The Impact of Frailty Indices on Predicting Complications and Functional Recovery in Proximal Humerus Fractures: A Comparative Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Study Design and Patient Selection
2.3. Treatment Allocation
2.4. Follow-Up Protocol
2.5. Fracture Severity and Classification
2.6. Inclusion and Exclusion Criteria
2.6.1. Inclusion Criteria
- Patients aged ≥ 60 years with unilateral PHF.
- Diagnosis confirmed via physical exam, radiography, and CT.
- First-time ipsilateral upper extremity fracture.
- Minimum of 24 months follow-up.
- Available and complete medical records.
2.6.2. Exclusion Criteria
- Multiple or bilateral fractures.
- Pathological fractures.
- History of previous shoulder or upper limb fractures.
- Preoperative systemic infection or sepsis.
- Neurological or malignant conditions causing pre-existing upper limb dysfunction.
- Incomplete follow-up or missing clinical data.
2.7. Patient Demographics
2.8. Frailty and Functional Assessment
2.8.1. ASA Score
2.8.2. mFI-5
2.8.3. EFS
2.8.4. CFS
2.8.5. TSFI
2.8.6. ASES Shoulder Score
2.9. Statistical Analysis
3. Results
3.1. Demographic Characteristics
3.2. Frailty Index Distribution
3.3. Relationship Between Frailty and Complications/Outcomes
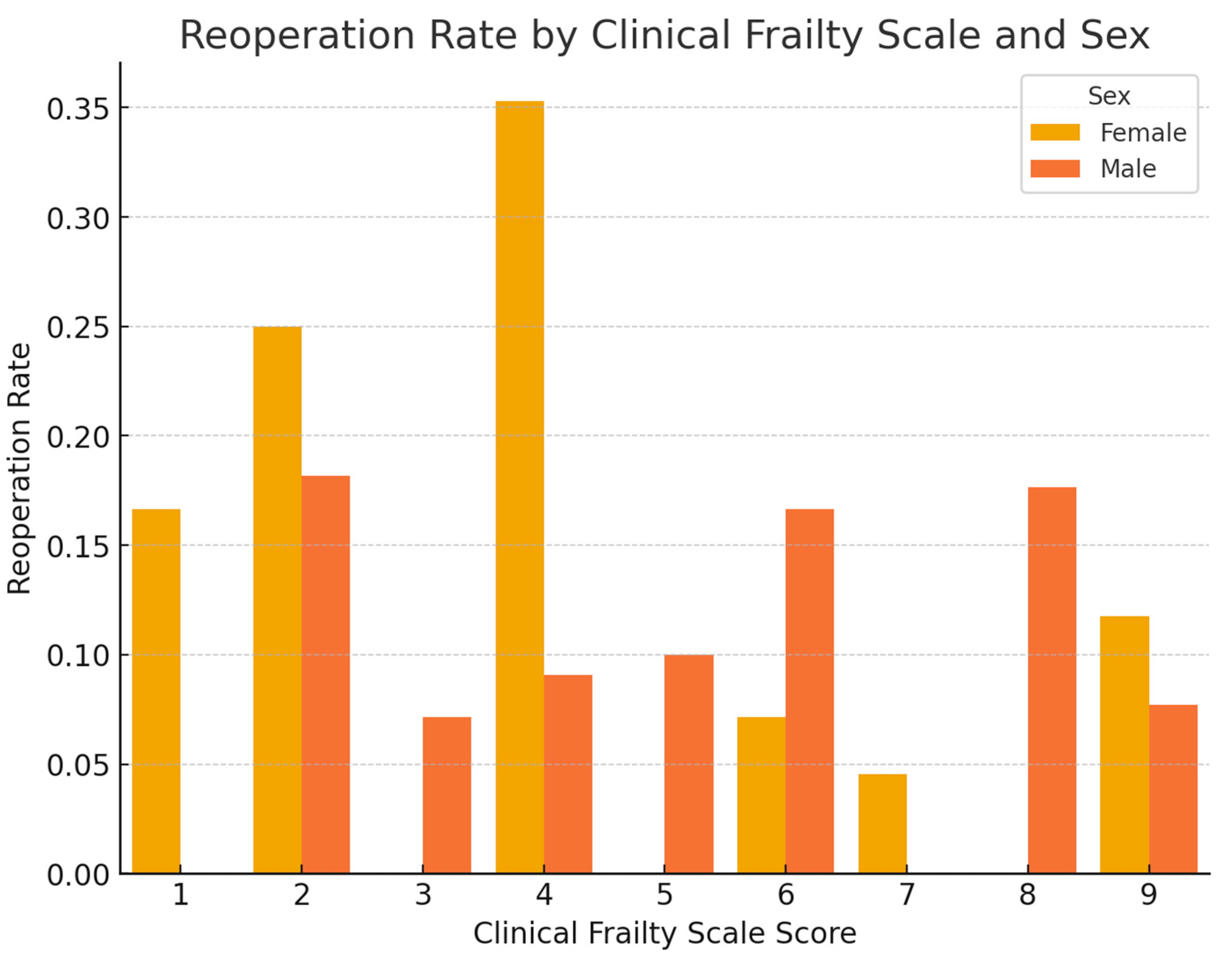
3.4. Visual Presentation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| mFI-5 | Modified Frailty Index-5 |
| EFS | Edmonton Frail Scale |
| CFS | Clinical Frailty Scale |
| TSFI | Trauma-Specific Frailty Index |
| PHFs | Proximal humerus fractures |
| ASA | American Society of Anesthesiologists |
| ASES | American Shoulder and Elbow Surgeons |
| ORIF | Open Reduction and Internal Fixation |
| CT | Computed Tomography |
| BMI | Body Mass Index |
References
- Bingol, I.; Kamaci, S.; Yilmaz, E.T.; Oral, M.; Yasar, N.E.; Dumlupinar, E.; Ata, N.; Ulgu, M.; Birinci, S.; Bayram, S.; et al. The epidemiology of geriatric fractures: A nationwide analysis of 1 million fractures. Injury 2024, 55, 111900. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.; McClung, M.R.; Tran, O.; Morrow, C.D.; Goldstein, S.; Kagan, R.; McDermott, M.; Yehoshua, A. Treatment rates and healthcare costs of patients with fragility fracture by site of care: A real-world data analysis. Arch. Osteoporos. 2023, 18, 42. [Google Scholar] [CrossRef] [PubMed]
- Spoden, M.; Dröge, P.; Günster, C.; Datzmann, T.; Helfen, T.; Schaser, K.-D.; Schmitt, J.; Schuler, E.; Katthagen, J.C.; Nowotny, J. A high hospital frailty risk score indicates an increased risk for complications following surgical treatment of proximal humerus fractures. Arch. Gerontol. Geriatr. 2025, 128, 105598. [Google Scholar] [CrossRef] [PubMed]
- Verboket, R.D.; Wendt, K.W.; Janko, M.; Marzi, I. Prosthetic treatment of proximal humerus fractures in the elderly. Eur. J. Trauma Emerg. Surg. 2025, 51, 1–12. [Google Scholar] [CrossRef]
