Prognostic Nutritional Index and a Blood-Based Prognostic Tool in Prostate Cancer Treated with Abiraterone, Enzalutamide or Cabazitaxel
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Prognostic Nutritional Index Calculation
2.3. Definition of Clinical Outcomes
2.4. Statistical Analyses
3. Results
3.1. Patient Characteristics
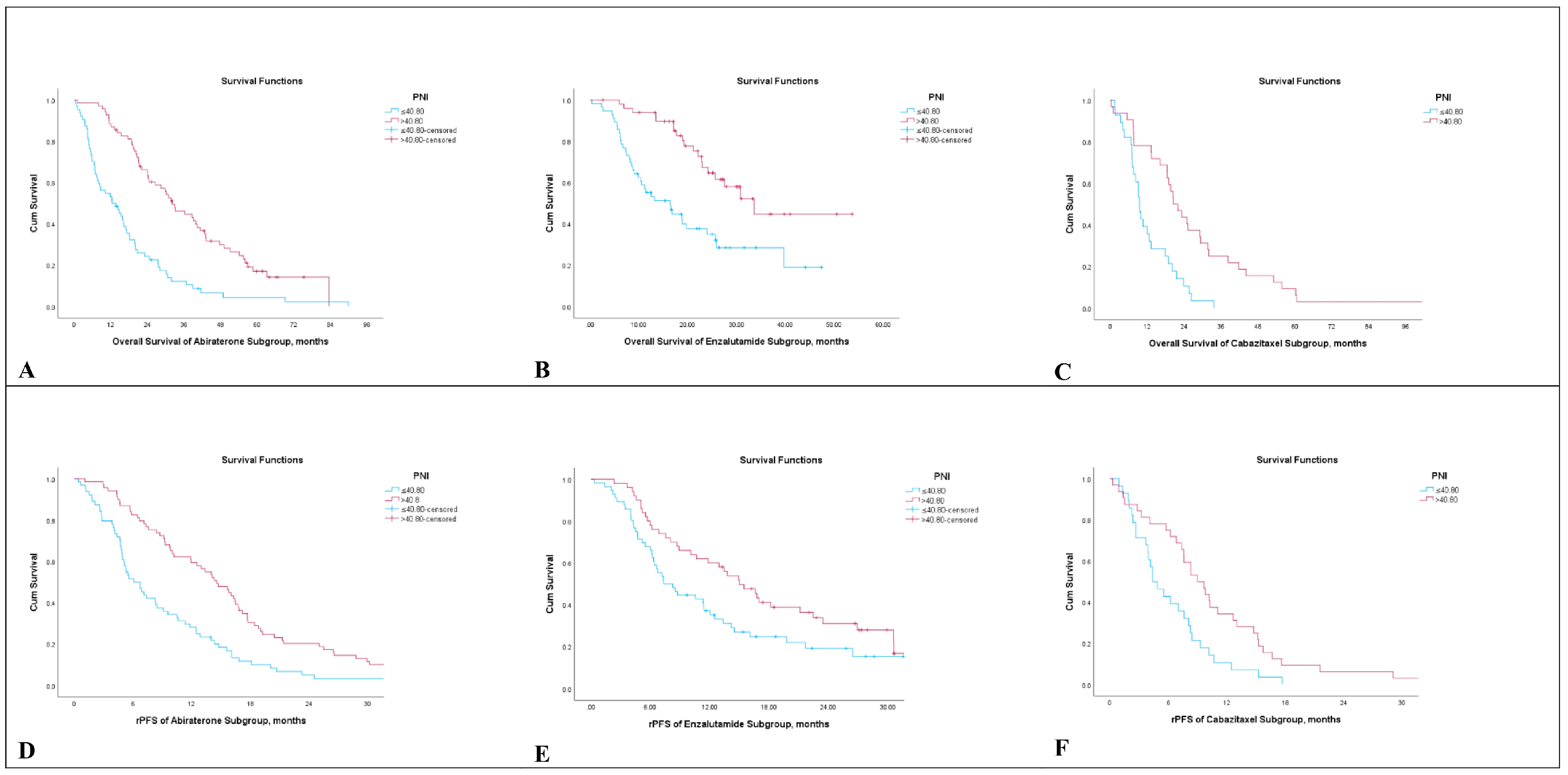
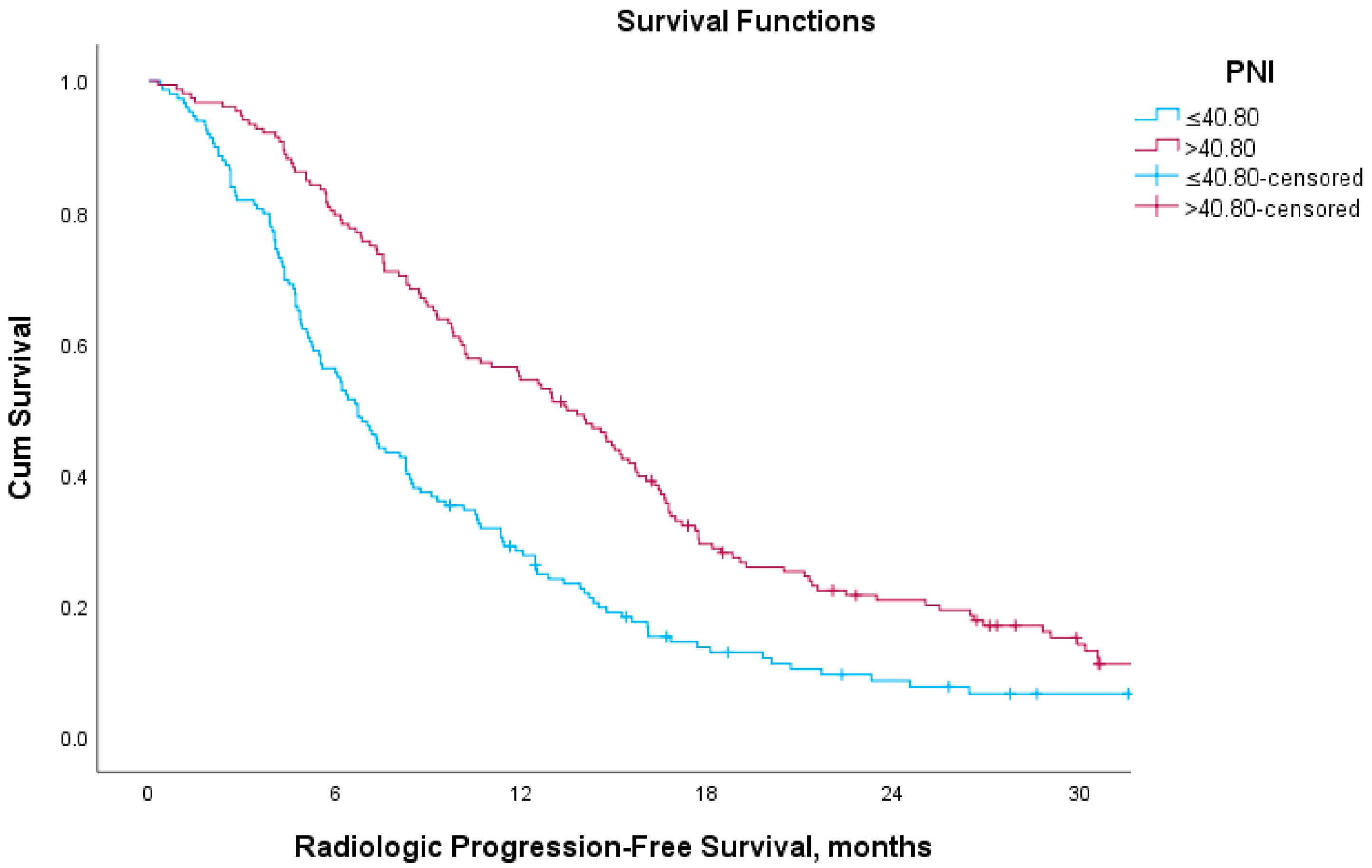
3.2. Overall Survival, Radiological Progression-Free Survival and PSA Response Analysis
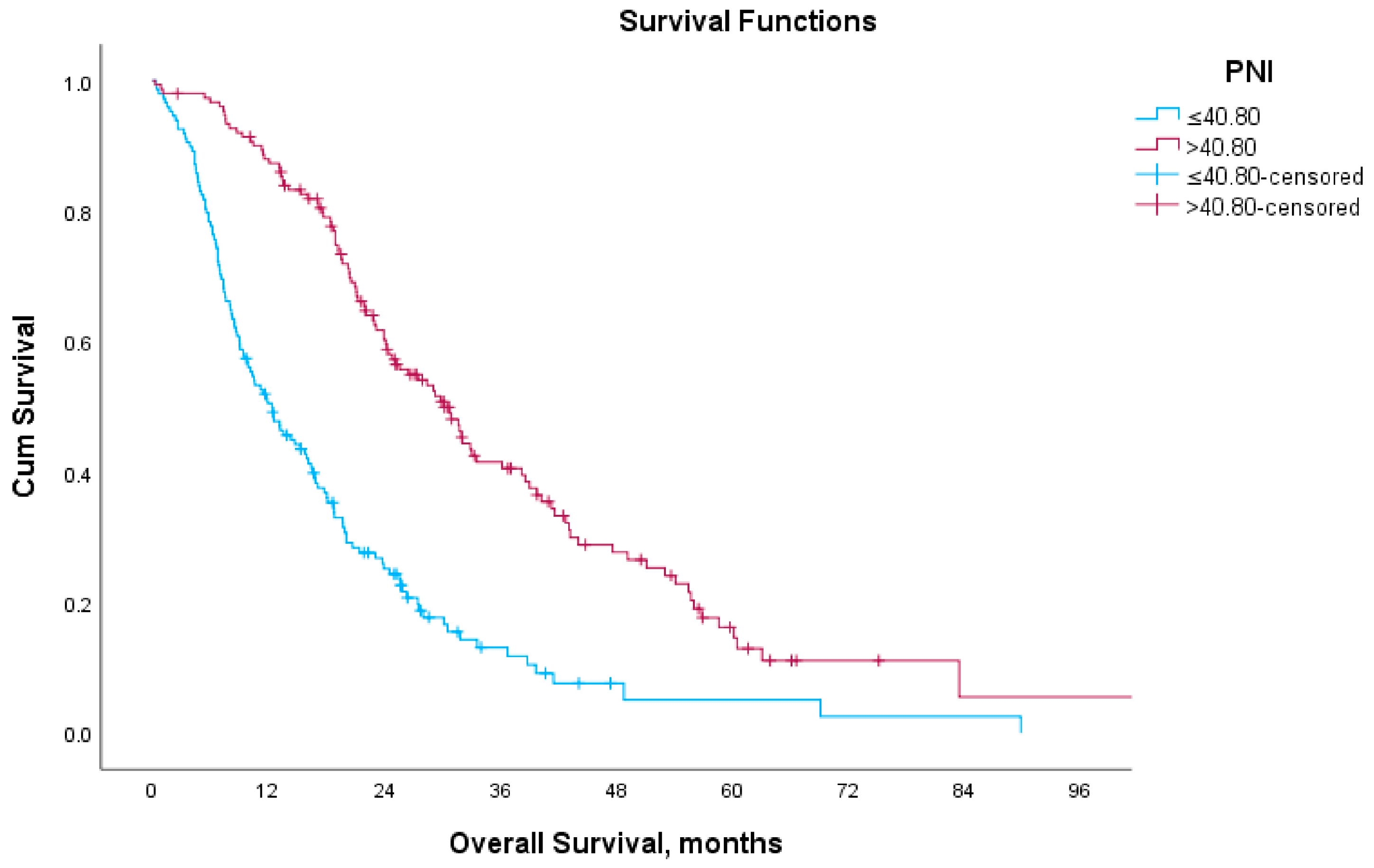
3.3. Survival Analysis According to Prognostic Nutritional Index
3.4. Multivariate Cox Regression Analysis
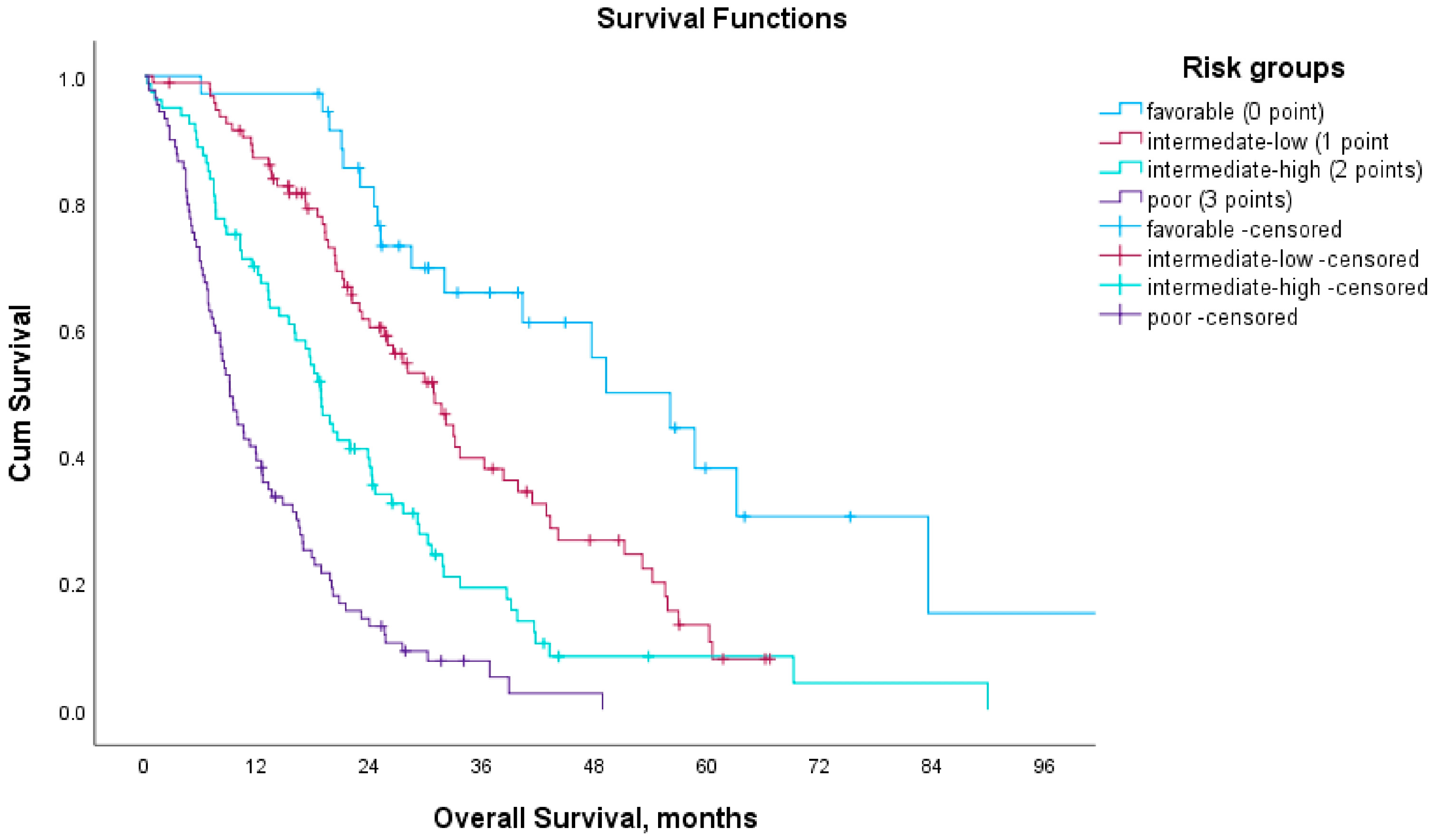
3.5. Prognostic Model Based on Blood-Based Biomarkers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Prostate Cancer (Version 2.2025). 2025. Available online: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdfhttps://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (accessed on 20 April 2025).
- Fizazi, K.; Scher, H.I.; Molina, A.; Logothetis, C.J.; Chi, K.N.; Jones, R.J.; Staffurth, J.N.; North, S.; Vogelzang, N.J.; Saad, F.; et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: Final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012, 13, 983–992. [Google Scholar] [CrossRef]
- Scher, H.I.; Fizazi, K.; Saad, F.; Taplin, M.-E.; Sternberg, C.N.; Miller, K.; de Wit, R.; Mulders, P.; Chi, K.N.; Shore, N.D.; et al. Increased Survival with Enzalutamide in Prostate Cancer after Chemotherapy. N. Engl. J. Med. 2012, 367, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- de Wit, R.; de Bono, J.; Sternberg, C.N.; Fizazi, K.; Tombal, B.; Wülfing, C.; Kramer, G.; Eymard, J.-C.; Bamias, A.; Carles, J.; et al. Cabazitaxel versus Abiraterone or Enzalutamide in Metastatic Prostate Cancer. N. Engl. J. Med. 2019, 381, 2506–2518. [Google Scholar] [CrossRef]
