Assessment of Pulmonary Function After Treatment of Scoliosis: Meta-Analysis and Review Article
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction
2.4. Quality Assessment
2.5. Data Analysis
3. Results
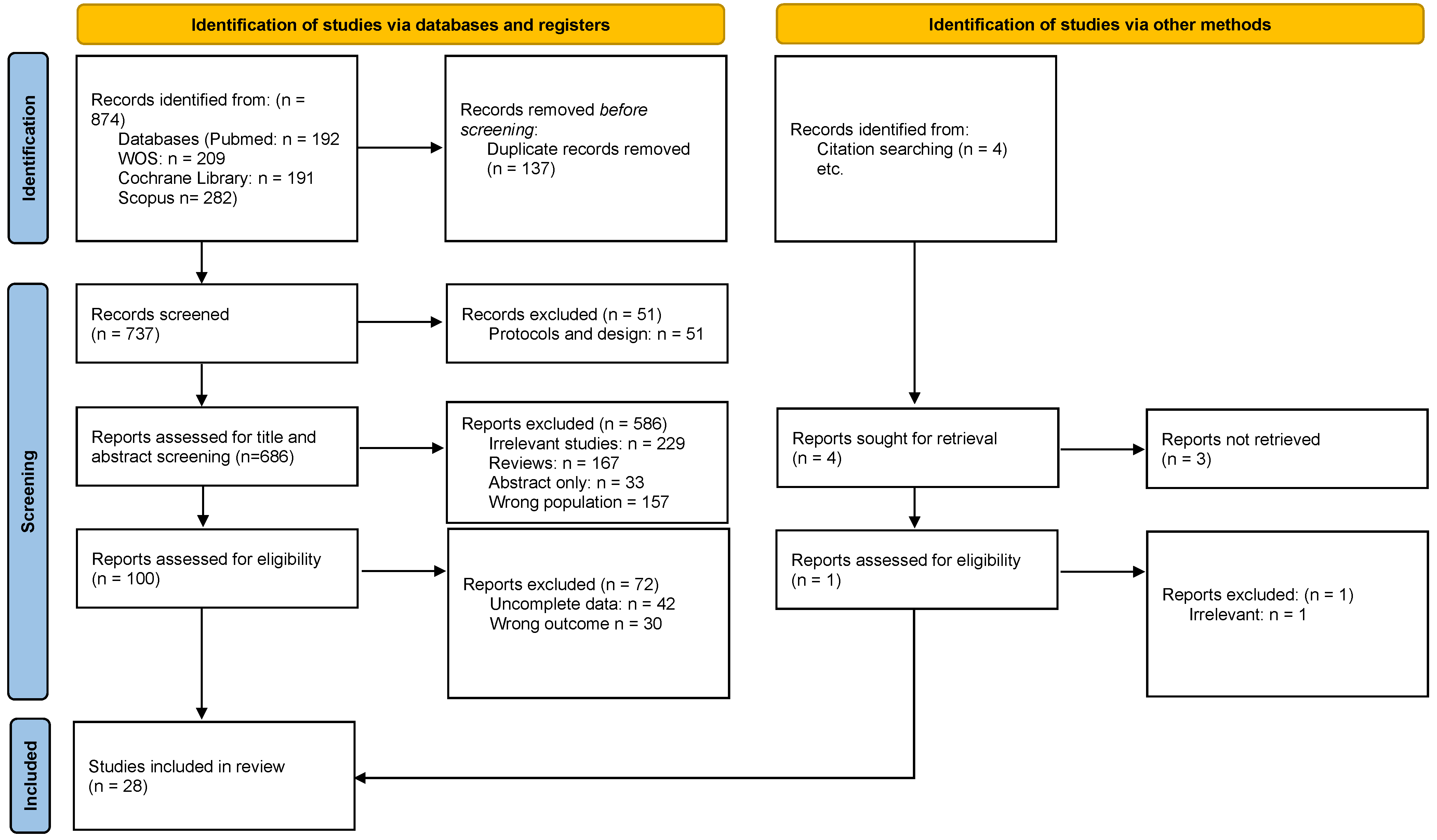
3.1. Study Selection and Characteristics
3.2. Demographic Characteristics of Included Studies
3.3. FVC Outcomes
3.4. FEV1 Outcomes
3.5. PEF Outcomes
3.6. Other Outcomes
3.7. Outcomes According to Intervention Type
3.7.1. Breathing and Exercise-Based Interventions
3.7.2. Surgical Interventions
3.8. Comparing Surgical Approaches
3.9. Scoliosis Surgery With or Without Breathing Exercises
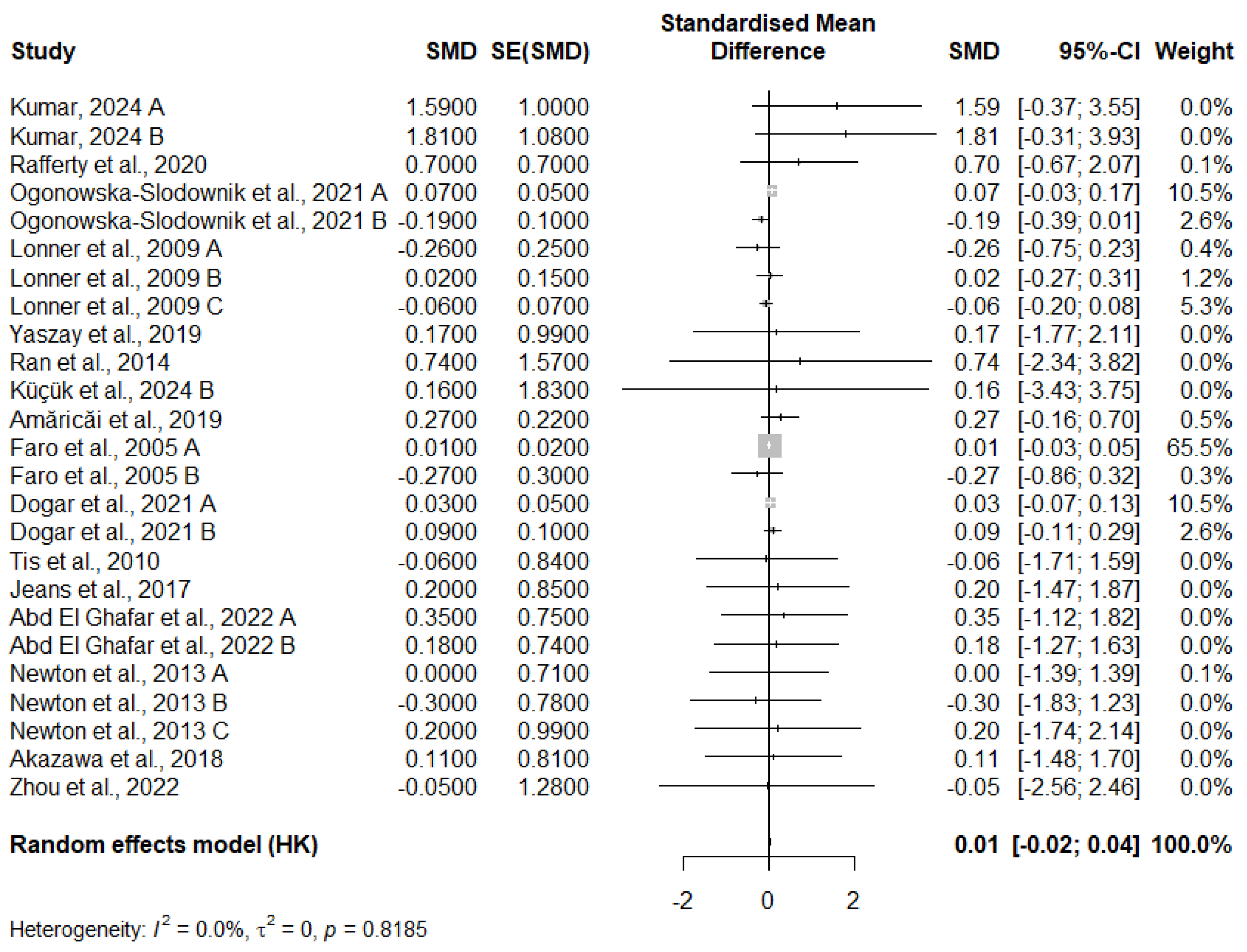
3.10. Meta-Analysis of FVC Change
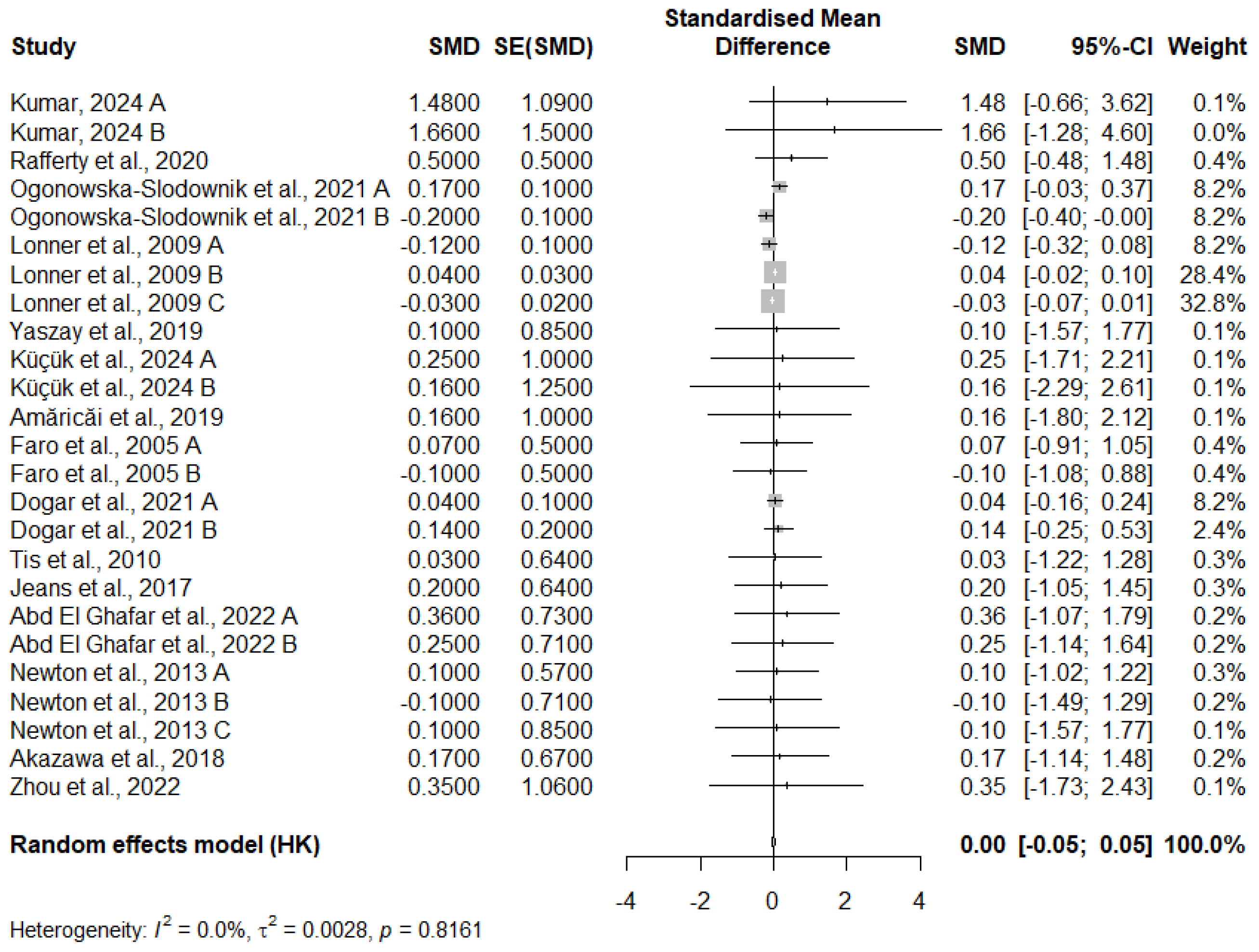
3.11. Meta-Analysis of FEV1 Change
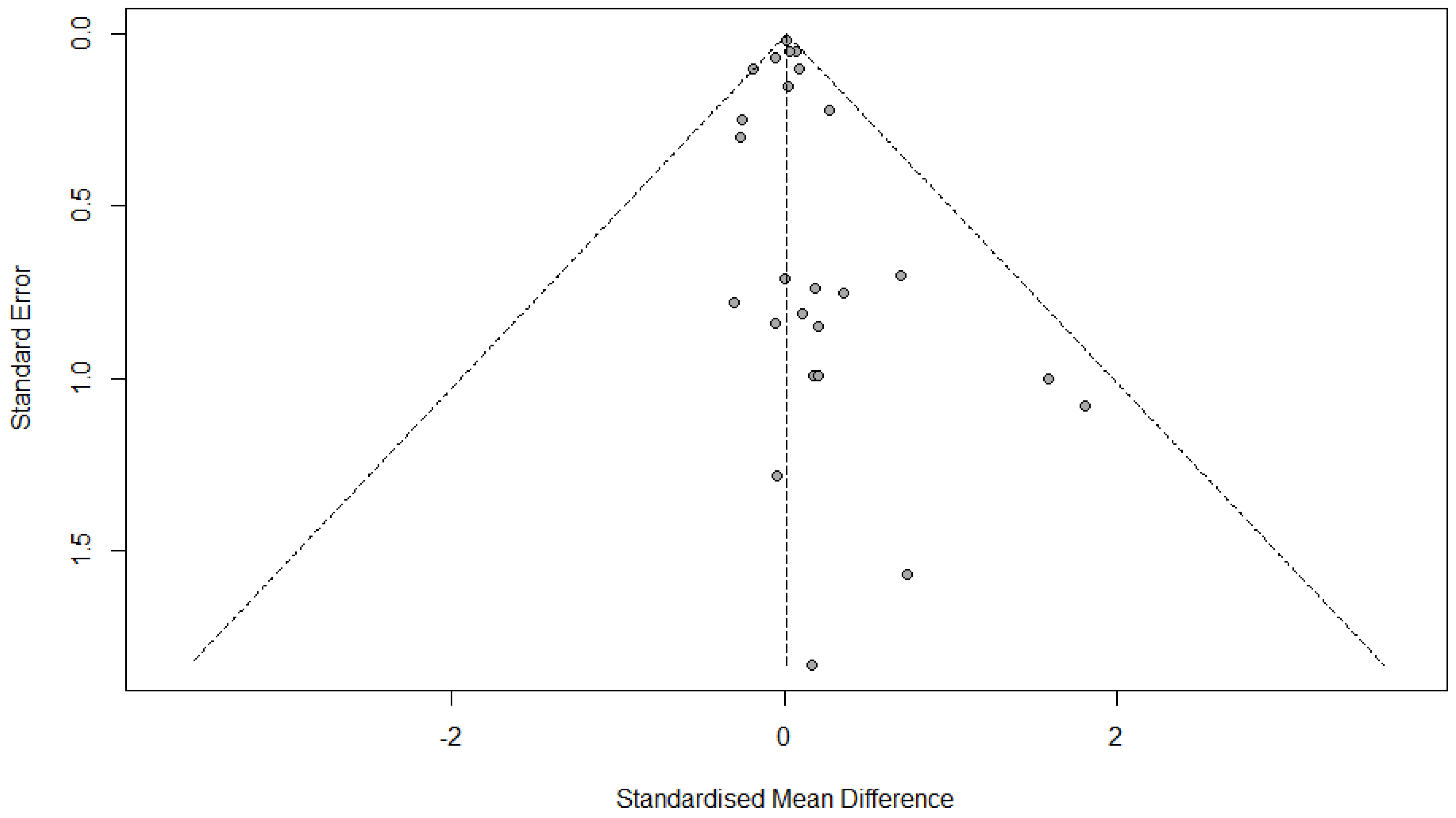
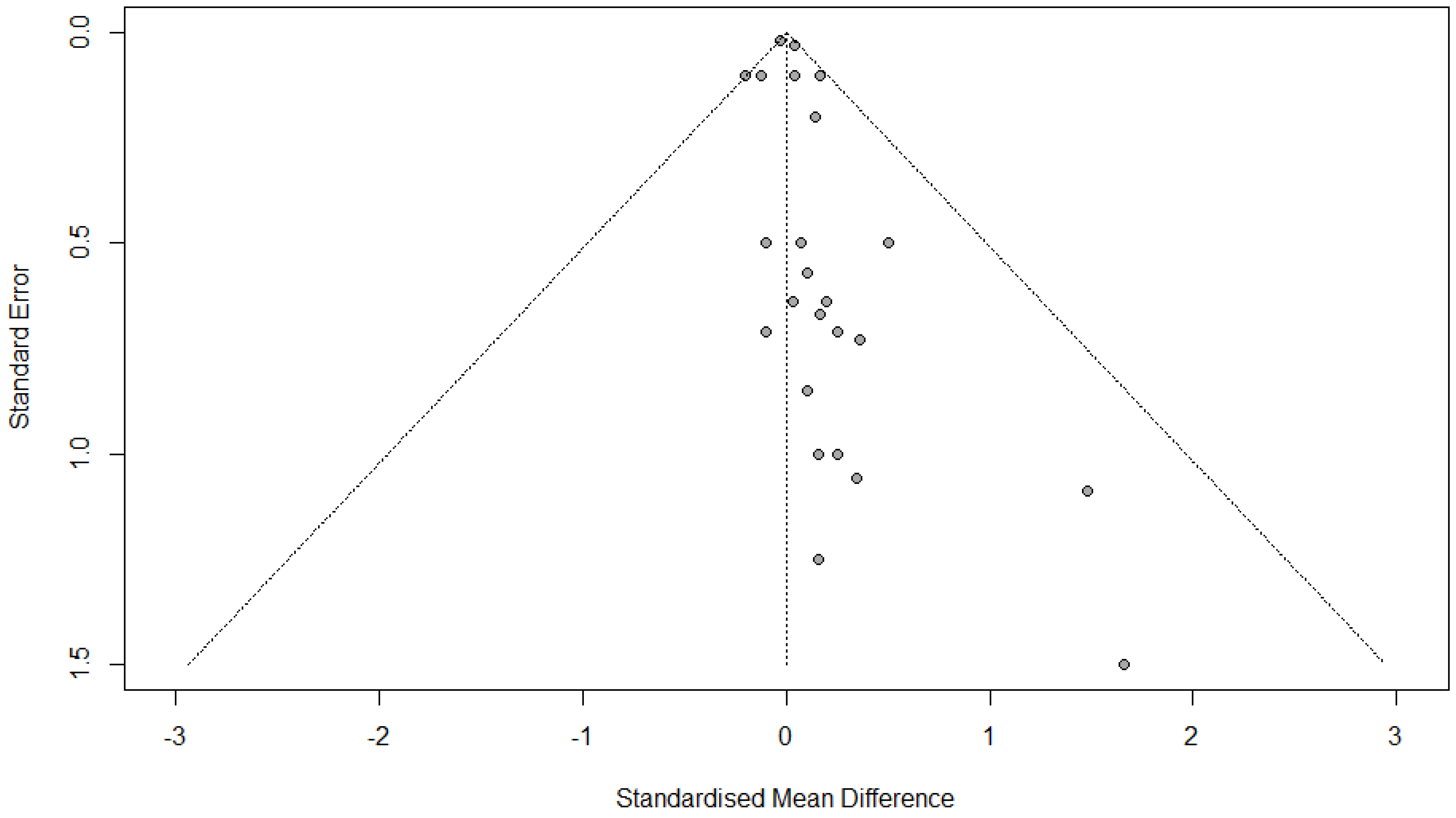
3.12. Publication Bias
3.12.1. FVC Change
3.12.2. FEV1 Change
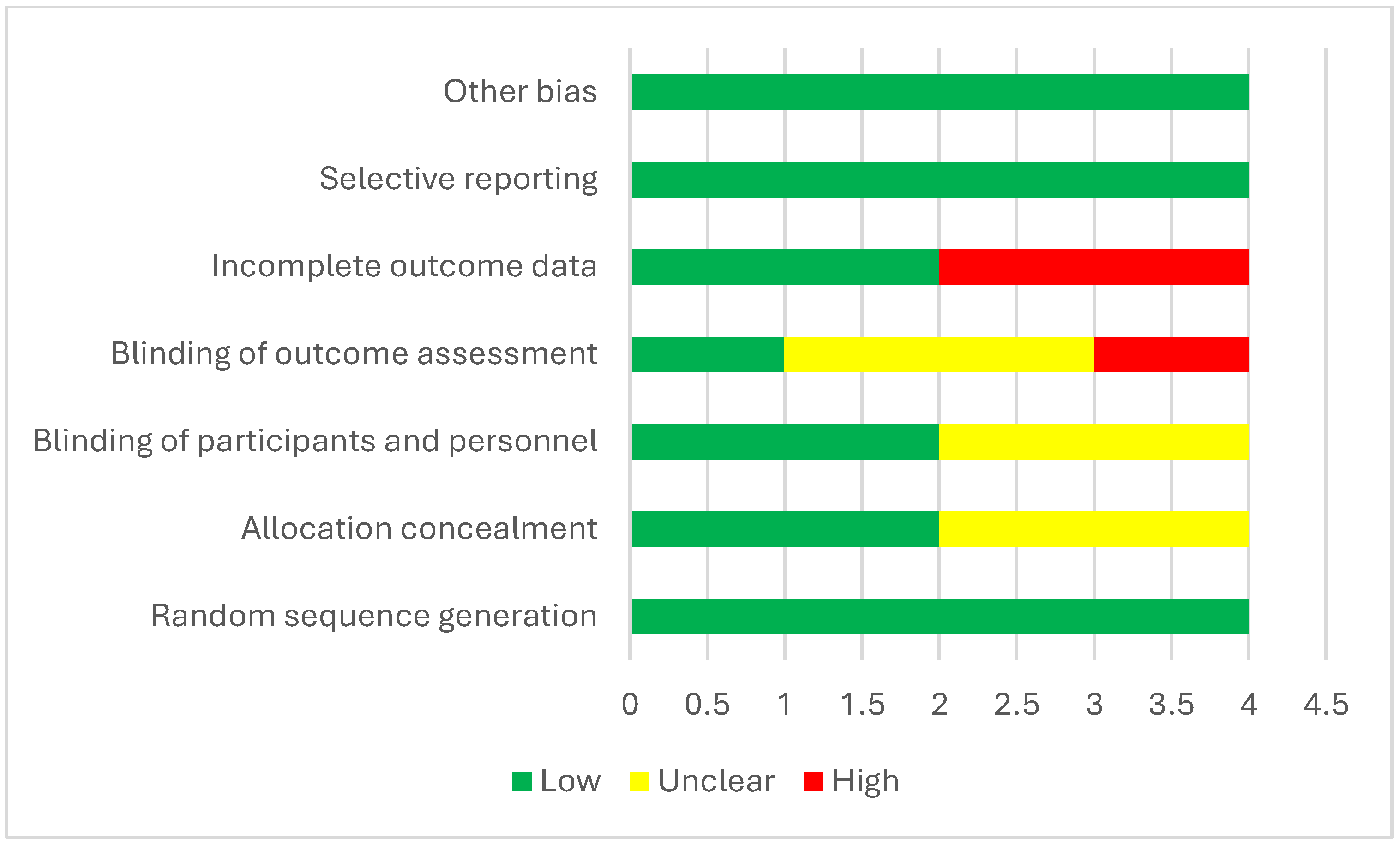
3.12.3. Quality Assessment
4. Discussion
4.1. Strengths
4.2. Limitations
4.3. Recommended Future Research
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DOAJ | Directory of open access journals |
| TLA | Three letter acronym |
| LD | Linear dichroism |
References
- Rolton, D.; Nnadi, C.; Fairbank, J. Scoliosis: A review. Paediatr. Child Health 2014, 24, 197–203. [Google Scholar] [CrossRef]
- Karol, L.A. The Natural History of Early-onset Scoliosis. J. Pediatr. Orthop. 2019, 39, S38–S43. [Google Scholar] [CrossRef] [PubMed]
- Behr, J.; Furst, D.E. Pulmonary function tests. Rheumatology 2008, 47, v65–v67. [Google Scholar] [CrossRef] [PubMed]
- Ruppel, G.L.; Enright, P.L. Pulmonary Function Testing. Respir. Care 2012, 57, 165. [Google Scholar] [CrossRef]
- Tsiligiannis, T.; Grivas, T. Pulmonary function in children with idiopathic scoliosis. Scoliosis 2012, 7, 7. [Google Scholar] [CrossRef]
- Zhang, J.G.; Wang, W.; Qiu, G.X.; Wang, Y.P.; Weng, X.S.; Xu, H.G. The Role of Preoperative Pulmonary Function Tests in the Surgical Treatment of Scoliosis. Spine 2005, 30, 218–221. [Google Scholar] [CrossRef]
- Newton, P.O.; Faro, F.D.; Gollogly, S.; Betz, R.R.; Lenke, L.G.; Lowe, T.G. Results of Preoperative Pulmonary Function Testing of Adolescents with Idiopathic Scoliosis: A Study of Six Hundred and Thirty-one Patients. JBJS 2005, 87, 1937–1946. [Google Scholar] [CrossRef]
- Newton, P.O.; Perry, A.; Bastrom, T.P.; Lenke, L.G.; Betz, R.R.; Clements, D.; D’Andrea, L. Predictors of Change in Postoperative Pulmonary Function in Adolescent Idiopathic Scoliosis: A Prospective Study of 254 Patients. Spine 2007, 32, 1875–1882. [Google Scholar] [CrossRef]
- Pehrsson, K.; Bake, B.; Larsson, S.; Nachemson, A. Lung function in adult idiopathic scoliosis: A 20 year follow up. Thorax 1991, 46, 474. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lenke, L.G.; Bridwell, K.H.; Kim, K.L.; Steger-May, K. Pulmonary Function in Adolescent Idiopathic Scoliosis Relative to the Surgical Procedure. JBJS 2005, 87, 1534–1541. [Google Scholar]
- Julian, P.T.H.; Douglas, G.A.; Peter, C.G.; Peter, J.; David, M.; Andrew, D.O.; Jelena, S.; Kenneth, F.S.; Laura, W.; Jonathan, A.C.S. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Kumar, A. Assessing the Impact of Rotational Angular Breathing on Lung Functions and Health-Related Quality of Life in Adolescent Idiopathic Scoliosis. Int. J. Physiother. 2024, 11, 47–51. [Google Scholar] [CrossRef]