- Gupta, N.K.; Dunivin, F.; Chmait, H.R.; Smitterberg, C.; Buttar, A.; Fazal-Ur-Rehman, M.; Manes, T.; Turnow, M.; Williamson, T.K.; Taylor, B.C.; et al. Orthopedic frailty risk stratification (OFRS): A systematic review of the frailty indices predicting adverse outcomes in orthopedics. J. Orthop. Surg. Res. 2025, 20, 247. [Google Scholar] [CrossRef]
- Launonen, A.P.; Lepola, V.; Flinkkilä, T.; Laitinen, M.; Paavola, M.; Malmivaara, A. Treatment of proximal humerus fractures in the elderly: A systemic review of 409 patients. Acta Orthop. 2015, 86, 280–285. [Google Scholar] [CrossRef]
- Dimitriou, D.; Waldmann, S.; Antoniadis, A.; Liebhauser, M.; Helmy, N.; Riede, U. Early locking plate removal following open reduction and internal fixation of proximal humeral fractures could prevent secondary implant-related complications. J. Orthop. 2020, 17, 106–109. [Google Scholar] [CrossRef]
- Pai, S.; Kotekar, M.F.; Pawaskar, S.M.; Kumar, M.A. Proximal Humerus Fractures in the Elderly. Indian J. Orthop. 2024, 59, 346–357. [Google Scholar] [CrossRef]
- Zhu, X.; Ding, C.; Zhu, Y.; Nian, S.; Tang, H. A comparative study of locking plate combined with minimally invasive plate osteosynthesis and intramedullary nail fixation in the treatment of Neer classification of two-part and three-part fractures of the proximal humerus. Eur. J. Orthop. Surg. Traumatol. 2024, 34, 2743–2749. [Google Scholar] [CrossRef]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older adults: Evidence for a phenotype. J. Gerontol. Ser. A 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Sagona, A.B.; Ortega, C.A.B.; Wang, L.; Brameier, D.T.B.; Selzer, F.; Zhou, L.; von Keudell, A. Frailty Is More Predictive of Mortality than Age in Patients with Hip Fractures. J. Orthop. Trauma 2024, 38, e278–e287. [Google Scholar] [CrossRef]
- Perea, L.L.; Fort, L.S.; Morgan, M.E.; Brown, C.T.; Wang, S.; Bradburn, E. Frailty Is Associated with Worse Outcomes in Geriatric Pelvic Fractures. Am. Surg. 2022, 88, 1573–1575. [Google Scholar] [CrossRef]
- Tan, B.H.; Poon, K.B. Perioperative and frailty outcomes after total knee arthroplasty: A retrospective cohort study. Singap. Med. J. 2024. [Google Scholar] [CrossRef]
- Herteleer, M. Navigation assisted removal of a broken cannulated sacroiliac screw: A technical note. Injury 2023, 54, 1222–1225. [Google Scholar] [CrossRef]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef]
- Cesari, M.; Gambassi, G.; van Kan, G.A.; Vellas, B. The frailty phenotype and the frailty index: Different instruments for different purposes. Age Ageing 2014, 43, 10–12. [Google Scholar] [CrossRef]
- Samuels, J.M.; Carmichael, H.; McIntyre, R.J.; Urban, S.B.; Ballow, S.D.; Dirks, R.C.; Spalding, M.D.; LaRiccia, A.D.; Farrell, M.S.; Stein, D.M.; et al. An observation-first strategy for liver injuries with “blush” on computed tomography is safe and effective. J. Trauma Acute Care Surg. 2022, 94, 281–287. [Google Scholar] [CrossRef]
