1. Introduction
Surgical site infections (SSIs) pose a significant challenge in the postoperative course of pediatric spinal deformity surgery, with considerable implications both medically for the patient and financially in terms of healthcare costs [
1,
2]. Indeed, SSIs are typically linked to necessary patient readmissions, additional surgical interventions, and extended antibiotic therapy [
3]. Consequently, SSIs are estimated to result in medical expenses ranging from USD 3.5 to USD 10 billion annually in the United States alone [
4,
5,
6]. The Centers for Disease Control (CDC) defines SSIs based on infection depth, distinguishing between superficial (skin and subcutaneous tissue) and deep (fascia and muscle) infections, both occurring within 30 days postsurgery [
7].
Recent literature has focused on identifying modifiable risk factors that could help reduce SSI rates following pediatric spinal fusion [
8]. As a result, a wide range of infection prevention strategies has been proposed [
9,
10,
11]. Preoperative, intraoperative, and postoperative measures include skin precleansing, correct antibiotic prophylaxis, soft tissue management, blood loss reduction, and optimized drain, closure, and dressing protocols [
1]. Furthermore, several authors have emphasized the critical role of preoperative nutrition in affecting SSI rates, particularly in neuromuscular scoliosis (NMS) patients, classifying it as a modifiable risk factor [
12,
13]. Nevertheless, the risk of these complications is strongly influenced also by non-modifiable factors, particularly the underlying etiology, with otherwise healthy patients with adolescent idiopathic scoliosis (AIS) exhibiting the lowest incidence rate and patients with NMS facing a significantly higher risk [
8]. For this reason, the selection of optimal prophylactic antibiotics for each etiology group has remained a subject of ongoing debate. While cefazolin, or clindamycin/vancomycin for allergic patients, is the standard of care in spinal deformity surgery due to its efficacy against common Gram-positive organisms, the rising incidence of Gram-negative infections has led some authors to recommend broader coverage by adding agents such as aztreonam, gentamicin, or single-dose fluoroquinolone to the standard penicillin- or vancomycin-based regimen [
14,
15,
16,
17]. As a result, dual antibiotic therapy has gained increasing attention, particularly for prophylaxis in patients with NMS, who are known to have a distinct skin microbiota that favors Gram-negative bacteria compared to AIS patients, especially in those who are non-ambulatory or have bowel and bladder incontinence [
18,
19]. Recently, Partridge et al. reviewed 399 spinal fusion cases, reporting a 2.5% deep SSI rate following antibiotic prophylaxis with vancomycin plus cefazolin, with AIS infections predominantly caused by Gram-negative organisms and NMS infections mainly involving polymicrobial extended-spectrum beta-lactamase (ESBL)-producing Gram-negative bacteria, all susceptible to amikacin [
20].
Therefore, this study aims to assess whether the use of a dual antibiotic regimen consisting of cefazolin and amikacin is associated with a reduction in the incidence of superficial and deep SSIs within 6 months of surgery in a cohort of patients with AIS, NMS, or SS treated at a single institution.
