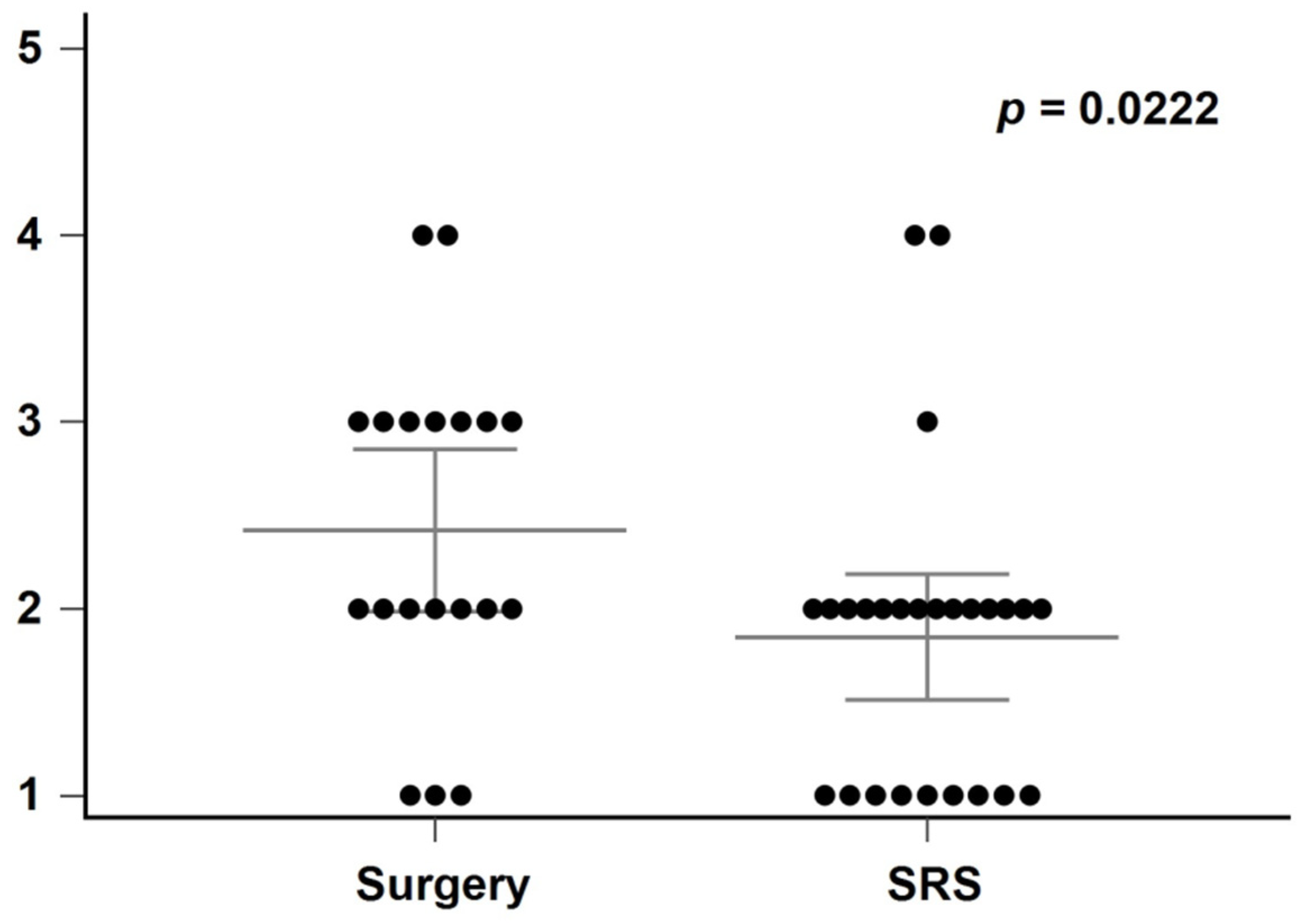
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antkowiak, L.; Putz, M.; Rogalska, M.; Mandera, M. Multimodal Treatment of Pediatric Ruptured Brain Arteriovenous Malformations: A Single-Center Study. Children 2021, 8, 215. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.D.; Staarmann, B.; Zuccarello, M. A Rational Approach to the Management of Cerebral Arteriovenous Malformations. World Neurosurg. 2022, 159, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Gross, B.A.; Du, R. Natural history of cerebral arteriovenous malformations: A meta-analysis. J. Neurosurg. 2013, 118, 437–443. [Google Scholar] [CrossRef]
- Brown, R.D., Jr. Simple risk predictions for arteriovenous malformation hemorrhage. Neurosurgery 2000, 46, 1024. [Google Scholar]
- Darsaut, T.E.; Guzman, R.; Marcellus, M.L.; Edwards, M.S.; Tian, L.; Do, H.M.; Chang, S.D.; Levy, R.P.; Adler, J.R.; Marks, M.P.; et al. Management of pediatric intracranial arteriovenous malformations: Experience with multimodality therapy. Neurosurgery 2011, 69, 540–556. [Google Scholar] [CrossRef]
- Gross, B.A.; Storey, A.; Orbach, D.B.; Scott, R.M.; Smith, E.R. Microsurgical treatment of arteriovenous malformations in pediatric patients: The Boston Children’s Hospital experience. J. Neurosurg. Pediatrics 2015, 15, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Sattari, S.A.; Shahbandi, A.; Yang, W.; Feghali, J.; Xu, R.; Huang, J. Microsurgery versus Microsurgery With Preoperative Embolization for Brain Arteriovenous Malformation Treatment: A Systematic Review and Meta-analysis. Neurosurgery 2023, 92, 27–41. [Google Scholar] [CrossRef]
- Brosnan, C.; Amoo, M.; Javadpour, M. Preoperative embolisation of brain arteriovenous malformations: A systematic review and meta-analysis. Neurosurg. Rev. 2022, 45, 2051–2063. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, C.J.; Ding, D.; Kano, H.; Mathieu, D.; Kondziolka, D.; Feliciano, C.; Rodriguez-Mercado, R.; Grills, I.S.; Barnett, G.; Lunsford, L.D.; et al. Stereotactic Radiosurgery for Pediatric Versus Adult Brain Arteriovenous Malformations. Stroke 2018, 49, 1939–1945. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Lee, C.C.; Kano, H.; Kearns, K.N.; Ding, D.; Tzeng, S.W.; Atik, A.; Joshi, K.; Barnett, G.H.; Huang, P.P.; et al. Stereotactic radiosurgery for pediatric brain arteriovenous malformations: Long-term outcomes. J. Neurosurg. Pediatr. 2020, 25, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.H.-C.; Kuo, Y.-H.; Guo, W.-Y.; Chung, W.-Y.; Wu, H.