- Michener, L.A.; McClure, P.W.; Sennett, B.J. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: Reliability, validity, and responsiveness. J. Shoulder Elb. Surg. 2022, 54, 587–594. [Google Scholar] [CrossRef]
- Stead, T.S.; Chen, T.H.; Maslow, A.; Asher, S. Utility of Frailty Index in Predicting Adverse Outcomes in Patients With the Same American Society of Anesthesiologists Class in Video-assisted Thoracoscopic Surgery. J. Cardiothorac Vasc. Anesth. 2025, 39, 187–195. [Google Scholar] [CrossRef]
- Bolliger, M.; Kroehnert, J.-A.; Molineus, F.; Kandioler, D.; Schindl, M.; Riss, P. Experiences with the standardized classification of surgical complications (Clavien-Dindo) in general surgery patients. Eur. Surg. 2018, 50, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, J.M.; Garmon, E.H. American Society of Anesthesiologists Physical Status Classification System. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Erne, F.; Wallmeier, V.; Ihle, C.; Braun, B.J.; Ehnert, S.; Histing, T.; Nüssler, A.K.; Maurer, E. The modified 5-item frailty index determines the length of hospital stay and accompanies with mortality rate in patients with bone and implant-associated infections after trauma and orthopedic surgery. Injury 2023, 54, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Aygör, H.E.; Fadıloğlu, Ç.; Şahin, S.; Aykar, F.Ş.; Akçiçek, F. Validation of edmonton frail scale into elderly turkish population. Arch. Gerontol. Geriatr. 2018, 76, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Church, S.; Rogers, E.; Rockwood, K.; Theou, O. A scoping review of the Clinical Frailty Scale. BMC Geriatr. 2020, 20, 393. [Google Scholar] [CrossRef]
- Joseph, B.; Pandit, V.; Zangbar, B.; Kulvatunyou, N.; Tang, A.; O’Keeffe, T.; Green, D.J.; Vercruysse, G.; Fain, M.J.; Friese, R.S.; et al. Validating Trauma-Specific Frailty Index for Geriatric Trauma Patients: A Prospective Analysis. J. Am. Coll. Surg. 2014, 219, 10–17e1. [Google Scholar] [CrossRef]
- Efremov, K.; Glass, E.A.; Swanson, D.P.; Bowler, A.R.; Le, K.; Kirsch, J.M.; Jawa, A. Outcomes after reverse shoulder arthroplasty for the treatment of glenohumeral osteoarthritis in patients under and over 70 years of age: A propensity score-matched analysis. J. Shoulder Elb. Surg. 2024, 33, 1547–1554. [Google Scholar] [CrossRef]
- Segal, D.N.; Wilson, J.M.; Staley, C.; Michael, K.W. The 5-Item Modified Frailty Index Is Predictive of 30-Day Postoperative Complications in Patients Undergoing Kyphoplasty Vertebral Augmentation. World Neurosurg. 2018, 116, e225–e231. [Google Scholar] [CrossRef]