2. Materials and Methods
2.1. Patient Population
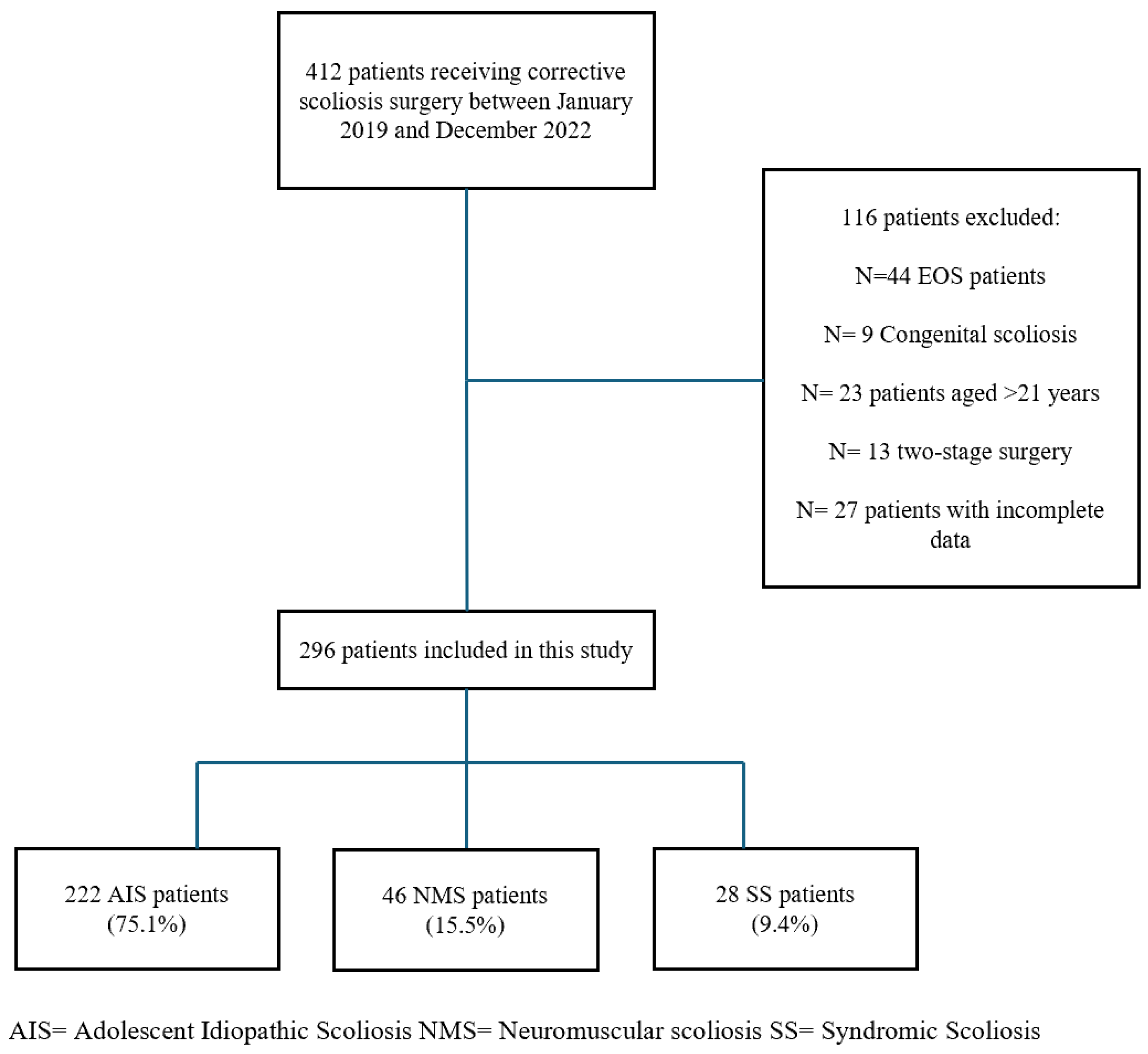
This retrospective, single-center study involved pediatric and adolescent patients under 21 years of age, diagnosed with AIS, NMS, or SS, who underwent posterior spinal fusion at a tertiary referral center for pediatric spinal deformities between January 2019 and December 2022. Patients underwent multidisciplinary preoperative evaluations, including clinical, pulmonary, cardiovascular, and anesthesiologic assessments, and were deemed fit for surgery before inclusion.
2.2. Study Design
This study was conducted according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [
21].
The primary aim of this retrospective single-center study was to evaluate the incidence of early SSIs and their correlation with the use of dual antibiotic prophylaxis in pediatric patients undergoing posterior spinal fusion for scoliosis. Secondary outcomes tried to assess the possible influence of other operative data (blood loss, hemoglobin levels, length of hospital stay (LOS), complications, pelvic fixation, and radiographic correction) as potential risk factors for SSIs.