-M.; Liu, K.-D.; Chang, Y.-C.; Wang, L.-W.; Wong, T.-T. Gamma Knife surgery for cerebral arteriovenous malformations in children: A 13-year experience. J. Neurosurg. Pediatr. 2008, 1, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Spetzler, R.F.; Martin, N.A. A proposed grading system for arteriovenous malformations. J. Neurosurg. 1986, 65, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Lawton, M.T.; Kim, H.; McCulloch, C.E.; Mikhak, B.; Young, W.L. A supplementary grading scale for selecting patients with brain arteriovenous malformations for surgery. Neurosurgery 2010, 66, 702–713; discussion 713. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nataraj, A.; Mohamed, M.B.; Gholkar, A.; Vivar, R.; Watkins, L.; Aspoas, R.; Gregson, B.; Mitchell, P.; Mendelow, A.D. Multimodality Treatment of Cerebral Arteriovenous Malformations. World Neurosurg. 2014, 82, 149–159. [Google Scholar] [CrossRef]
- Kırış, T.; Sencer, A.; Şahinbaş, M.; Sencer, S.; İmer, M.; İzgi, N. Surgical results in pediatric Spetzler-Martin grades I–III intracranial arteriovenous malformations. Childs Nerv. Syst. 2005, 21, 69–74; discussion 75–76. [Google Scholar] [CrossRef] [PubMed]
- Reyns, N.; Blond, S.; Gauvrit, J.Y.; Touzet, G.; Coche, B.; Pruvo, J.P.; Dhellemmes, P. Role of radiosurgery in the management of cerebral arteriovenous malformations in the pediatric age group: Data from a 100-patient series. Neurosurgery 2007, 60, 268–276; discussion 276. [Google Scholar] [CrossRef]
- Yen, C.P.; Monteith, S.J.; Nguyen, J.H.; Rainey, J.; Schlesinger, D.J.; Sheehan, J.P. Gamma Knife surgery for arteriovenous malformations in children. J. Neurosurg. Pediatr. 2010, 6, 426–434. [Google Scholar] [CrossRef]
- Börcek, A.Ö.; Çeltikçi, E.; Aksoğan, Y.; Rousseau, M.J. Clinical Outcomes of Stereotactic Radiosurgery for Cerebral Arteriovenous Malformations in Pediatric Patients: Systematic Review and Meta-Analysis. Neurosurgery 2019, 85, E629–E640. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-J.; Ding, D.; Kumar, J.S.; Kearns, K.N.; Ironside, N.; Yang, H.-C.; Ogino, A.; Kano, H.; Liscak, R.; May, J.; et al. Hemorrhage and Recurrence of Obliterated Brain Arteriovenous Malformations Treated With Stereotactic Radiosurgery. Stroke 2022, 53, e363–e368. [Google Scholar] [CrossRef]
- Ali, M.J.; Bendok, B.R.; Rosenblatt, S.; Rose, J.E.; Getch, C.C.; Batjer, H.H. Recurrence of pediatric cerebral arteriovenous malformations after angiographically documented resection. Pediatr. Neurosurg. 2003, 39, 32–38. [Google Scholar] [CrossRef]
