Comparative Analysis of Open, Laparoscopic, and Robotic Pancreaticoduodenectomy: A Systematic Review of Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Registration and Reporting
2.2. Search Strategy
2.3. Search Keywords
2.4. Article Selection Criteria
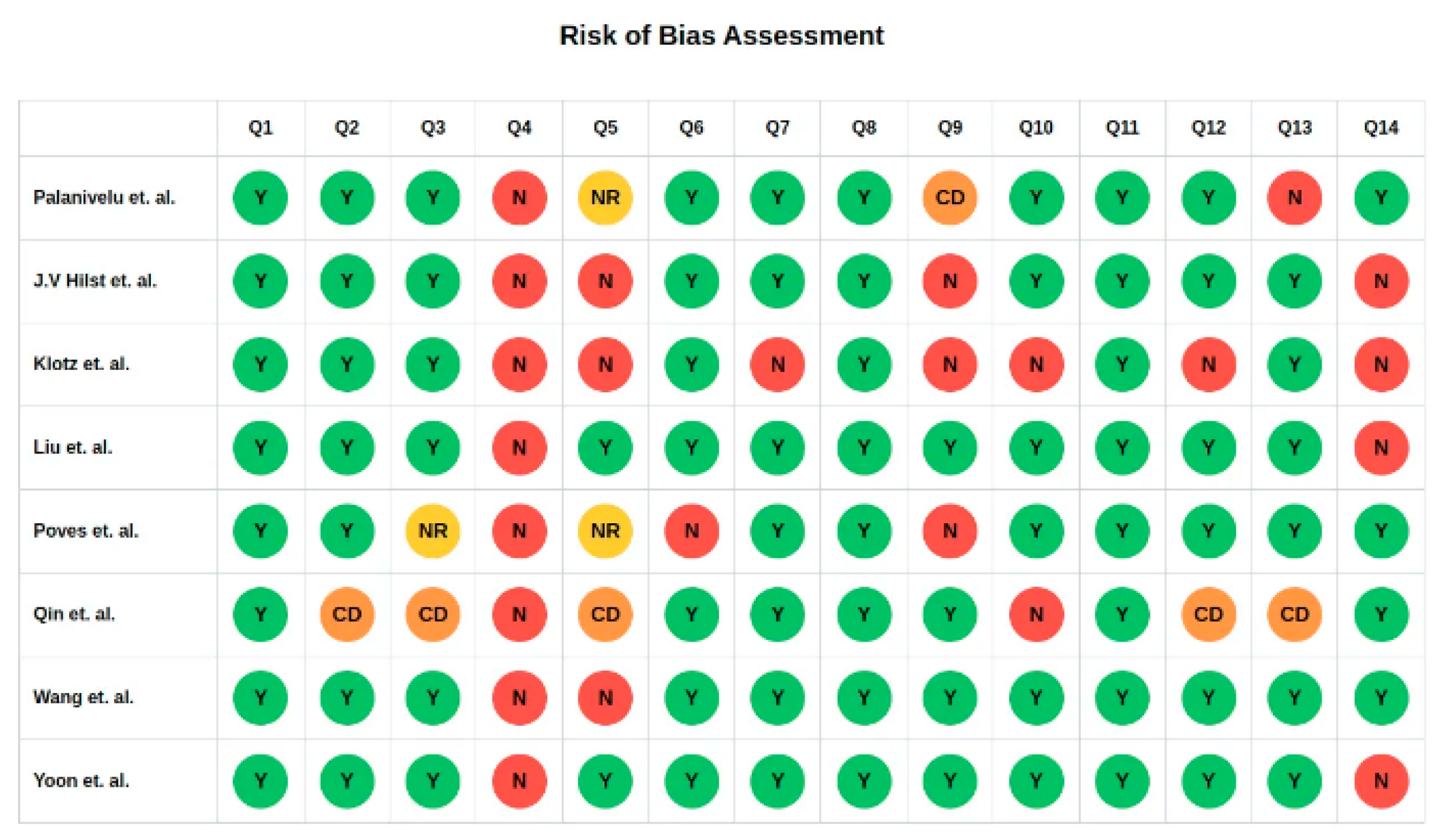
2.5. Assessment of Risk of Bias
2.6. Data Extraction
2.7. Statistical Analysis
3. Results
3.1. Study and Patients’ Characteristics
3.2. Quality Assessment and Certainty of Evidence
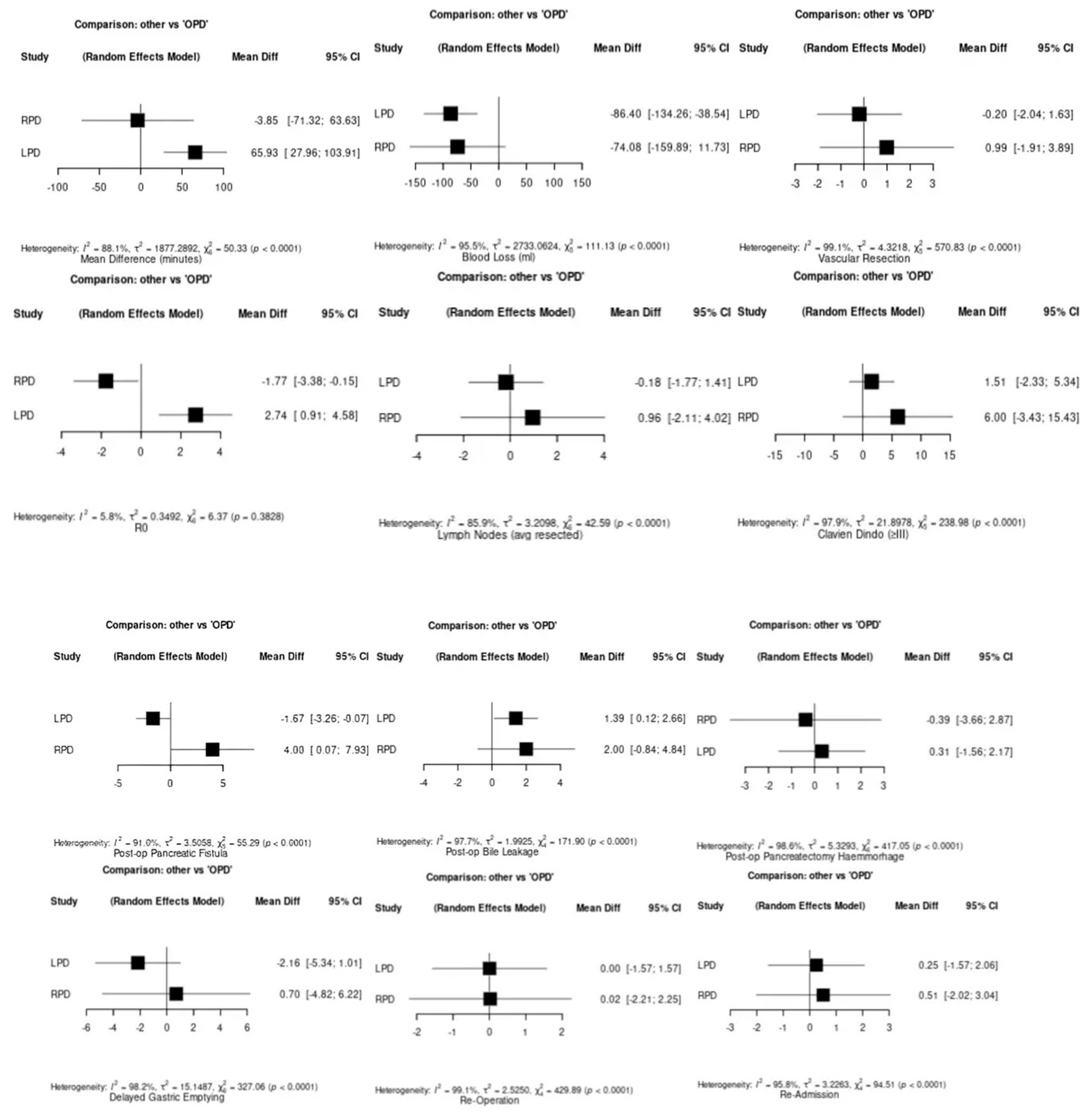
3.3. Operative Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PD | Pancreaticoduodenectomy |
| OPD | Open Pancreaticoduodenectomy |
| LPD | Laparoscopic Pancreaticoduodenectomy |
| RPD | Robotic Pancreaticoduodenectomy |
| RCT | Randomized Controlled Trial |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| NIH | National Institutes of Health |
| BMI | Body Mass Index |
| ASA | American Society of Anesthesiologists |
| CI | Confidence Interval |
| NMA | Network Meta-Analysis |
| MD | Mean Difference |
| SD | Standard Deviation |
| OR | Odds Ratio |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- McGuigan, A.; Kelly, P.; Turkington, R.C.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 2018, 24, 4846–4861. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.M.; Cameron, J.L.; Campbell, K.A.; Arnold, M.A.; Chang, D.C.; Coleman, J.; Hodgin, M.B.; Sauter, P.K.; Hruban, R.H.; Riall, T.S.; et al. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J. Gastrointest. Surg. 2006, 10, 1199–1210; discussion 1210–1211. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, C.S.; Gladhaug, I.P. Resection margin involvement and tumour origin in pancreatic head cancer. Br. J. Surg. 2012, 99, 1036–1049. [Google Scholar] [CrossRef]
- Tempero, M.A.; Malafa, M.P.; Al-Hawary, M.; Behrman, S.W.; Benson, A.B.; Cardin, D.B.; Chiorean, E.G.; Chung, V.; Czito, B.; Del Chiaro, M.; et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 439–457. [Google Scholar] [CrossRef]
- Klotz, R.; Larmann, J.; Klose, C.; Bruckner, T.; Benner, L.; Doerr-Harim, C.; Tenckhoff, S.; Lock, J.F.; Brede, E.-M.; Salvia, R.; et al. Gastrointestinal Complications After Pancreatoduodenectomy With Epidural vs Patient-Controlled Intravenous Analgesia: A Randomized Clinical Trial. JAMA Surg. 2020, 155, e200794. [Google Scholar] [CrossRef]
- Simon, R. Complications After Pancreaticoduodenectomy. Surg. Clin. N. Am. 2021, 101, 865–874. [Google Scholar] [CrossRef]