- Panayi, A.; Orkaby, A.; Sakthivel, D.; Endo, Y.; Varon, D.; Roh, D.; Orgill, D.; Neppl, R.; Javedan, H.; Bhasin, S.; et al. Impact of frailty on outcomes in surgical patients: A systematic review and meta-analysis. Am. J. Surg. 2019, 218, 393–400. [Google Scholar] [CrossRef]
- Han, D.; Li, J.; Cui, P.; Wang, S.-K.; Wang, P.; Lu, S.-B. Comparison of three frailty evaluation tools in predicting postoperative adverse events in older patients undergoing lumbar fusion surgery: A prospective cohort study of 240 patients. Eur. Spine J. 2025, 34, 1714–1749. [Google Scholar] [CrossRef]
- Ruzbarsky, J.J.; Rauck, R.C.; Manzi, J.; Khormaee, S.; Jivanelli, B.; Warren, R.F. The fragility of findings of randomized controlled trials in shoulder and elbow surgery. J. Shoulder Elbow Surg. 2019, 28, 2409–2417. [Google Scholar] [CrossRef]
- Maman, G.; Chechik, O.; Kazum, E.; Bivas, A.; Maman, E.; Rotman, D. 5-Year Mortality After Complex Displaced Proximal Humerus Fractures in Elderly Patients: Conservative Versus Reverse Total Shoulder Arthroplasty. J. Clin. Med. 2024, 14, 167. [Google Scholar] [CrossRef] [PubMed]
- Holierook, M.; Henstra, M.J.; Dolman, D.J.; Chekanova, E.V.; Veenis, L.; Beijk, M.A.; de Winter, R.J.; Baan, J.; Vis, M.M.; Lemkes, J.S.; et al. Higher Edmonton Frail Scale prior to transcatheter Aortic Valve Implantation is related to longer hospital stay and mortality. Int. J. Cardiol. 2024, 399, 131637. [Google Scholar] [CrossRef]
- Evans, D.R.; Saltzman, E.B.; Anastasio, A.T.; Guisse, N.F.; Belay, E.S.; Pidgeon, T.S.; Richard, M.J.; Ruch, D.S.; Anakwenze, O.A.; Gage, M.J.; et al. Use of a 5-item modified Fragility Index for risk stratification in patients undergoing surgical management of proximal humerus fractures. JSES Int. 2020, 5, 212–219. [Google Scholar] [CrossRef]
- Niedermeyer, S.; Leiber, M.; Stöcklein, S.; Weig, T.; Terpolilli, N.A. Impact of frailty in elderly patients undergoing decompressive craniectomy after traumatic brain injury. Neurosurg. Rev. 2025, 48, 297. [Google Scholar] [CrossRef]
- Migliorini, F.; Giorgino, R.; Hildebrand, F.; Spiezia, F.; Peretti, G.M.; Alessandri-Bonetti, M.; Eschweiler, J.; Maffulli, N. Fragility Fractures: Risk Factors and Management in the Elderly. Medicina 2021, 57, 1119. [Google Scholar] [CrossRef]
- Joseph, B.; Saljuqi, A.T.; Amos, J.D.; Teichman, A.; Whitmill, M.L.; Anand, T.; Hosseinpour, H.; Burruss, S.K.; Dunn, J.A.; Najafi, K.; et al. Prospective validation and application of the Trauma-Specific Frailty Index: Results of an American Association for the Surgery of Trauma multi-institutional observational trial. J. Trauma Acute Care Surg. 2023, 94, 36–44. [Google Scholar] [CrossRef]
- Baker, H.P.; Gutbrod, J.; Cahill, M.; Shi, L. Optimal Treatment of Proximal Humeral Fractures in the Elderly: Risks and Management Challenges. Orthop. Res. Rev. 2023, 15, 129–137. [Google Scholar] [CrossRef]