- Küçük, E.; Öten, E.; Coskun, G. Effects of spinal mobilisation in adolescent idiopathic scoliosis: A randomised controlled trial. J. Paediatr. Child Health 2024, 60, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Lenke, L.G.; Bridwell, K.H.; Cheh, G.; Sides, B.; Whorton, J. Prospective pulmonary function comparison of anterior spinal fusion in adolescent idiopathic scoliosis: Thoracotomy versus thoracoabdominal approach. Spine 2008, 33, 1055–1060. [Google Scholar] [CrossRef]
- Abdel Ghafar, M.A.; Abdelraouf, O.R.; Abdel-Aziem, A.A.; Elnegamy, T.E.; Mohamed, M.E.; Yehia, A.M.; Samir Mousa, G. Pulmonary function and aerobic capacity responses to equine-assisted therapy in adolescents with idiopathic scoliosis: A randomized controlled trial. J. Rehabil. Med. 2022, 54, 1085. [Google Scholar] [CrossRef]
- Akazawa, T.; Kuroya, S.; Iinuma, M.; Asano, K.; Torii, Y.; Umehara, T.; Kotani, T.; Sakuma, T.; Minami, S.; Orita, S.; et al. Pulmonary function and thoracic deformities in adolescent idiopathic scoliosis 27 years or longer after spinal fusion with Harrington instrument. J. Orthop. Sci. 2018, 23, 45–50. [Google Scholar] [CrossRef]
- Amăricăi, E.; Suciu, O.; Onofrei, R.R.; Miclaus, R.S.; Iacob, R.E.; Catan, L.; Popoiu, C.M.; Cerbu, S.; Boia, E. Respiratory function, functional capacity, and physical activity behaviours in children and adolescents with scoliosis. J. Int. Med. Res. 2020, 48, 0300060519895093. [Google Scholar] [CrossRef]
- Byun, Y.M.; Iida, T.; Yamada, K.; Abumi, K.; Kokabu, T.; Iwata, A.; Iwasaki, N.; Sudo, H. Long-term pulmonary function after posterior spinal fusion in main thoracic adolescent idiopathic scoliosis. PLoS ONE 2020, 15, e0235123. [Google Scholar] [CrossRef]
- Dogar, F.; Argun, M.; Erdem, S.; Gurbuz, K.; Argun, A.S.; Kafadar, I.H. Clinical and radiological results of surgically treated patients with adolescent idiopathic scoliosis and the effects of pulmonary rehabilitation on respiration functions. Medicine 2021, 100, e24675. [Google Scholar] [CrossRef]
- Faro, F.D.; Marks, M.C.; Newton, P.O.; Blanke, K.; Lenke, L.G. Perioperative changes in pulmonary function after anterior scoliosis instrumentation: Thoracoscopic versus open approaches. Spine 2005, 30, 1058–1063. [Google Scholar] [CrossRef]
- Fu, J.; Liu, C.; Zhang, Y.G.; Zheng, G.Q.; Zhang, G.Y.; Song, K.; Tang, X.Y.; Wang, Y. Three-dimensional Computed Tomography for Assessing Lung Morphology in Adolescent Idiopathic Scoliosis following Posterior Spinal Fusion Surgery. Orthop. Surg. 2015, 7, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Grabala, P.; Helenius, I.J.; Buchowski, J.M.; Shah, S.A. The Efficacy of a Posterior Approach to Surgical Correction for Neglected Idiopathic Scoliosis: A Comparative Analysis According to Health-Related Quality of Life, Pulmonary Function, Back Pain and Sexual Function. Children 2023, 10, 299. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.M.; Hui, H.; Zhang, H.P.; Huang, D.G.; Liu, Z.K.; Zhao, Y.T.; He, S.M.; Zhang, X.F.; He, B.R.; Hao, D.J. The impact of posterior temporary internal distraction on stepwise corrective surgery for extremely severe and rigid scoliosis greater than 130°. Eur. Spine J. 2016, 25, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Jeans, K.A.; Lovejoy, J.F.; Karol, L.A.; McClung, A.M. How Is Pulmonary Function and Exercise Tolerance Affected in Patients With AIS Who Have Undergone Spinal Fusion? Spine Deform. 2017, 5, 416–423. [Google Scholar] [CrossRef]
- Lonner, B.S.; Auerbach, J.D.; Estreicher, M.B.; Betz, R.R.; Crawford, A.H.; Lenke, L.G.; Newton, P.O. Pulmonary function changes after various anterior approaches in the treatment of adolescent idiopathic scoliosis. J. Spinal Disord. Tech. 2009, 22, 551–558. [Google Scholar] [CrossRef]