- Gagner, M.; Pomp, A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg. Endosc. 1994, 8, 408–410. [Google Scholar] [CrossRef]
- Tan, Y.; Tang, T.; Zhang, Y.; Zu, G.; An, Y.; Chen, W.; Wu, D.; Sun, D.; Chen, X. Laparoscopic vs. open pancreaticoduodenectomy: A comparative study in elderly people. Updates Surg. 2020, 72, 701–707. [Google Scholar] [CrossRef]
- Adam, M.A.; Choudhury, K.; Dinan, M.A.; Reed, S.D.; Scheri, R.P.; Blazer, D.G.; Roman, S.A.; Sosa, J.A. Minimally Invasive Versus Open Pancreaticoduodenectomy for Cancer: Practice Patterns and Short-term Outcomes Among 7061 Patients. Ann. Surg. 2015, 262, 372–377. [Google Scholar] [CrossRef]
- Van Hilst, J.; de Rooij, T.; Bosscha, K.; Brinkman, D.J.; van Dieren, S.; Dijkgraaf, M.G.; Gerhards, M.F.; de Hingh, I.H.; Karsten, T.M.; Lips, D.J.; et al. Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours (LEOPARD-2): A multicentre, patient-blinded, randomised controlled phase 2/3 trial. Lancet Gastroenterol. Hepatol. 2019, 4, 199–207. [Google Scholar] [CrossRef]
- Giulianotti, P.C.; Mangano, A.; Bustos, R.E.; Fernandes, E.; Masrur, M.A.; Valle, V.; Gangemi, A.; Bianco, F.M. Educational step-by-step surgical video about operative technique in robotic pancreaticoduodenectomy (RPD) at University of Illinois at Chicago (UIC): 17 steps standardized technique—Lessons learned since the first worldwide RPD performed in the year 2001. Surg. Endosc. 2020, 34, 2758–2762. [Google Scholar] [CrossRef]
- Mantzavinou, A.; Uppara, M.; Chan, J.; Patel, B. Robotic versus open pancreaticoduodenectomy, comparing therapeutic indexes; a systematic review. Int. J. Surg. 2022, 101, 106633. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Palanivelu, C.; Senthilnathan, P.; Sabnis, S.C.; Babu, N.S.; Gurumurthy, S.S.; Vijai, N.A.; Nalankilli, V.P.; Raj, P.P.; Parthasarathy, R.; Rajapandian, S. Randomized clinical trial of laparoscopic versus open pancreatoduodenectomy for periampullary tumours. Br. J. Surg. 2017, 104, 1443–1450. [Google Scholar] [CrossRef]
- Poves, I.; Burdío, F.; Morató, O.; Iglesias, M.; Radosevic, A.; Ilzarbe, L.; Visa, L.; Grande, L. Comparison of Perioperative Outcomes Between Laparoscopic and Open Approach for Pancreatoduodenectomy: The PADULAP Randomized Controlled Trial. Ann. Surg. 2018, 268, 731–739. [Google Scholar] [CrossRef]