- Conteduca, V.; Mosca, A.; Brighi, N.; de Giorgi, U.; Rescigno, P. New Prognostic Biomarkers in Metastatic Castration-Resistant Prostate Cancer. Cells 2021, 10, 193. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.W.; Hung, S.C.; Li, J.R.; Chiu, K.Y.; Yang, C.K.; Chen, C.S.; Lu, K.; Chen, C.C.; Wang, S.C.; Lin, C.Y.; et al. Geriatric Nutritional Risk Index as a Prognostic Marker for Patients With Metastatic Castration-Resistant Prostate Cancer Receiving Docetaxel. Front. Pharmacol. 2020, 11, 601513. [Google Scholar] [CrossRef]
- Küçükarda, A.; Gökyer, A.; Gökmen, I.; Özcan, E.; Hacıoğlu, M.B.; Erdoğan, B.; Uzunoğlu, S.; Çiçin, I. Prognostic nutritional index is an independent prognostic factor for treatment response, survival and drug choice in metastatic castration-resistant prostate cancer treated with abiraterone acetate or enzalutamide. Actas Urológicas Españolas (Engl. Ed.) 2022, 46, 301–309. [Google Scholar] [CrossRef]
- Fan, L.; Wang, X.; Chi, C.; Wang, Y.; Cai, W.; Shao, X.; Xu, F.; Pan, J.; Zhu, Y.; Shangguan, X.; et al. Prognostic nutritional index predicts initial response to treatment and prognosis in metastatic castration-resistant prostate cancer patients treated with abiraterone. Prostate 2017, 77, 1233–1241. [Google Scholar] [CrossRef]
- Fan, L.; Wang, R.; Chi, C.; Cai, W.; Zhang, Y.; Qian, H.; Shao, X.; Wang, Y.; Xu, F.; Pan, J.; et al. Systemic immune-inflammation index predicts the combined clinical outcome after sequential therapy with abiraterone and docetaxel for metastatic castration-resistant prostate cancer patients. Prostate 2018, 78, 250–256. [Google Scholar] [CrossRef]
- Guan, Y.; Xiong, H.; Feng, Y.; Liao, G.; Tong, T.; Pang, J. Revealing the prognostic landscape of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in metastatic castration-resistant prostate cancer patients treated with abiraterone or enzalutamide: A meta-analysis. Prostate Cancer Prostatic Dis. 2020, 23, 220–231. [Google Scholar] [CrossRef]