- Kweh, B.T.S.; Lee, H.Q.; Tan, T.; Liew, S.; Hunn, M.; Wee Tee, J. Posterior Instrumented Spinal Surgery Outcomes in the Elderly: A Comparison of the 5-Item and 11-Item Modified Frailty Indices. Global Spine J. 2024, 14, 593–602. [Google Scholar] [CrossRef]
- Yi, B.C.; Gowd, A.K.; Agarwalla, A.; Chen, E.; Amin, N.H.; Nicholson, G.P.; Romeo, A.A.; Liu, J.N. Efficacy of the modified Frailty Index and the modified Charlson Comorbidity Index in predicting complications in patients undergoing operative management of proximal humerus fracture. J. Shoulder Elbow Surg. 2021, 30, 658–667. [Google Scholar] [CrossRef]
- Kyriakides, P.W.; Schultz, B.J.; Egol, K.; Leucht, P. The fragility and reverse fragility indices of proximal humerus fracture randomized controlled trials: A systematic review. Eur. J. Trauma Emerg. Surg. 2022, 48, 4545–4552. [Google Scholar] [CrossRef]





| Variable | Category | Surgical (n) | Conservative (n) | p-Value |
|---|---|---|---|---|
| Age Group | 65–74 | 49 | 55 | 0.6140 |
| 75–84 | 33 | 35 | 0.6140 | |
| 85 and above | 9 | 12 | 0.6140 | |
| Under 65 | 19 | 32 | 0.6140 | |
| Sex | Female | 65 | 76 | 0.8077 |
| Male | 45 | 58 | 0.8077 | |
| Smoking | No | 80 | 121 | <0.001 |
| Yes | 30 | 13 | <0.001 | |
| ASA Classification | ASA I | 9 | 0 | 1.0000 |
| ASA II | 62 | 0 | 1.0000 | |
| ASA III | 36 | 0 | 1.0000 | |
| ASA IV | 3 | 0 | 1.0000 | |
| BMI Category | Normal weight | 26 | 39 | 0.3049 |
| Obese | 43 | 60 | 0.3049 | |
| Overweight | 21 | 19 | 0.3049 | |
| Underweight | 20 | 16 | 0.3049 | |
| mFI-5 Classification | High frailty | 42 | 40 | 0.2750 |
| Low frailty | 28 | 45 | 0.2750 | |
| Moderate frailty | 40 | 49 | 0.2750 | |
| EFS Classification | Mild frailty | 11 | 19 | 0.4622 |
| Moderate frailty | 17 | 14 | 0.4622 | |
| No/minimal frailty | 33 | 46 | 0.4622 | |
| Severe frailty | 49 | 55 | 0.4622 | |
| CFS Classification | Fit/Well | 35 | 48 | 0.8268 |
| Mild frailty | 22 | 28 | 0.8268 | |
| Moderate-severe frailty | 23 | 28 | 0.8268 | |
| Very severe frailty | 30 | 30 | 0.8268 | |
| TSFI Classification | Frail | 86 | 100 | 0.1148 |
| Non-frail | 16 | 30 | 0.1148 | |
| Pre-frail | 8 | 4 | 0.1148 | |
| ASES Functional Outcome | Excellent | 17 | 36 | 0.0060 |
| Fair | 42 | 33 | 0.0060 | |
| Good | 35 | 56 | 0.0060 | |
| Poor | 16 | 9 | 0.0060 |
| Frailty Index | Category | Age (Mean ± SD) | Male Count (%) | Female Count (%) | Smoking (%) | Length of Stay (Mean ± SD) | Reoperation Rate (%) | Readmission Rate (%) | Complication Rate (%) | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|
| mFI-5 | Low | 71.8 ± 9.3 | 20 (55.6%) | 16 (44.4%) | 19.4% | 1.9 ± 2.7 | 2.8% | 11.1% | 11.1% | 0.007 |
| Moderate | 71.9 ± 8.0 | 33 (37.1%) | 56 (62.9%) | 14.6% | 3.5 ± 7.7 | 7.9% | 13.5% | 11.2% | 0.023 | |
| High | 72.6 ± 8.5 | 31 (37.8%) | 51 (62.2%) | 24.4% | 4.7 ± 8.3 | 22.0% | 37.8% | 34.1% | 0.001 | |