- Wang, M.; Pan, S.; Qin, T.; Xu, X.; Huang, X.; Liu, J.; Chen, X.; Zhao, W.; Li, J.; Liu, C.; et al. Short-Term Outcomes Following Laparoscopic vs Open Pancreaticoduodenectomy in Patients With Pancreatic Ductal Adenocarcinoma: A Randomized Clinical Trial. JAMA Surg. 2023, 158, 1245–1253. [Google Scholar] [CrossRef]
- Qin, T.; Zhang, H.; Pan, S.; Liu, J.; Li, D.; Chen, R.; Huang, X.; Liu, Y.; Liu, J.; Cheng, W.; et al. Effect of Laparoscopic and Open Pancreaticoduodenectomy for Pancreatic or Periampullary Tumors: Three-year Follow-up of a Randomized Clinical Trial. Ann. Surg. 2024, 279, 605–612. [Google Scholar] [CrossRef]
- Yoon, Y.-S.; Lee, W.; Kang, C.M.; Hong, T.; Shin, S.H.; Hwang, D.W.; Song, K.B.; Kwon, J.W.; Sung, M.K.; Shim, I.K.; et al. Laparoscopic versus open pancreatoduodenectomy for periampullary tumors: A randomized clinical trial. Int. J. Surg. 2024, 110, 7011–7019. [Google Scholar] [CrossRef]
- Liu, Q.; Li, M.; Gao, Y.; Jiang, T.; Han, B.; Zhao, G.; Lin, C.; Lau, W.Y.; Zhao, Z.; Liu, R. Effect of robotic versus open pancreaticoduodenectomy on postoperative length of hospital stay and complications for pancreatic head or periampullary tumours: A multicentre, open-label randomised controlled trial. Lancet Gastroenterol. Hepatol. 2024, 9, 428–437. [Google Scholar] [CrossRef]
- Klotz, R.; Mihaljevic, A.L.; Kulu, Y.; Sander, A.; Klose, C.; Behnisch, R.; Joos, M.C.; Kalkum, E.; Nickel, F.; Knebel, P.; et al. Robotic versus open partial pancreatoduodenectomy (EUROPA): A randomised controlled stage 2b trial. Lancet Reg. Health—Eur. 2024, 39, 100864. [Google Scholar] [CrossRef] [PubMed]
- Cameron, J.L.; Pitt, H.A.; Yeo, C.J.; Lillemoe, K.D.; Kaufman, H.S.; Coleman, J. One hundred and forty-five consecutive pancreaticoduodenectomies without mortality. Ann. Surg. 1993, 217, 430–435; discussion 435–438. [Google Scholar] [CrossRef] [PubMed]
- Lillemoe, K.D.; Kaushal, S.; Cameron, J.L.; Sohn, T.A.; Pitt, H.A.; Yeo, C.J. Distal pancreatectomy: Indications and outcomes in 235 patients. Ann. Surg. 1999, 229, 693–698; discussion 698–700. [Google Scholar] [CrossRef]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef]
- Birkmeyer, J.D.; Siewers, A.E.; Finlayson, E.V.A.; Stukel, T.A.; Lucas, F.L.; Batista, I.; Welch, H.G.; Wennberg, D.E. Hospital volume and surgical mortality in the United States. N. Engl. J. Med. 2002, 346, 1128–1137. [Google Scholar] [CrossRef]