- Zhang, Q.; Qian, L.; Liu, T.; Ding, J.S.; Zhang, X.; Song, M.M.; Wang, Z.W.; Ge, Y.Z.; Hu, C.L.; Li, X.R.; et al. Prevalence and Prognostic Value of Malnutrition Among Elderly Cancer Patients Using Three Scoring Systems. Front. Nutr. 2021, 8, 738550. [Google Scholar] [CrossRef]
- Onodera, T.; Goseki, N.; Kosaki, G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi 1984, 85, 1001–1005. [Google Scholar] [PubMed]
- Mirili, C.; Yılmaz, A.; Demirkan, S.; Bilici, M.; Basol Tekin, S. Clinical significance of prognostic nutritional index (PNI) in malignant melanoma. Int. J. Clin. Oncol. 2019, 24, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Luan, C.W.; Tsai, Y.T.; Yang, H.Y.; Chen, K.Y.; Chen, P.H.; Chou, H.H. Pretreatment prognostic nutritional index as a prognostic marker in head and neck cancer: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 17117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Bao, J.; Zhu, Z.Y.; Jin, M.X. Prognostic nutritional index as a prognostic factor in lung cancer patients receiving chemotherapy: A systematic review and meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 5636–5652. [Google Scholar]
- Yasar, H.A.; Bir Yucel, K.; Arslan, C.; Ucar, G.; Karakaya, S.; Bilgin, B.; Taban, H.; Kucukarda, A.; Erturk, I.; Hızal, M.; et al. The relationship between prognostic nutritional index and treatment response in patients with metastatic renal cell cancer. J. Oncol. Pharm. Pr. 2020, 26, 1110–1116. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y. The prognostic nutritional index is prognostic factor of gynecological cancer: A systematic review and meta-analysis. Int. J. Surg. 2019, 67, 79–86. [Google Scholar] [CrossRef]
- Ucar, G.; Ergun, Y.; Acikgoz, Y.; Uncu, D. The prognostic value of the prognostic nutritional index in patients with metastatic colorectal cancer. Asia-Pac. J. Clin. Oncol. 2020, 16, e179–e184. [Google Scholar] [CrossRef]
- Keskinkilic, M.; Semiz, H.S.; Ataca, E.; Yavuzsen, T. The prognostic value of immune-nutritional status in metastatic colorectal cancer: Prognostic Nutritional Index (PNI). Support. Care Cancer 2024, 32, 374. [Google Scholar] [CrossRef]