| Edmonton | No/Mild | 72.3 ± 8.2 | 21 (35.6%) | 38 (64.4%) | 11.9% | 2.9 ± 6.1 | 3.4% | 6.8% | 10.2% | 0.000 |
| Mild | 74.3 ± 9.5 | 9 (30.0%) | 21 (70.0%) | 30.0% | 2.3 ± 8.4 | 3.3% | 16.7% | 10.0% | 0.085 | |
| Moderate | 70.4 ± 7.5 | 18 (58.1%) | 13 (41.9%) | 19.4% | 3.2 ± 5.7 | 9.7% | 32.3% | 16.1% | 0.408 | |
| Severe | 72.1 ± 8.4 | 44 (42.3%) | 60 (57.7%) | 17.3% | 4.0 ± 7.7 | 16.3% | 22.1% | 21.2% | 0.288 | |
| CFS | Fit | 70.9 ± 8.0 | 37 (44.6%) | 46 (55.4%) | 18.1% | 2.5 ± 4.6 | 10.8% | 16.9% | 14.5% | 0.022 |
| Mild | 73.2 ± 8.8 | 21 (42.0%) | 29 (58.0%) | 22.0% | 3.3 ± 8.1 | 16.0% | 28.0% | 22.0% | ||
| Moderate | 72.8 ± 7.8 | 15 (29.4%) | 36 (70.6%) | 13.7% | 3.8 ± 8.3 | 5.9% | 17.6% | 11.8% | ||
| Severe | 72.4 ± 8.4 | 30 (50.0%) | 30 (50.0%) | 16.7% | 3.9 ± 7.4 | 10.0% | 20.0% | 21.7% | ||
| TSFI | Non-frail | 70.9 ± 8.1 | 20 (43.5%) | 26 (56.5%) | 21.7% | 4.0 ± 9.5 | 8.7% | 17.4% | 15.2% | 0.785 |
| Prefrail | 69.7 ± 7.6 | 7 (50.0%) | 7 (50.0%) | 14.3% | 5.8 ± 12.0 | 14.3% | 28.6% | 14.3% | ||
| Frail | 72.6 ± 8.3 | 76 (41.3%) | 108 (58.7%) | 16.8% | 2.9 ± 5.5 | 10.9% | 20.1% | 17.9% |
| Complication Type | Conservative (n) | Surgical (n) | Surgical (%) | Conservative (%) | p-Value |
|---|---|---|---|---|---|
| Acute Kidney Failure | 0 | 3 | 10.7% | 0.0% | 0.5390 |
| Deep Vein Thrombosis | 0 | 3 | 10.7% | 0.0% | 0.5390 |
| Implant Failure | 0 | 2 | 7.1% | 0.0% | 0.5447 |
| Nonunion | 2 | 6 | 21.4% | 14.3% | 0.6969 |
| Osteonecrosis | 0 | 3 | 10.7% | 0.0% | 0.5390 |
| Pneumonia | 1 | 4 | 14.3% | 7.1% | 0.6496 |
| Pulmonary Embolism | 1 | 3 | 10.7% | 7.1% | 1.0000 |
| Wound Dehiscence | 0 | 4 | 14.3% | 0.0% | 0.2829 |
| ASES Score (Mean ± SD) | 75.0 ± 17.2 | 66.8 ± 20.1 | 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özdemir, E.; Özdeş, O.O.; Topsakal, F.E.; Altay, N.; Demirel, E. The Impact of Frailty Indices on Predicting Complications and Functional Recovery in Proximal Humerus Fractures: A Comparative Study. Medicina 2025, 61, 1169. https://doi.org/10.3390/medicina61071169
Özdemir E, Özdeş OO, Topsakal FE, Altay N, Demirel E. The Impact of Frailty Indices on Predicting Complications and Functional Recovery in Proximal Humerus Fractures: A Comparative Study. Medicina. 2025; 61(7):1169. https://doi.org/10.3390/medicina61071169
Chicago/Turabian StyleÖzdemir, Ekrem, Oya Olcay Özdeş, Fatih Emre Topsakal, Nasuhi Altay, and Esra Demirel. 2025. "The Impact of Frailty Indices on Predicting Complications and Functional Recovery in Proximal Humerus Fractures: A Comparative Study" Medicina 61, no. 7: 1169. https://doi.org/10.3390/medicina61071169
APA StyleÖzdemir, E., Özdeş, O. O., Topsakal, F. E., Altay, N., & Demirel, E. (2025). The Impact of Frailty Indices on Predicting Complications and Functional Recovery in Proximal Humerus Fractures: A Comparative Study. Medicina, 61(7), 1169. https://doi.org/10.3390/medicina61071169