- Elta, G.H.; Enestvedt, B.K.; Sauer, B.G.; Lennon, A.M. ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am. J. Gastroenterol. 2018, 113, 464–479. [Google Scholar] [CrossRef]
- Chen, L.; Yuan, S.; Xu, Q.; Cui, M.; Li, P.; Liu, W.; Lin, C.; Chen, W.; Chen, H.; Hu, Y.; et al. Outcomes evaluation of robotic versus laparoscopic pancreaticoduodenectomy: A propensity score matching and learning curve analysis. Surg. Endosc. 2025, 39, 3681–3690. [Google Scholar] [CrossRef] [PubMed]
- The First Affiliated Hospital of University of South China A Multicenter Prospective Randomized Controlled Study of Robot Pancreaticoduodenectomy Versus Laparoscopic Pancreaticoduodenectomy. 2023. Available online: https://clinicaltrials.gov (accessed on 9 January 2025).
- McCulloch, P.; Altman, D.G.; Campbell, W.B.; Flum, D.R.; Glasziou, P.; Marshall, J.C.; Nicholl, J. No surgical innovation without evaluation: The IDEAL recommendations. Lancet Lond. Engl. 2009, 374, 1105–1112. [Google Scholar] [CrossRef]
- Zureikat, A.H.; Beane, J.D.; Zenati, M.S.; Al Abbas, A.I.; Boone, B.A.; Moser, A.J.; Bartlett, D.L.; Hogg, M.E.; Zeh, H.J. 500 Minimally Invasive Robotic Pancreatoduodenectomies: One Decade of Optimizing Performance. Ann. Surg. 2021, 273, 966–972. [Google Scholar] [CrossRef]
- Nickel, F.; Haney, C.M.; Kowalewski, K.F.; Probst, P.; Limen, E.F.; Kalkum, E.; Diener, M.K.; Strobel, O.; Müller-Stich, B.P.; Hackert, T. Laparoscopic Versus Open Pancreaticoduodenectomy: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Ann. Surg. 2020, 271, 54–66. [Google Scholar] [CrossRef]
- Kabir, T.; Tan, H.L.; Syn, N.L.; Wu, E.J.; Kam, J.H.; Goh, B.K.P. Outcomes of laparoscopic, robotic, and open pancreatoduodenectomy: A network meta-analysis of randomized controlled trials and propensity-score matched studies. Surgery 2022, 171, 476–489. [Google Scholar] [CrossRef] [PubMed]
- Aiolfi, A.; Lombardo, F.; Bonitta, G.; Danelli, P.; Bona, D. Systematic review and updated network meta-analysis comparing open, laparoscopic, and robotic pancreaticoduodenectomy. Updates Surg. 2021, 73, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Joseph, N.; Varghese, C.; Lucocq, J.; McGuinness, M.J.; Tingle, S.; Marchegiani, G.; Soreide, K.; Abu-Hilal, M.; Samra, J.; Besselink, M.; et al. Network Meta-Analysis and Trial Sequential Analysis of Randomised Controlled Trials Comparing Robotic, Laparoscopic, and Open Pancreatoduodenectomy. Ann. Surg. Open Perspect. Surg. Hist. Educ. Clin. Approaches 2024, 5, e507. [Google Scholar] [CrossRef] [PubMed]