- Tobing, E.; Tansol, C.; Tania, C.; Sihombing, A.T. Prognostic Nutritional Index (PNI) as Independent Predictor of Poor Survival in Prostate Cancer: A Systematic Review and Meta-Analysis. Clin. Genitourin. Cancer 2024, 22, 102142. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, K.; Ou, Y.; Hu, X.; Wang, Z.; Wang, D.; Li, X.; Ren, S. Prognostic value of a baseline prognostic nutritional index for patients with prostate cancer: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2024, 27, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Halabi, S.; Tannock, I.; Morris, M.; Sternberg, C.N.; Carducci, M.A.; Eisenberger, M.A.; Higano, C.; Bubley, G.J.; Dreicer, R.; et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J. Clin. Oncol. 2008, 26, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.J.; Smith, M.R.; de Bono, J.S.; Molina, A.; Logothetis, C.J.; de Souza, P.; Fizazi, K.; Mainwaring, P.; Piulats, J.M.; Ng, S.; et al. Abiraterone in Metastatic Prostate Cancer without Previous Chemotherapy. N. Engl. J. Med. 2012, 368, 138–148. [Google Scholar] [CrossRef]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Evans, C.P.; Kim, C.-S.; Kimura, G.; et al. Enzalutamide in Men with Chemotherapy-naïve Metastatic Castration-resistant Prostate Cancer: Extended Analysis of the Phase 3 PREVAIL Study. Eur. Urol. 2017, 71, 151–154. [Google Scholar] [CrossRef]
- de Bono, J.S.; Oudard, S.; Ozguroglu, M.; Hansen, S.; Machiels, J.-P.; Kocak, I.; Gravis, G.; Bodrogi, I.; Mackenzie, M.J.; Shen, L.; et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet 2010, 376, 1147–1154. [Google Scholar] [CrossRef]
- Roxburgh, C.S.D.; McMillan, D.C. Cancer and systemic inflammation: Treat the tumour and treat the host. Br. J. Cancer 2014, 110, 1409–1412. [Google Scholar] [CrossRef]