- Laurentowska, M.; Glowacki, M.; Michalak, E.; Deskur-Śmielecka, E.; Barinow-Wojewódzki, A. Effects of rehabilitation based on endurance training in adolescent ghtls with surgically treated scoliosis. Biol. Sport 2009, 26, 45–53. [Google Scholar] [CrossRef]
- Min, K.; Haefeli, M.; Mueller, D.; Klammer, G.; Hahn, F. Anterior short correction in thoracic adolescent idiopathic scoliosis with mini-open thoracotomy approach: Prospective clinical, radiological and pulmonary function results. Eur. Spine J. 2012, 21, S765–S772. [Google Scholar] [CrossRef][Green Version]
- Mallepally, A.R.; Iyengar, R.S.; Patnala, C.S. Analysis of Adolescent Idiopathic Thoracic Scoliosis Treated with Posterior Instrumentation and Fusion: Our Experience. J. Clin. Diagn. Res. 2018, 12, RC1–RC5. [Google Scholar] [CrossRef]
- Newton, P.O.; Marks, M.C.; Bastrom, T.P.; Betz, R.; Clements, D.; Lonner, B.; Crawford, A.; Shufflebarger, H.; O’Brien, M.; Yaszay, B.; et al. Surgical Treatment of Lenke 1 Main Thoracic Idiopathic Scoliosis Results of a Prospective, Multicenter Study. Spine 2013, 38, 328–338. [Google Scholar] [CrossRef]
- Ogonowska-Slodownik, A.; Kaczmarczyk, K.; Kokowicz, G.; Morgulec-Adamowicz, N. Does the Aquatic Breathing Program Improve Lung Function in Adolescents with Scoliosis? Phys. Occup. Ther. Pediatr. 2020, 41, 259–270. [Google Scholar] [CrossRef]
- Ran, B.; Li, Q.; Li, C.; Li, M.; Chen, J.Y.; Wang, L.X.; Qiao, Y.H.; Guan, J.H.; Wang, Z.W. Effect of Anterior Thoracoscopic Release Combined with the Posterior Correction Operation on the Pulmonary Function of Patients with Idiopathic Scoliosis. Thorac. Cardiovasc. Surg. 2014, 63, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Rafferty, A.; Donne, B.; Kiely, P.; Fleming, N. Functional deficits in post-operative adolescent idiopathic scoliosis. Physiother. Pract. Res. 2020, 41, 133–141. [Google Scholar] [CrossRef]
- Tis, J.E.; O’Brien, M.F.; Newton, P.O.; Lenke, L.G.; Clements, D.H.; Harms, J.; Betz, R.R. Adolescent idiopathic scoliosis treated with open instrumented anterior spinal fusion: Five-year follow-up. Spine 2010, 35, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Vitale, M.G.; Matsumoto, H.; Bye, M.R.; Gomez, J.A.; Booker, W.A.; Hyman, J.E.; Roye Jr, D.P. A retrospective cohort study of pulmonary function, radiographic measures, and quality of life in children with congenital scoliosis: An evaluation of patient outcomes after early spinal fusion. Spine 2008, 33, 1242–1249. [Google Scholar] [CrossRef]
- Yaszay, B.; Jankowski, P.P.; Bastrom, T.P.; Lonner, B.; Betz, R.; Shah, S.; Asghar, J.; Miyanji, F.; Samdani, A.; Newton, P.O. Progressive decline in pulmonary function 5 years post-operatively in patients who underwent anterior instrumentation for surgical correction of adolescent idiopathic scoliosis. Eur. Spine J. 2019, 28, 1322–1330. [Google Scholar] [CrossRef]
- Yu, C.G.; Grant, C.A.; Izatt, M.T.; Labrom, R.D.; Askin, G.N.; Adam, C.J.; Little, J.P. Change in Lung Volume Following Thoracoscopic Anterior Spinal Fusion Surgery. Spine 2017, 42, 909–916. [Google Scholar] [CrossRef]
- Zhou, X.; Li, X.; Wu, Q.; Liang, J.; Guo, H.; Jin, M.; Zhu, X.; Du, Q. Three-dimensional corrective exercise therapy for idiopathic scoliosis: Study protocol for a prospective non-randomized trial. BMC Musculoskelet. Disord. 2022, 23, 118. [Google Scholar] [CrossRef]