- Coolsen, M.M.E.; van Dam, R.M.; van der Wilt, A.A.; Slim, K.; Lassen, K.; Dejong, C.H.C. Systematic review and meta-analysis of enhanced recovery after pancreatic surgery with particular emphasis on pancreaticoduodenectomies. World J. Surg. 2013, 37, 1909–1918. [Google Scholar] [CrossRef]
- Melloul, E.; Lassen, K.; Roulin, D.; Grass, F.; Perinel, J.; Adham, M.; Wellge, E.B.; Kunzler, F.; Besselink, M.G.; Asbun, H.; et al. Guidelines for Perioperative Care for Pancreatoduodenectomy: Enhanced Recovery After Surgery (ERAS) Recommendations 2019. World J. Surg. 2020, 44, 2056–2084. [Google Scholar] [CrossRef]
- Spanjersberg, W.R.; van Sambeeck, J.D.P.; Bremers, A.; Rosman, C.; van Laarhoven, C.J.H.M. Systematic review and meta-analysis for laparoscopic versus open colon surgery with or without an ERAS programme. Surg. Endosc. 2015, 29, 3443–3453. [Google Scholar] [CrossRef]
- Van Den Hoven, P.; Osterkamp, J.; Nerup, N.; Svendsen, M.B.S.; Vahrmeijer, A.; Van Der Vorst, J.R.; Achiam, M.P. Quantitative perfusion assessment using indocyanine green during surgery—Current applications and recommendations for future use. Langenbecks Arch. Surg. 2023, 408, 67. [Google Scholar] [CrossRef]
- Morales-Conde, S.; Licardie, E.; Alarcón, I.; Balla, A. Indocyanine green (ICG) fluorescence guide for the use and indications in general surgery: Recommendations based on the descriptive review of the literature and the analysis of experience. Cir. Esp. 2022, 100, 534–554. [Google Scholar] [CrossRef]
- Partridge, J.S.L.; Harari, D.; Martin, F.C.; Dhesi, J.K. The impact of pre-operative comprehensive geriatric assessment on postoperative outcomes in older patients undergoing scheduled surgery: A systematic review. Anaesthesia 2014, 69 (Suppl. S1), 8–16. [Google Scholar] [CrossRef]
- Cunha, A.I.L.; Veronese, N.; de Melo Borges, S.; Ricci, N.A. Frailty as a predictor of adverse outcomes in hospitalized older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2019, 56, 100960. [Google Scholar] [CrossRef]
- Baimas-George, M.; Watson, M.; Elhage, S.; Parala-Metz, A.; Vrochides, D.; Davis, B.R. Prehabilitation in Frail Surgical Patients: A Systematic Review. World J. Surg. 2020, 44, 3668–3678. [Google Scholar] [CrossRef]
- Boone, B.A.; Zenati, M.; Hogg, M.E.; Steve, J.; Moser, A.J.; Bartlett, D.L.; Zeh, H.J.; Zureikat, A.H. Assessment of Quality Outcomes for Robotic Pancreaticoduodenectomy: Identification of the Learning Curve. JAMA Surg. 2015, 150, 416–422. [Google Scholar] [CrossRef]