- Gupta, D.; Lis, C.G. Pretreatment serum albumin as a predictor of cancer survival: A systematic review of the epidemiological literature. Nutr. J. 2010, 9, 69. [Google Scholar] [CrossRef]
- Guven, D.C.; Aktepe, O.H.; Taban, H.; Aktas, B.Y.; Guner, G.; Yildirim, H.C.; Sahin, T.K.; Aksun, M.S.; Dizdar, O.; Aksoy, S.; et al. Lower prognostic nutritional index is associated with poorer survival in patients receiving immune checkpoint inhibitors. Biomark. Med. 2021, 15, 1123–1130. [Google Scholar] [CrossRef]
- Guven, D.C.; Yildirim, H.C.; Bilgin, E.; Aktepe, O.H.; Taban, H.; Sahin, T.K.; Cakir, I.Y.; Akin, S.; Dizdar, O.; Aksoy, S.; et al. PILE: A candidate prognostic score in cancer patients treated with immunotherapy. Clin. Transl. Oncol. 2021, 23, 1630–1636. [Google Scholar] [CrossRef]
- Li, B.; Lu, Z.; Wang, S.; Hou, J.; Xia, G.; Li, H.; Yin, B.; Lu, W. Pretreatment elevated prognostic nutritional index predicts a favorable prognosis in patients with prostate cancer. BMC Cancer 2020, 20, 361. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Song, Q.; Cheng, Y.; Wang, N. Prognostic Significance of Preoperative Prognostic Nutritional Index for Overall Survival and Postoperative Complications in Esophageal Cancer Patients. Cancer Manag. Res. 2021, 13, 8585–8597. [Google Scholar] [CrossRef] [PubMed]
- Hacioglu, M.B.; Kucukarda, A.; Gokmen, I.; Gurbuz, A.F.; Araz, M.; Kahvecioglu, F.A.; Hacibekiroglu, I.; Akdoğan, O.; Yazıcı, O.; Akkus, F.A.; et al. Prognostic Nutritional Index as a Biomarker in Metastatic Hormone-Sensitive Prostate Cancer: Impact on Survival and Treatment Optimization. Prostate 2025, 85, 693–702. [Google Scholar] [CrossRef]
- Evans, C.P.; Higano, C.S.; Keane, T.; Andriole, G.; Saad, F.; Iversen, P.; Miller, K.; Kim, C.