- Lauzier, D.C.; Vellimana, A.K.; Chatterjee, A.R.; Osbun, J.W.; Moran, C.J.; Zipfel, G.J.; Kansagra, A.P. Return of the lesion: A meta-analysis of 1134 angiographically cured pediatric arteriovenous malformations. J. Neurosurg. Pediatr. 2021, 28, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Sonstein, W.J.; Kader, A.; Michelsen, W.J.; Llena, J.F.; Hirano, A.; Casper, D. Expression of vascular endothelial growth factor in pediatric and adult cerebral arteriovenous malformations: An immunocytochemical study. J. Neurosurg. 1996, 85, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Gross, B.A.; Lai, P.M.R.; Du, R. Hydrocephalus after arteriovenous malformation rupture. Neurosurg. Focus 2013, 34, E11. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.P.; Varady, P.; Sheehan, J.; Steiner, M.; Steiner, L. Subtotal obliteration of cerebral arteriovenous malformations after Gamma Knife surgery. J. Neurosurg. 2007, 106, 361–369. [Google Scholar] [CrossRef]
- Bristol, R.E.; Albuquerque, F.C.; Spetzler, R.F.; Rekate, H.L.; McDougall, C.G.; Zabramski, J.M. Surgical management of arteriovenous malformations in children. J. Neurosurg. 2006, 105 (Suppl. S2), 88–93. [Google Scholar] [CrossRef]
- Blauwblomme, T.; Bourgeois, M.; Meyer, P.; Puget, S.; Di Rocco, F.; Boddaert, N.; Zerah, M.; Brunelle, F.; Rose, C.S.; Naggara, O. Long-term outcome of 106 consecutive pediatric ruptured brain arteriovenous malformations after combined treatment. Stroke 2014, 45, 1664–1671. [Google Scholar] [CrossRef]
- Stricker, S.; Boulouis, G.; Benichi, S.; Gariel, F.; Garzelli, L.; Beccaria, K.; Chivet, A.; Denis, T.d.S.; James, S.; Paternoster, G.; et al. Hydrocephalus in children with ruptured cerebral arteriovenous malformation. J. Neurosurg. Pediatr. 2020, 26, 283–287. [Google Scholar] [CrossRef]
- Pribil, S.; Boone, S.C.; Waley, R. Obstructive hydrocephalus at the anterior third ventricle caused by dilated veins from an arteriovenous malformation. Surg. Neurol. 1983, 20, 487–492. [Google Scholar] [CrossRef]
- Orešković, D.; Radoš, M.; Klarica, M. New Concepts of Cerebrospinal Fluid Physiology and Development of Hydrocephalus. Pediatr. Neurosurg. 2017, 52, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Theologou, M.; Natsis, K.; Kouskouras, K.; Chatzinikolaou, F.; Varoutis, P.; Skoulios, N.; Tsitouras, V.; Tsonidis, C. Cerebrospinal Fluid Homeostasis and Hydrodynamics: A Review of Facts and Theories. Eur. Neurol. 2022, 85, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Mindea, S.A.; Yang, B.P.; Batjer, H.H. Unruptured arteriovenous malformation in a patient presenting with obstructive hydrocephalus. Case report and review of the literature. Neurosurg. Focus. 