- Figueras, J.; Sabater, L.; Planellas, P.; Muñoz-Forner, E.; Lopez-Ben, S.; Falgueras, L.; Sala-Palau, C.; Albiol, M.; Ortega-Serrano, J.; Castro-Gutierrez, E. Randomized clinical trial of pancreaticogastrostomy versus pancreaticojejunostomy on the rate and severity of pancreatic fistula after pancreaticoduodenectomy. Br. J. Surg. 2013, 100, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Diener, M.K.; Seiler, C.M.; Rossion, I.; Kleeff, J.; Glanemann, M.; Butturini, G.; Tomazic, A.; Bruns, C.J.; Busch, O.R.C.; Farkas, S.; et al. Efficacy of stapler versus hand-sewn closure after distal pancreatectomy (DISPACT): A randomised, controlled multicentre trial. Lancet 2011, 377, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Braga, M.; Pecorelli, N.; Ariotti, R.; Capretti, G.; Greco, M.; Balzano, G.; Castoldi, R.; Beretta, L. Enhanced recovery after surgery pathway in patients undergoing pancreaticoduodenectomy. World J. Surg. 2014, 38, 2960–2966. [Google Scholar] [CrossRef]
- Klek, S.; Sierzega, M.; Szybinski, P.; Szczepanek, K.; Scislo, L.; Walewska, E.; Kulig, J. Perioperative nutrition in malnourished surgical cancer patients—A prospective, randomized, controlled clinical trial. Clin. Nutr. 2011, 30, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, S.B.; Jamieson, N.B.; Upstill-Goddard, R.; Bailey, P.J.; McKay, C.J.; Australian Pancreatic Cancer Genome Initiative; Biankin, A.V.; Chang, D.K. Defining the molecular pathology of pancreatic body and tail adenocarcinoma. Br. J. Surg. 2018, 105, e183–e191. [Google Scholar] [CrossRef]
- Pietrasz, D.; Pécuchet, N.; Garlan, F.; Didelot, A.; Dubreuil, O.; Doat, S.; Imbert-Bismut, F.; Karoui, M.; Vaillant, J.-C.; Taly, V.; et al. Plasma Circulating Tumor DNA in Pancreatic Cancer Patients Is a Prognostic Marker. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 116–123. [Google Scholar] [CrossRef]
- Kraftician, J.D.; Kuchta, K.; Zenati, M.S.; Hays, S.B.; AlMasri, S.; Khachfe, H.H.; Maalouf, M.; Desilva, A.; Hammad, A.Y.; Paniccia, A.; et al. Biotissue Curriculum Translates to Performance in the Operating Room for Gastrojejunostomy and Hepaticojejunostomy in Robotic Pancreaticoduodenectomy. J. Surg. Educ. 2025, 82, 103395. [Google Scholar] [CrossRef]