-S.; Kimura, G.; Armstrong, A.J.; et al. The PREVAIL Study: Primary Outcomes by Site and Extent of Baseline Disease for Enzalutamide-treated Men with Chemotherapy-naïve Metastatic Castration-resistant Prostate Cancer. Eur. Urol. 2016, 70, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Chi, K.N.; Kheoh, T.; Ryan, C.J.; Molina, A.; Bellmunt, J.; Vogelzang, N.J.; Rathkopf, D.E.; Fizazi, K.; Kantoff, P.W.; Li, J.; et al. A prognostic index model for predicting overall survival in patients with metastatic castration-resistant prostate cancer treated with abiraterone acetate after docetaxel. Ann. Oncol. 2016, 27, 454–460. [Google Scholar] [CrossRef]
| Characteristic | n = 299 (%) |
|---|---|
| Age of diagnosis (mean, SD) | 65.2 (±9.0) |
| <70 yr | 207 (69.2%) |
| ≥70 yr | 92 (30.8%) |
| PSA level ng/mL (median, IQR) | |
| (n = 186) | 58.5 (16.8–180.8) |
| M1 disease at diagnosis | |
| Yes | 179 (59.9%) |
| No | 120 (40.1%) |
| Gleason score (n = 247) | |
| <8 | 52 (21.1%) |
| ≥8 | 195 (78.9%) |
| Prostate Surgery | |
| Yes | 61 (20.4%) |
| No | 238 (79.6%) |
| Definitive or salvage radiotherapy | |
| Yes | 81 (27.1%) |
| No | 218 (72.9%) |
| Orchiectomy | |
| Yes | 65 (21.7%) |
| No | 234 (78.3%) |
| Comorbidities | |
| Hypertension | 107 (35.8%) |
| Cardiovascular disease | 82 (27.4%) |
| Diabetes | 60 (20.1%) |
| Chronic kidney disease | 22 (7.4%) |
| Previous docetaxel treatment | |
| Yes | 234 (78.3%) |
| No | 65 (21.7%) |
| Number of docetaxel cycles | |
| Median (range) | 6 (1–32) |
| Treatment Subgroups | |
| Abiraterone acetate | 133 (44.5%) |
| Enzalutamide | 106 (35.5%) |
| Cabazitaxel | 60 (20.0%) |
| PNI (median) | |
| PNI ≤ 40.8 | 148 (49.5%) |
| PNI > 40.8 | 151 (50.5%) |
| Parameter | Low PNI (PNI ≤ 40.8) n = 148 (%) | High PNI (PNI > 40.8) n = 151 (%) | p-Value |
|---|---|---|---|
| Abiraterone or Enzalutamide or Cabazitaxel starting age, years Mean (SD) | 71.9 (±8.9) | 68.3 (±9.4) | <0.001 |
| Abiraterone or Enzalutamide or Cabazitaxel starting age | 0.145 | ||
| <70 years | 64 (43.2%) | 78 (51.7%) | |
| ≥70 years | 84 (56.8%) | 73 (48.3%) | |
| PSA level-ng/mL (before treatment) (median/IQR) | 70.1 (16.7–202.5) | 24.7 (9.5–70.2) | <0.001 |
| M1 disease at diagnosis | 0.741 | ||
| M0 | 58 (39.2%) | 62 (41.1%) | |