- Yildirim, S.; Ozyilmaz, S.; Elmadag, N.M.; Yabaci, A. Effects of Core Stabilization Exercises on Pulmonary Function, Respiratory Muscle Strength, Peripheral Muscle Strength, Functional Capacity, and Perceived Appearance in Children With Adolescent Idiopathic Scoliosis: A Randomized Controlled Trial. Am. J. Phys. Med. Rehabil. 2022, 101, 719–725. [Google Scholar] [CrossRef]
- Yuan, N.; Fraire, J.A.; Margetis, M.M.; Skaggs, D.L.; Tolo, V.T.; Keens, T.G. The effect of scoliosis surgery on lung function in the immediate postoperative period. Spine 2005, 30, 2182–2185. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |
| Studies | Study Design | Treatment | Country of the Patients | Number of Participants | Age | Gender (Female/Male %) |
|---|---|---|---|---|---|---|
| Kumar, 2024 A [12] | Pre and post test | Breathing exercise protocol | India | 18 | 10 and 15 years | 47.2/52.8% |
| Kumar, 2024 B [12] | Pre and post test | Conventional exercise therapy | India | 18 | 10 and 15 years | |
| Mallepally et al., 2018 [28] | Retrospective, observational study | Posterior instrumentation and posterior spinal fusion | India | 41 | 11 to 19 years | 61/39% |
| Rafferty et al., 2020 [32] | Retrospective, case control study | Surgery | Ireland | 20 | 18 (2) | _ |
| Ogonowska-Slodownik et al., 2021 A [30] | Pre and post test | Aquatic Breathing Program | Poland | 13 | 14.2 (1.4) | _ |
| Ogonowska-Slodownik et al., 2021 B [30] | Pre and post test | Corrective swimming | Poland | 10 | 14.2 (1.4) | _ |
| Lonner et al., 2009 A [25] | Prospective chart review | Thoracotomy | USA | 68 | 14.3 (10–21) | 89.7/10.3 |
| Lonner et al., 2009 B [25] | Prospective chart review | Thoracoscopic | USA | 44 | 13.9 (10–18) | 88.6/11.4% |
| Lonner et al., 2009 C [25] | Prospective chart review | Thoracoabdominal | USA | 19 | 14.8 (13–18) | 94.7/5.3% |
| Yaszay et al., 2019 [35] | Retrospective study | Surgery | USA | 262 | 10–21 years | 82.8/17.2% |
| Ran et al., 2014 [31] | Retrospective study | Anterior thoracoscopic release combined with posterior correction | China | 28 | 16.3 (12–27) | 57.1/42.9% |
| Yu et al., 2017 [36] | Cohort | Thoracoscopic anterior spinal fusion | _ | 23 | 15.7 (3.1) | 100/0% |
| Küçük et al., 2024 A [13] | RCT | spinal mobilisation followed by core stabilisation exercises | Turkey | 20 | 12.9 ± 1.8 | 60/40% |
| Küçük et al., 2024 B [13] | RCT | Core stabilisation exercises | Turkey | 20 | 12.85 ± 1.81 | 65/35% |
| Amăricăi et al., 2019 [17] | Non-randomized | Rehabilitation programme | Romania | 40 | 13.10 ± 2.8 | 60/40% |
| Faro et al., 2005 A [20] | Prospective study | Thoracoscopic | USA | 31 | 13.3 (1.5) | 96.8/3.2% |
| Faro et al., 2005 B [20] | Prospective study | Open thoracotomy for anterior spinal fusion | USA | 23 | 14.8 (2.3) | 82.6/17.4% |
| Dogar et al., 2021 A [19] | Randomized trial | Scoliosis surgery and diaphragmatic breathing and pursed lip exercises | Turkey | 15 | 16.3 ± 3.48 | _ |
| Dogar et al., 2021 B [19] | Randomized trial | Scoliosis surgery | Turkey | 15 | _ | |
| Hu et al., 2015 [23] | Retrospective study | Posterior temporary internal distraction correction and definitive posterior spinal correction with posterior pedicle screw instrumentation | China | 11 | mean: 17.8 (15–23) | 72.7/27.8% |
| Tis et al., 2010 [33] | Prospective study | Open instrumented anterior spinal fusion | _ | 85 | _ | _ |
| Fu et al., 2015 [21] | Prospective study | Posterior spinal fusion surgery | China | 30 | 10–16 years | 70/30% |
| Jeans et al., 2017 [24] | Prospective study | Spinal fusion | USA | 37 | 14.5 (11.3–18.7) | 81/19% |
| Min et al., 2012 [27] | Prospective study | Anterior short correction | Switzerland | 62 | 15.2 (SD 2.5) | _ |