- Müller, P.C.; Kuemmerli, C.; Billeter, A.T.; Shen, B.; Jin, J.; Nickel, F.; Guidetti, C.; Kauffmann, E.; Purchla, J.; Tschuor, C.; et al. Competency, Proficiency, and Mastery: Learning Curves for Robotic Distal Pancreatectomy at 16 International Expert Centers. Ann. Surg. 2024. [Google Scholar] [CrossRef]
- Tang, Y.; Peng, X.; He, Y.; Li, J.; Zheng, L.; Huang, X. A novel laparoscopic pancreaticoduodenal training model: Optimization of the learning curve and simplification of postoperative complications. Int. J. Surg. 2024, 110, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, R.; Imamura, H.; Ichida, H.; Gyoda, Y.; Mizuno, T.; Mise, Y.; Saiura, A.; Chen, R.J. Simulation training in pancreatico-jejunostomy using an inanimate biotissue model improves the technical skills of hepatobiliary-pancreatic surgical fellows. PLoS ONE 2021, 16, e0244915. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, C.; Zureikat, A.H. The Role of Simulation in Attaining Proficiency in Minimally Invasive Hepatopancreatobiliary Surgery. J. Laparoendosc. Adv. Surg. Tech. A 2021, 31, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Neshan, M.; Padmanaban, V.; Chick, R.C.; Pawlik, T.M. Open vs robotic-assisted pancreaticoduodenectomy, cost-effectiveness and long-term oncologic outcomes: A systematic review and meta-analysis. J. Gastrointest. Surg. 2024, 28, 1933–1942. [Google Scholar] [CrossRef]
- Baker, E.H.; Ross, S.W.; Seshadri, R.; Swan, R.Z.; Iannitti, D.A.; Vrochides, D.; Martinie, J.B. Robotic pancreaticoduodenectomy: Comparison of complications and cost to the open approach. Int. J. Med. Robot. Comput. Assist. Surg. 2016, 12, 554–560. [Google Scholar] [CrossRef]
- Hashimoto, D.A.; Rosman, G.; Rus, D.; Meireles, O.R. Artificial Intelligence in Surgery: Promises and Perils. Ann. Surg. 2018, 268, 70–76. [Google Scholar] [CrossRef]
- Mangano, A.; Valle, V.; Dreifuss, N.H.; Aguiluz, G.; Masrur, M.A. Role of Artificial Intelligence (AI) in Surgery: Introduction, General Principles, and Potential Applications. Surg. Technol. Int. 2020, 38, 17–21. [Google Scholar] [CrossRef]


| Study | Country | Total No. | OPD No. | LPD No. | RPD No. | Age (y) OPD | Age LPD | Age RPD | BMI OPD (kg/m2) | BMI LPD | BMI RPD | ASA I OPD/LPD/RPD | ASA II | ASA III | BD OPD (n) | BD LPD (n) | BD RPD (n) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Palanivelu et al., 2017 [15] | India | 64 | 32 | 32 | - | 58.6 | 57.8 | - | 22.4 | 24.9 | - | 11/13/- | 18/17/- | 3/2/- | 11 | 8 | - |