| M1 | 90 (60.8%) | 89 (58.9%) | |
| Gleason score | 0.974 | ||
| <8 | 26 (21.0%) | 26 (21.1%) | |
| ≥8 | 98 (79.0%) | 97 (78.9%) | |
| Previous docetaxel treatment | 0.056 | ||
| Yes | 39 (26.4%) | 26 (17.2%) | |
| No | 109 (73.6%) | 125 (82.8%) | |
| Treatment groups Abiraterone Acetate Enzalutamide Cabazitaxel | 0.682 | ||
| 64 (43.2%) | 69 (45.7%) | ||
| 56 (37.8%) | 50 (33.1%) | ||
| 28 (18.9) | 32 (21.2%) | ||
| Metastasis sites | 0.144 | ||
| Bone | |||
| Yes | 131 (88.5%) | 123 (82.6%) | |
| No | 17 (11.5%) | 26 (17.4%) | |
| Lymph nodes | 0.581 | ||
| Yes | 94 (63.5%) | 90 (60.4%) | |
| No | 54 (36.5%) | 59 (39.6%) | |
| Visceral | 0.023 | ||
| Yes | 30 (20.3%) | 16 (10.7%) | |
| No | 118 (79.7%) | 133 (89.3%) | |
| Hemoglobin level (g/dL) | <0.001 | ||
| <13.5 | 134 (90.5%) | 98 (64.9%) | |
| ≥13.5 | 14 (9.5%) | 53 (35.1%) | |
| Neutrophil level (x103/µL) | 0.421 | ||
| Low/Normal | 115 (77.7%) | 123 (81.5%) | |
| High | 33 (22.3%) | 28 (18.5%) | |
| Lymphocyte level (x103/µL) | 0.034 | ||
| Low | 44 (29.7%) | 29 (19.2%) | |
| Normal/High | 104 (70.3%) | 122 (80.8%) | |
| Albumin level | <0.001 | ||
| <4 g/dL | 126 (85.1%) | 0 (0%) | |
| ≥4 g/dL | 22 (14.9%) | 151 (100%) | |
| Alkaline phosphatase (ALP) | <0.001 | ||
| Normal | 58 (39.2%) | 100 (66.2%) | |
| High (>120 U/L) | 90 (60.8%) | 51 (33.8%) | |
| LDH level | 0.001 | ||
| Normal | 40 (36.0%) | 64 (59.3%) | |
| High (>249 U/L) | 71 (64.0%) | 44 (40.7%) |
| Parameter | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age (<70 vs. ≥70) | 1.5 (1.15–2.02) | 0.003 | 1.3 (0.90–1.88) | 0.162 |
| Prostate surgery | 0.6 (0.41–0.85) | 0.004 | 0.6 (0.40–0.95) | 0.029 |
| Prostate radiotherapy | 0.7 (0.51–0.93) | 0.017 | 1.2 (0.81–1.88) | 0.321 |
| Anemia | 2.7 (1.93–3.92) | <0.001 | 1.4 (0.88–2.30) | 0.149 |
| Neutrophilia | 1.4 (1.04–1.90) | 0.027 | 1.3 (0.89–1.91) | 0.169 |
| ALP level (normal vs. > ULN) | 2.5 (1.91–3.27) | <0.001 | 1.6 (1.11–2.28) | 0.012 |