Patients were identified retrospectively through institutional surgical databases and were included if they had a minimum follow-up of 24 months and complete clinical and radiographic data. The patients were consecutively enrolled in the database.
The following inclusion criteria were applied:
- -
Diagnosis of AIS, NMS, or SS;
- -
Age < 21 years;
- -
Posterior-only spinal fusion with or without pelvic fixation;
- -
Availability of complete perioperative data and ≥24 months of follow-up;
- -
Dual antibiotic therapy.
The following exclusion criteria were applied:
- -
Congenital or early-onset scoliosis (EOS);
- -
Age > 21 years;
- -
Prior spinal surgery;
- -
Two-stage procedures;
- -
Missing data on antibiotic protocol or complications.
2.3. Antibiotic Prophylaxis Protocol
All patients received standardized perioperative antibiotic prophylaxis to minimize the risk of SSI. This study systematically employed a dual antibiotic regimen in all cases. This consisted of a first-generation cephalosporin (cefazolin) and an aminoglycoside (amikacin). Depending on patient allergies and local microbiological risk profiles, cefazolin could be replaced with amoxicillin, clavulanic acid, or clarithromycin. At the same time, amikacin could be substituted with a different aminoglycoside or tigecycline. The primary prophylactic protocol is outlined below.
2.3.1. Preoperative Administration
The prophylactic antibiotic regimen consisted of cefazolin at 50 mg/kg and amikacin at 15 mg/kg, both administered 60 min prior to the skin incision. In cases where the surgical procedure was prolonged, an additional half-dose of cefazolin was given 4 h after the initial dose to maintain adequate antibiotic coverage.
2.3.2. Postoperative Administration
Postoperatively, cefazolin was given at a dose of 30 mg/kg/day and amikacin at 15 mg/kg/day, for a general duration of 5 days and 2 days, respectively, with adjustments made based on the individual case. Cefazolin was given in divided doses, while amikacin was administered once daily.
This dual antibiotic regimen was selected based on institutional protocols and local microbiological profiles. It aims to provide broad bacterial coverage and reduce the risk of postoperative SSIs.
During the postoperative period, renal function was evaluated biochemically every 48 h for amikacin use.
2.4. Surgical Technique
All surgeries were conducted by the same surgical team, with the patient under general anesthesia and intraoperative neurophysiological monitoring, including motor-evoked potentials (MEPs) and sensory-evoked potentials (SEPs). Preoperative preparation included a skin scrub with chlorhexidine gluconate (CHG) soap left on the site for approximately three minutes. The surgical site was then disinfected using two consecutive applications of ChloraPrep™ (2% CHG and 70% isopropyl alcohol) antiseptic solution for 30 s. A non-povidone–iodine sterile drape was used to cover the surgical field before skin incision. After exposing the bony landmarks, pedicle screws were inserted using a free-hand technique, with fluoroscopic guidance for verification. Ponte osteotomies (POs) were selectively performed to improve deformity flexibility, particularly in rigid curves [
22]. Instrumentation was completed using cobalt–chrome rods with single- or double-rod derotation and segmental correction maneuvers. Bone autografts from the facets and spinous processes were used for fusion. A suction drain was placed sub-fascially and routinely removed on the third postoperative day. Multiple washes were performed with 1 L of saline solution before skin closure. The skin was closed using intradermal sutures with rapid 2-0 in AIS patients, while surgical staples were used for NMS and SS patients. All patients were treated with absorbent foam dressings for wound coverage, which were changed on the third postoperative day and at discharge. All patients were monitored in the intensive care unit (ICU) for at least 24 h before transfer to the orthopedic ward.
2.5. Study Variables and Outcome Measures
Study variables are detailed in
Table 1.
The following outcome measures were utilized in this study.
Curve flexibility of the main and minor curves, which was considered the main predictor of curve stiffness, was calculated as follows:
Implant density was calculated as the total number of screws divided by the number of fused levels.