| Abd El Ghafar et al., 2022 A [15] | RCT | Hippotherapy combined with Schroth exercises | KSA | 22 | 14.68 ± 1.72 | 59/41% |
| Abd El Ghafar et al., 2022 B [15] | RCT | Schroth exercises | KSA | 23 | 15.04 ± 1.66 | 65.2/34.8% |
| Vitale et al., 2008 [34] | Retrospective study | Spinal fusion | USA | 21 | 12.6 (3.5) | 57.1/42.9% |
| Laurentowska et al., 2009 [26] | Cohort | Cotrel-Dubousset plus Rehabilitation program | Poland | 16 | 16.6 (1.5) | _ |
| Yuan et al., 2005 [39] | Prospective study | Surgery | USA | 24 | 12.7 (2.7) | 58.3/41.7% |
| Newton et al., 2013 A [29] | Prospective study | Thoracoscopic anterior spinal fusion | USA | 55 | 14.5 ± 2 | 84/16% |
| Newton et al., 2013 B [29] | Prospective study | Open anterior spinal fusion | USA | 17 | ||
| Newton et al., 2013 C [29] | Prospective study | Posterior spinal fusion | USA | 64 | ||
| Garbala et al., 2023 SG [22] | Retrospective study | HGT | Poland | 88 | 14.3 (2.8) | 88.6/11.4% |
| Garbala et al., 2023 MG [22] | Retrospective study | HGT | Poland | 107 | 15 (2.6) | 86.9/13.1% |
| Yildirim et al., 2019 A [38] | RCT | Core stabilization exercises, plus structured and coached recreational activities plus usual care | Turkey | 25 | 16.68 ± 0.74 | 0/100% |
| Yildirim et al., 2019 B [38] | RCT | Structured, coached recreational activities plus usual care | Turkey | 24 | 16.79 ± 0.58 | 0/100% |
| Akazawa et al., 2018 [16] | Cohort | Posterior Spinal Fusion with Thoracoplasty | Japan | 24 | 15.5 ± 2.0 | 95.3/4.7% |
| Zhou et al., 2022 [37] | Non-randomized | Halo gravity traction (HGT) | China | 47 | 29.5 (5.8) | 66/34% |
| Kim et al., 2008 A [14] | Prospective study | Thoracotomy | USA | 64 | 14.9 (2.3) | 90.8/9.2% |
| Kim et al., 2008 B [14] | Prospective study | Thoracoabdominal Approach | USA | 55 | 15.2 (1.8) | |
| Byun et al., 2020 [18] | Cohort | Posterior spinal fusion | Japan | 35 | 14.9 ± 2.0 | 80/20% |
| Study | Treatment | FVC | FEV1 | PEF | Other Outcomes |
|---|---|---|---|---|---|
| Kumar, 2024 A [12] | Breathing exercise protocol | FVC increased by 1.59 L from baseline | FEV1 improved by 1.48 L | PEF increased by 3.77 L per second | FEV1/FVC ratio changed by 85.12%; VC improved by 1.78 L |
| Kumar, 2024 B [12] | Conventional exercise therapy | FVC increased by 1.81 L from baseline | FEV1 improved by 1.66 L | PEF increased by 4.11 L per second | FEV1/FVC ratio changed by 81.32%; VC improved by 2.06 L |
| Mallepally et al., 2018 [28] | Posterior instrumentation and posterior spinal fusion | Preop: 90.6; Postop: 86.2 (2-year follow-up) | Preop: 86.67; Postop: 85 (2-year follow-up) | _ | TLC: Preop 97.8, Postop 100 (2-year); SRS-30 Scores: 1-year 93 ± 17, 2-year 98 ± 22 |
| Rafferty et al., 2020 [32] | Surgery | AIS cohort: 2.6 ± 0.5 L vs. 3.3 ± 0.5 L (p < 0.001) | 2.8 ± 0.6 L vs. 3.3 ± 0.5 L (p < 0.001) | _ | - |
| Ogonowska-Slodownik et al., 2021 A [30] | Aquatic breathing program | FVCex: Increased from 3.54 L to 3.61 L | FEV1: Increased from 2.88 L to 3.05 L | _ | FEV1%VC: Increased from 81.84% to 86.57%; FEV1%VC%pred: Increased from 95.69% to 101.77%; MEF25 increased from 2.07 L to 2.30 L |
| Ogonowska-Slodownik et al., 2021 B [30] | Corrective swimming | FVCex: Decreased from 3.51 L to 3.32 L | FEV1: Decreased from 2.98 L to 2.78 L | _ | FEV1%VC: Decreased from 81.39% to 81.22%; MEF25 decreased from 2.13 L to 2.04 L |
| Lonner et al., 2009 A [25] | Thoracotomy | Preop: 2.96 L; Follow-up: 2.70 L, Change −0.26 L (p < 0.001) | Preop: 2.48 L; Follow-up: 2.36 L, Change −0.12 L (p = 0.004) | _ | TLC: Preop: 3.71 L; Follow-up: 3.71 L (no significant change) |