2007, 22, E11. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Ai, X.; Hu, X.; Fang, F.; You, C. Clinical features and prognostic factors in patients with intraventricular hemorrhage caused by ruptured arteriovenous malformations. Medicine 2017, 96, e8544. [Google Scholar] [CrossRef] [PubMed]

| Variable | Patients (n = 45) | Number of Patients (%) | ||
|---|---|---|---|---|
| Male | 28 | 62.2 | ||
| Female | 17 | 37.8 | ||
| Age in years at diagnosis | 11.8 | - | ||
| Follow-up time in years | 6.4 | - | ||
| Initial presentation | ||||
| Consciousness disturbance | 21 | 46.6 | ||
| Seizure | 10 | 22.2 | ||
| Neurologic deficit | 6 | 13.3 | ||
| Limbs weakness | 3 | 6.7 | ||
| Unsteady gait | 2 | 4.4 | ||
| Aphasia | 1 | 2.2 | ||
| Headache | 7 | 15.5 | ||
| Vomiting | 1 | 2.2 | ||
| Spetzler-Martin grade | ||||
| I | 12 | 26.6 | ||
| II | 21 | 46.6 | ||
| III | 8 | 17.8 | ||
| IV | 4 | 8.8 | ||
| V | 0 | 0 | ||
| Modified Rankin Scale | Admission | Discharge |
|---|---|---|
| 0 | 1 (2.2) | 8 (17.8) |
| 1 | 19 (42.2) | 34 (75.6) |
| 2 | 11 (24.4) | 2 (4.4) |
| 3 | 4 (8.8) | 1 (2.2) |
| 4 | 4 (8.8) | 0 |
| 5 | 6 (13.3) | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiao, M.-C.; Tsuei, Y.-S.; Pan, H.-C.; Sun, M.-H.; Chen, W.-H.; Chen, H.-C.; Shen, C.-C.; Li, C.-R.; Chou, Y.-C. Long-Term Outcomes of Pediatric Cerebral Arteriovenous Malformations: A Ten-Year Single-Center Retrospective Study. Medicina 2025, 61, 1177. https://doi.org/10.3390/medicina61071177
Hsiao M-C, Tsuei Y-S, Pan H-C, Sun M-H, Chen W-H, Chen H-C, Shen C-C, Li C-R, Chou Y-C. Long-Term Outcomes of Pediatric Cerebral Arteriovenous Malformations: A Ten-Year Single-Center Retrospective Study. Medicina. 2025; 61(7):1177. https://doi.org/10.3390/medicina61071177
Chicago/Turabian StyleHsiao, Mei-Cheng, Yuang-Seng Tsuei, Hung-Chuan Pan, Ming-Hsi Sun, Wen-Hsien Chen, Hung-Chieh Chen, Chiung-Chyi Shen, Chi-Ruei Li, and Yu-Cheng Chou. 2025. "Long-Term Outcomes of Pediatric Cerebral Arteriovenous Malformations: A Ten-Year Single-Center Retrospective Study" Medicina 61, no. 7: 1177. https://doi.org/10.3390/medicina61071177
APA StyleHsiao, M.-C., Tsuei, Y.-S., Pan, H.-C., Sun, M.-H., Chen, W.-H., Chen, H.-C., Shen, C.-C., Li, C.-R., & Chou, Y.-C. (2025). Long-Term Outcomes of Pediatric Cerebral Arteriovenous Malformations: A Ten-Year Single-Center Retrospective Study. Medicina, 61(7), 1177. https://doi.org/10.3390/medicina61071177