Patients were stratified into high-density (HD) and low-density (LD) groups using the cohort mean value (1.64 screws per level) as a cutoff.
Regarding clinical data, the following measures were applied:
Postoperative hemoglobin levels were determined using the initial blood tests conducted after surgery, compared to the preoperative blood tests.
The operative time was measured from the surgical incision to the completion of the immediate postoperative X-ray.
The LOS indicated patients’ recovery duration, from the day they were admitted to the day of discharge.
All complications were recorded, analyzed, and classified according to the Clavien–Dindo–Sink classification (CDSC) for pediatric scoliosis patients [
23]. Major complications were classified as >IIIB. SSIs developed within the first 90 days postoperatively were classified based on the depth of the infection as superficial (skin and subcutaneous tissue) and deep (fascia and muscle) [
7].
The definition of late SSIs after spinal fusion is controversial. They have been described as occurring more than 1 month, 2 months, 3 months, 6 months, 9 months, and 1 year after the initial procedure [
24]. For this study, we defined late infections as those that occurred more than 6 months after the procedure.
2.6. Statistical Analysis and Ethics
Descriptive and inferential statistics assessed associations between antibiotic regimen, SSI risk, and other perioperative variables. Categorical variables were compared using the chi-squared test and continuous variables with the Mann–Whitney U test or ANOVA, as appropriate. A univariate Cox regression was used to explore predictors of perioperative complications, with odds ratios (ORs) and 95% confidence intervals (CIs) reported. A p-value < 0.05 was considered statistically significant.
This study was conducted under international ethical guidelines for clinical research as outlined in the Helsinki and Istanbul Declarations. Due to the anonymized and retrospective nature of the data, the local ethics committee waived ethical approval.
4. Discussion
This study evaluated and compared the effectiveness and safety of dual antibiotic prophylaxis with cefazolin and amikacin in minimizing the incidence of SSIs in pediatric patients undergoing posterior spinal fusion for scoliosis.
The primary finding of this study is that the dual antibiotic prophylaxis protocol showed an acceptable rate of both 90-day superficial and deep SSIs across the three groups examined. Notably, the cumulative infection rate in the AIS group was 1.2%, with 0.8% of cases being deep infections, within a cohort of 222 patients. The NMS group exhibited an overall infection rate of 6.5%, while the SS group had a rate of 3.5%, all of which were superficial SSIs, which did not require surgical intervention. Univariate Cox regression analysis did not identify any statistically significant predictors of SSI; however, older age at surgery trended toward being a protective factor (p = 0.051; OR = 0.658).
SSIs are a common complication following posterior spinal surgery, often leading to devastating consequences [
25]. It has been estimated that SSIs are associated with increased surgical costs, ranging from USD 80,000 to USD 100,000 per case, due to the need for patient readmission, additional surgical procedures for wound debridement, and prolonged antibiotic therapy [
3]. For this reason, numerous efforts have been made in recent years to optimize antibiotic therapies for surgical patients and identify potential risk factors associated with these complications [
26].
In the 2011 Scoliosis Research Society (SRS) report, the overall rates of superficial and deep infections for pediatric scoliosis (n = 20,424) were 1% and 1.7%, respectively [
27]. When stratified by etiology, idiopathic patients (n = 11,741) demonstrated a 0.6% rate of superficial infections and a 0.9% rate of deep infections, for a total of 1.5%. In comparison, NMS patients (n = 4855) showed an overall rate of 1.7% for superficial SSIs and 3.8% for deep SSIs, resulting in a total of 5.5% [
27]. However, in numerous other studies, the infection rates associated with posterior scoliosis correction appear significantly higher. Rudic et al. recently analyzed a national AIS database of 9801 patients to evaluate SSI rates at 7, 30, and 90 days, reporting an overall SSI rate of 1.9% at 30 days and 2.7% at 90 days [
26]. Furthermore, infection rates for NMS corrective surgery have widely been reported to range from 4.2% to 20% [
28,
29]. In the present study, the cumulative rate of postoperative infections appears lower than the averages reported in previous studies.