| Lonner et al., 2009 B [25] | Thoracoscopic | Preop: 2.88 L; Follow-up: 2.90 L, Change +0.02 L (NS) | Preop: 2.49 L; Follow-up: 2.53 L, Change +0.04 L (NS) | _ | TLC: Preop: 3.46 L; Follow-up: 3.90 L, Change +0.44 L (p < 0.001, significant) |
| Lonner et al., 2009 C [25] | Thoracoabdominal | Preop: 3.26 L; Follow-up: 3.20 L, Change −0.06 L (NS) | Preop: 2.84 L; Follow-up: 2.81 L, Change −0.03 L (NS) | _ | TLC: Preop: 4.25 L; Follow-up: 4.48 L, Change +0.23 L |
| Yaszay et al., 2019 [35] | Surgery | Preop: 2.98 ± 0.7 L; 5-year: 3.15 ± 0.7 L (p = 0.008) | Preop: 2.48 ± 0.6 L; 5-year: 2.58 ± 0.6 L (p = 0.10) | _ | FVC% Predicted: Preop 87 ± 16%; 5-year 81 ± 13% (p = 0.004 significant) |
| Ran et al., 2014 [31] | Anterior thoracoscopic release + posterior correction | Preop: 2.47 ± 0.33 L; 2 years: 3.21 ± 1.18 L | _ | _ | FRC: Preop 2.86 ± 0.47; 2 years: 2.68 ± 0.90; RV: Preop 1.22 ± 0.39; 2 years: 1.13 ± 0.38; TLC: Preop 3.68 ± 0.36; 2 years: 4.32 ± 1.41 |
| Yu et al., 2017 [36] | Thoracoscopic anterior spinal fusion | TLC: Preop: 2056.8 ± 388.4 mL; Postop: 2228.5 ± 368.9 mL (p = 0.01) | _ | _ | Right Lung Volume: Preop: 1126.3 ± 221.0 mL; Postop: 1207.6 ± 183.2 mL (p = 0.09); Left Lung Volume: Preop: 930.3 ± 183.3 mL; Postop: 1020.9 ± 199.9 mL (p = 0.01 significant) |
| Küçük et al., 2024 A [13] | Spinal mobilisation + core stabilisation | _ | FEV1 Pre-treatment: Mean ± S.D. = 2.33 ± 0.76; Post: 2.58 ± 0.74 | PEF Pre-treatment: 243.75 ± 69.77, Post: 289.25 ± 83.92 (p = 0.001) | Change in FEV1: Mean Δ 0.25 ± 0.62 (p = 0.02); Median Δ 0.22 |
| Küçük et al., 2024 B [13] | Core stabilisation exercises | Pre: 2.74 ± 1.07 L; Post: 2.9 ± 1.27 L | Pre: 2.37 ± 0.79 L; Post: 2.53 ± 0.96 L | Pre: 237.25 ± 87.68 L/min; Post: 276.5 ± 103.68 L/min | FEV1/FVC: Pre = 88.5 ± 11.64%, Post = 90% |
| Amăricăi et al., 2019 [17] | Rehabilitation programme | T1: 3.18 L; T2: 3.45 L | T1: 3.03 L; T2: 3.19 L | T1: 5.12 L/min; T2: 5.22 L/min | FEV1/FVC: T1 = 87.90%, T2 = 88.50% |
| Faro et al., 2005 A [20] | Thoracoscopic | Pre: 2.81 ± 0.49 L; 3 months: 2.42 ± 0.55 L; 1 year: 2.82 ± 0.52 L | Pre: 2.42 ± 0.45 L; 3 months: 2.12 ± 0.49 L; 1 year: 2.49 ± 0.44 L | _ | p = 0.025 for FEV1, p = 0.001 for FVC |
| Faro et al., 2005 B [20] | Open thoracotomy for anterior spinal fusion | Pre: 3.09 ± 0.64 L; 3 months: 2.50 ± 0.57 L; 1 year: 2.83 ± 0.61 L | Pre: 2.59 ± 0.53 L; 3 months: 2.32 ± 0.53 L; 1 year: 2.49 ± 0.52 L | _ | p = 0.025 for FEV1, p = 0.001 for FVC |
| Dogar et al., 2021 A [19] | Scoliosis surgery and diaphragmatic breathing and pursed lip exercises | Pre: 3.28 ± 1.00 L; 1 month: 2.73 ± 0.79 L; 6 months: 3.31 ± 0.87 L | Pre: 2.89 ± 0.86 L; 1 month: 2.47 ± 0.72 L; 6 months: 2.93 ± 0.78 L | _ | FEV1/FVC ratio: Pre = 89%, 6 months = 88.8% |
| Dogar et al., 2021 B [19] | Scoliosis surgery | Pre: 2.57 ± 0.76 L; 1 month: 2.28 ± 0.64 L; 6 months: 2.66 ± 0.66 L | Pre: 2.30 ± 0.67 L; 1 month: 2.15 ± 0.59 L; 6 months: 2.44 ± 0.56 L | _ | FEV1/FVC ratio: Pre = 88.93%, 6 months = 92.26% |
| Hu et al., 2015 [23] | Posterior temporary internal distraction correction and definitive posterior spinal correction with posterior pedicle screw instrumentation | Pre: 59.3% ± 11.6; After internal distraction: 68.7% ± 13.7; After final correction: 71.2% ± 8.3; Final follow-up: 73.1% ± 11.9 | Pre: 61.4% ± 13.6; After internal distraction: 71.3% ± 9.3; After final correction: 76.3% ± 16.7; Final follow-up: 75.5% ± 13.8 | _ | FEV1/FVC: Pre = 61.4%, final follow-up = 75.5% |