| Poves et al., 2018 [16] | Spain | 66 | 32 | 34 | - | 70 | 69 | - | 26 | 24 | - | 1/1/- | 13/18/- | 15/13/- | NA | NA | - |
| Hilst et al., 2019 [11] | Netherlands | 99 | 49 | 50 | - | 66 | 67 | - | 26 | 25 | - | 7/5/- | 26/32/- | 16/13/- | 26 | 25 | - |
| Wang et al., 2023 [17] | China | 200 | 100 | 100 | - | 60.7 | 61.9 | - | 22.3 | 22.9 | - | 22/32/- | 54/41/- | 24/27/- | 37 | 44 | - |
| Qin et al., 2024 [18] | China | 529 | 261 | 268 | - | 57.8 | 59.2 | - | 22 | 22.4 | - | 44/47/- | 168/158/- | 49/63/- | 68 | 63 | - |
| Yoon et al., 2024 [19] | South Korea | 235 | 119 | 116 | - | 63.2 | 62.5 | - | 23.6 | 23.9 | - | 6/8/- | 104/102/- | 17/15/- | 45 | 55 | - |
| Klotz et al., 2024 [21] | Germany | 62 | 33 | - | 29 | 62.6 | - | 64.7 | 26.6 | - | 26.9 | 0/-/0 | 20/-/18 | 13/-/8 | 7 | - | 9 |
| Liu et al., 2024 [20] | China | 161 | 80 | - | 81 | 60.0 | - | 62.0 | 23.3 | - | 23.3 | 8/-/9 | 64/-/66 | 8/-/6 | 36 | - | 35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valle, V.; Pakataridis, P.; Marchese, T.; Ferrari, C.; Chelmis, F.; Sorotou, I.N.; Gianniou, M.-A.; Dimova, A.; Tcholakov, O.; Ielpo, B. Comparative Analysis of Open, Laparoscopic, and Robotic Pancreaticoduodenectomy: A Systematic Review of Randomized Controlled Trials. Medicina 2025, 61, 1121. https://doi.org/10.3390/medicina61071121
Valle V, Pakataridis P, Marchese T, Ferrari C, Chelmis F, Sorotou IN, Gianniou M-A, Dimova A, Tcholakov O, Ielpo B. Comparative Analysis of Open, Laparoscopic, and Robotic Pancreaticoduodenectomy: A Systematic Review of Randomized Controlled Trials. Medicina. 2025; 61(7):1121. https://doi.org/10.3390/medicina61071121
Chicago/Turabian StyleValle, Valentina, Paraskevas Pakataridis, Tiziana Marchese, Cecilia Ferrari, Filippos Chelmis, Iliana N. Sorotou, Maria-Anna Gianniou, Aleksandra Dimova, Oleg Tcholakov, and Benedetto Ielpo. 2025. "Comparative Analysis of Open, Laparoscopic, and Robotic Pancreaticoduodenectomy: A Systematic Review of Randomized Controlled Trials" Medicina 61, no. 7: 1121. https://doi.org/10.3390/medicina61071121
APA StyleValle, V., Pakataridis, P., Marchese, T., Ferrari, C., Chelmis, F., Sorotou, I. N., Gianniou, M.-A., Dimova, A., Tcholakov, O., & Ielpo, B. (2025). Comparative Analysis of Open, Laparoscopic, and Robotic Pancreaticoduodenectomy: A Systematic Review of Randomized Controlled Trials. Medicina, 61(7), 1121. https://doi.org/10.3390/medicina61071121