| LDH level (normal vs. > ULN) | 1.9 (1.42–2.65) | <0.001 | 1.3 (0.89–1.84) | 0.185 |
| Visceral metastasis | 1.5 (1.04–2.12) | 0.028 | 1.3 (0.77–2.04) | 0.363 |
| Bone metastasis | 1.9 (1.31–2.89) | 0.001 | 1.9 (1.08–3.35) | 0.025 |
| PSA response (absent vs. present) | 0.4 (0.31–0.53) | <0.001 | 0.5 (0.33–0.65) | <0.001 |
| PNI (low vs. high) | 0.4 (0.29–0.49) | <0.001 | 0.5 (0.33–0.70) | <0.001 |
| Score | Prognosis | mOS (Months) | mPFS (Months) |
|---|---|---|---|
| 0 | Favorable | 49.1 | 18.2 |
| 1 | Intermediate-low | 30.8 | 14.2 |
| 2 | Intermediate-high | 18.8 | 8.4 |
| 3 | Poor | 9.1 | 5.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taban, H.; Erman, M.; Guven, D.C.; Aktas, B.Y.; Yilmaz, F.; Yaşar, S.; Yildirim, H.C.; Aslan, F.; Aksoy, S. Prognostic Nutritional Index and a Blood-Based Prognostic Tool in Prostate Cancer Treated with Abiraterone, Enzalutamide or Cabazitaxel. Medicina 2025, 61, 1105. https://doi.org/10.3390/medicina61061105
Taban H, Erman M, Guven DC, Aktas BY, Yilmaz F, Yaşar S, Yildirim HC, Aslan F, Aksoy S. Prognostic Nutritional Index and a Blood-Based Prognostic Tool in Prostate Cancer Treated with Abiraterone, Enzalutamide or Cabazitaxel. Medicina. 2025; 61(6):1105. https://doi.org/10.3390/medicina61061105
Chicago/Turabian StyleTaban, Hakan, Mustafa Erman, Deniz Can Guven, Burak Yasin Aktas, Feride Yilmaz, Serkan Yaşar, Hasan Cagri Yildirim, Ferit Aslan, and Sercan Aksoy. 2025. "Prognostic Nutritional Index and a Blood-Based Prognostic Tool in Prostate Cancer Treated with Abiraterone, Enzalutamide or Cabazitaxel" Medicina 61, no. 6: 1105. https://doi.org/10.3390/medicina61061105
APA StyleTaban, H., Erman, M., Guven, D. C., Aktas, B. Y., Yilmaz, F., Yaşar, S., Yildirim, H. C., Aslan, F., & Aksoy, S. (2025). Prognostic Nutritional Index and a Blood-Based Prognostic Tool in Prostate Cancer Treated with Abiraterone, Enzalutamide or Cabazitaxel. Medicina, 61(6), 1105. https://doi.org/10.3390/medicina61061105