| Tis et al., 2010 [33] | Open instrumented anterior spinal fusion | Preop: 2.68 ± 0.62; Postop: 2.62 ± 0.56 | Preop: 2.28 ± 0.49; Postop: 2.31 ± 0.42 | _ | % Predicted FEV1: Preop 75.5 ± 13; Postop 68.8 ± 12 (↓ significant); % Predicted FVC: Preop 81.6 ± 16; Postop 69.8 ± 13 (↓ significant) |
| Fu et al., 2015 [21] | Posterior spinal fusion surgery | _ | _ | _ | LLV, RLV, TLV, CCLVR showed no change; LLH, RLH (↑ significant); PCSA, CCPCSAR (↓ significant) |
| Jeans et al., 2017 [24] | Spinal fusion | Preop: 2.8 ± 0.6; Postop: 3.0 ± 0.6 (↑) | Preop: 2.3 ± 0.4; Postop: 2.5 ± 0.5 (↑) | _ | FVC%: No change; FEV1%: No significant change |
| Min et al., 2012 [27] | Anterior short correction | Preop: 2842 mL; Last: 2812 mL (no change) | - | _ | FVC%: No significant change between Preop and Last follow-up |
| Abd El Ghafar et al., 2022 A [15] | Hippotherapy + Schroth exercises | Preop: 2.62 ± 0.49; Postop: 2.97 ± 0.57 (↑) | Preop: 2.28 ± 0.44; Postop: 2.64 ± 0.58 (↑) | _ | MVV: ↑ significant; FEV1/FVC: ↑ significant |
| Abd El Ghafar et al., 2022 B [15] | Schroth exercises | Preop: 2.47 ± 0.45; Postop: 2.65 ± 0.59 (↑) | Preop: 2.31 ± 0.41; Postop: 2.56 ± 0.58 (↑) | _ | MVV: ↑ significant; FEV1/FVC: ↑ significant |
| Vitale et al., 2008 [34] | Spinal fusion | FVC: 74.4 ± 19.4 (↓) | FEV1: 73.0 ± 20.2 (↓) | _ | TLC: 88.5 ± 17.0 (↓); VC: 75.6 ± 19.6 (↓) |
| Laurentowska et al., 2009 [26] | Cotrel-Dubousset + Rehab program | VC: 3.05 ± 0.439 | FEV1%: 92.18 ± 5.002 | _ | % Predicted FEV1: 108.27 ± 5.781; MVV: 87.60 ± 13.876; MVV%pred: 70.54 ± 13.201 |
| Yuan et al., 2005 [39] | Surgery | Declined up to 60% post-surgery, nadir at 3 days, recovery to 70% baseline by 6 months. | Not significantly correlated with scoliosis etiology or surgery type. | _ | No statistical differences in recovery trends based on scoliosis etiology or surgery type. |
| Newton et al., 2013 A [29] | Thoracoscopic anterior spinal fusion | Pre: 2.7 ± 0.5 L; Post: 2.7 ± 0.5 L; Change: +0.02 ± 0.5. | Pre: 2.3 ± 0.4 L; Post: 2.4 ± 0.4 L; Change: +0.1 ± 0.4. | _ | TLC (%predicted) Pre: 86 ± 10; Post: 87 ± 9; Change: +1 ± 7. |
| Newton et al., 2013 B [29] | Open anterior spinal fusion | Pre: 2.8 ± 0.6 L; Post: 2.5 ± 0.5 L; Change: −0.2 ± 0.3. | Pre: 2.4 ± 0.5 L; Post: 2.3 ± 0.5 L; Change: −0.2 ± 0.4. | _ | TLC (%predicted) Pre: 88 ± 17; Post: 73 ± 7; Change: −15 ± 13. |
| Newton et al., 2013 C [29] | Posterior spinal fusion | Pre: 3.1 ± 0.7 L; Post: 3.3 ± 0.7 L; Change: +0.2 ± 0.4. | Pre: 2.6 ± 0.6 L; Post: 2.7 ± 0.6 L; Change: +0.1 ± 0.4. | _ | TLC (%predicted) Pre: 90 ± 13; Post: 78 ± 26; Change: −20 ± 36. |
| Garbala et al., 2023 SG [22] | Halo gravity traction (HGT) for severe group | Pre: 51.2 ± 12.8%; Follow-up: 69.9 ± 11.2% (p < 0.001). | Pre: 60.8 ± 13.9%; Follow-up: 76.9 ± 14.5% (p < 0.001). | _ | Improved pulmonary function observed for severe group. |
| Garbala et al., 2023 MG [22] | Halo gravity traction (HGT) for moderate group | Pre: 83 ± 11.2%; Follow-up: 79 ± 13.2% (p = 0.12). | Pre: 77 ± 12.8%; Follow-up: 81 ± 12.8% (p = 0.09). | _ | Minimal changes in pulmonary function in moderate group. |
| Yildirim et al., 2019 A [38] | Core stabilization exercises + recreational activities + usual care | Baseline: 85.70 ± 11.62%; Post: 91.51 ± 13.22%; Change: +5.8 (p < 0.0001). | Baseline: 92.74 ± 13.18%; Post: 100.08 ± 13.77%; Change: +7.35 (p < 0.0001). | Baseline: 75.00 ± 18.34%; Post: 88.32 ± 15.03%; Change: +13.32 (p < 0.0001). | Improved FEF25–75% from 97.68 ± 19.66% to 109.52 ± 18.20% (p = 0.0002). |
| Yildirim et al., 2019 B [38] | Recreational activities + usual care | Baseline: 88.29 ± 13.29%; Post: 89.25 ± 12.55%; Change: +0.96 (p = 0.064). | Baseline: 95.00 ± 11.56%; Post: 97.91 ± 11.37%; Change: +2.92 (p = 0.035). | Baseline: 78.79 ± 17.77%; Post: 83.45 ± 19.62%; Change: +4.67 (p < 0.0001). | Improved FEF25–75% from 98.88 ± 19.37% to 105.12 ± 14.09% (p = 0.015). |
| Akazawa et al., 2018 [16] | Posterior spinal fusion with thoracoplasty | Pre: 2.27 ± 0.64 L; Final: 2.38 ± 0.50 L; Change: +0.11 (p = 0.240). | Pre: 1.88 ± 0.52 L; Final: 2.05 ± 0.42 L; Change: +0.17 (p = 0.045). | Pre: 3.67 ± 1.51 L/s; Final: 4.38 ± 1.18 L/s; Change: +0.71 (p = 0.029). | Significant improvements in %FEV1 (p = 0.001), FEV1/FVC (p = 0.019), and other flow measures like V25 and V50. |
| Zhou et al., 2022 [37] | Halo gravity traction (HGT) |
|
| _ |
|
| Kim et al., 2018 A [14] | Thoracotomy |
|
| _ |
|
| Kim et al., 2018 B [14] | Thoracoabdominal Approach |
|
| _ |
|
| Byun et al., 2020 [18] | Posterior spinal fusion |
|
| _ |
|
| Study | Random Sequence Generation | Allocation Concealment | Blinding of Participants and Personnel | Blinding of Outcome Assessment | Incomplete Outcome Data | Selective Reporting | Other Bias |
|---|---|---|---|---|---|---|---|
| Küçük et al., 2024 [13] | Low | Low | Low | Low | Low | Low | Low |
| Doger et al., 2021 [19] | Low | Unclear | Unclear | Unclear | Low | Low | Low |
| Abdel Ghafar et al., 2022 [15] | Low | Low | Low | High | High | Low | Low |
| Yildirim et al., 2019 [38] | Low | Unclear | Unclear | Unclear | High | Low | Low |
| Studies | Selection | Comparability | Exposure/Outcome | Overall Star Rating |
|---|---|---|---|---|
| Kumar, 2024 [12] | ** | ** | * | 5 |
| Mallepally et al., 2018 [28] | *** | * | ** | 6 |
| Rafferty et al., 2020 [32] | ** | ** | *** | 7 |
| Ogonowska-Slodownik et al., 2020 [30] | *** | ** | * | 6 |
| Lonner et al., 2009 [25] | **** | ** | ** | 8 |
| Yaszay et al., 2019 [35] | *** | * | ** | 6 |
| Ran et al., 2019 [31] | *** | ** | * | 6 |
| Yu et al., 2017 [36] | ** | * | *** | 6 |
| Amăricăi et al., 2019 [17] | ** | * | ** | 5 |
| Faro et al., 2005 [20] | ** | * | ** | 5 |
| Hu et al., 2015 [23] | * | ** | * | 4 |
| Tis et al., 2010 [33] | *** | ** | *** | 8 |
| Fu et al., 2015 [21] | ** | ** | 4 | |
| Jeans et al., 2017 [24] | *** | ** | ** | 7 |
| Min et al., 2012 [27] | ** | ** | ** | 6 |
| Vitale et al., 2008 [34] | *** | ** | ** | 7 |
| Laurentowska et al., 2009 [26] | ** | * | * | 4 |
| Yuan et al., 2005 [39] | *** | * | ** | 6 |
| Newton et al., 2013 [29] | **** | ** | ** | 8 |
| Grabala et al., 2023 [22] | *** | ** | *** | 8 |
| Akazawa et al., 2018 [16] | *** | * | *** | 7 |
| Zhou et al., 2022 [37] | *** | * | *** | 7 |
| Kim et al., 2008 [14] | **** | ** | ** | 8 |
| Byun et al., 2020 [18] | *** | ** | ** | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashem, M. Assessment of Pulmonary Function After Treatment of Scoliosis: Meta-Analysis and Review Article. Medicina 2025, 61, 1127. https://doi.org/10.3390/medicina61071127
Hashem M. Assessment of Pulmonary Function After Treatment of Scoliosis: Meta-Analysis and Review Article. Medicina. 2025; 61(7):1127. https://doi.org/10.3390/medicina61071127
Chicago/Turabian StyleHashem, Majdi. 2025. "Assessment of Pulmonary Function After Treatment of Scoliosis: Meta-Analysis and Review Article" Medicina 61, no. 7: 1127. https://doi.org/10.3390/medicina61071127
APA StyleHashem, M. (2025). Assessment of Pulmonary Function After Treatment of Scoliosis: Meta-Analysis and Review Article. Medicina, 61(7), 1127. https://doi.org/10.3390/medicina61071127