Cefazolin, or clindamycin/vancomycin for patients with allergies, is generally regarded as the standard prophylactic antibiotic in spinal deformity surgery because of its effectiveness against common Gram-positive bacteria. However, the recommended dosage, timing, and duration of treatment can vary between institutions and clinical practices [
8,
30]. Nevertheless, these antibiotic agents do not provide significant coverage against Gram-negative organisms, which are frequently isolated in SSIs, with an incidence rate ranging from 18% to 25% [
31,
32]. Most Gram-negative infections are predominantly observed in NMS patients, accounting for up to 60% of all cases, likely due to a unique skin microbiota predisposing them to Gram-negative bacteria compared to AIS patients [
18,
32,
33,
34]. This factor has prompted many centers to adopt the use of Gram-negative agents, particularly for NMS patients, with a gradual increase over time, while some authors have proposed extending this coverage to AIS patients as well, using aminoglycosides, third or fourth generation cephalosporins, monobactams, or quinolones [
6]. Among these agents, amikacin is the most commonly utilized semisynthetic aminoglycoside, frequently employed in the treatment of pediatric infections, including those in neonates, caused by a broad spectrum of Gram-negative bacteria, as well as mycobacteria and nocardia [
35,
36,
37]. It is widely considered the most resistant to the action of aminoglycoside-modifying enzymes [
38,
39]. Adverse effects, although less common compared to other aminoglycosides, include ototoxicity, caused by damage to the sensory hair cells in the inner ear, particularly the high-frequency outer hair cells, and nephrotoxicity, a reversible side effect that leads to non-oliguric acute kidney injury due to decreased glomerular filtration [
40,
41]. In the present study, no such side effects were observed in the patient sample, confirming the safety of amikacin as a widely used medication in the pediatric population.
Partridge et al. highlighted that Gram-negative organisms predominantly caused infections in their cohort of 399 spinal fusion cases, all susceptible to amikacin, suggesting a potential for its more widespread use [
20]. In the current study, no Gram-negative microorganisms were isolated in the microbiological samples in cases of wound infection. Indeed, all organisms identified as causing SSIs were MRSA. To reduce such occurrences, recent literature has highlighted that the use of intravenous vancomycin at the time of wound closure has been associated with a reduction in infection rates by approximately 10-fold [
42]. However, intravenous vancomycin can be linked to a significant number of postinfusion complications, known as “red man syndrome,” a condition that can be prevented only with slow infusion [
1]. Using vancomycin powder during wound closure has become increasingly common to reduce the risk of such complications. This practice has proven particularly effective in adult spinal deformity surgery and is also considered safe in the pediatric population [
43,
44]. A recent meta-analysis involving 6701 patients treated with vancomycin powder reported an overall complication rate of 0.3%, including nephropathy, ototoxicity, and seroma formation, concluding that this treatment can be considered safe [
45]. The standard of care typically involves administering cephalosporin, such as cefazolin, within one hour before incision and continuing for 24 h postoperatively [
45]. However, the optimal duration of postoperative antibiotic therapy remains controversial. Some studies, including a randomized trial by Takemoto et al., found no added benefit in extending antibiotics beyond drain removal in adult spinal surgery [
43]. Similarly, pediatric studies have shown no significant difference in infection rates between short (<24 h) and extended (>48 h) antibiotic use in patients with suction drains [
1,
34,
44]. Labbè et al. noted that infections were more likely when prophylaxis was improperly timed or dosed [
33], suggesting prolonged coverage may partly offset earlier errors. Still, prolonged use raises concerns about antibiotic resistance [
45]. Nevertheless, incorporating topical antibiotics may have further improved postoperative infection outcomes in the analyzed study groups of this study, providing additional coverage against Gram-positive microorganisms resistant to penicillins.
The present findings may support the potential role of dual antibiotic prophylaxis with cefazolin and amikacin in reducing early surgical site infections in pediatric scoliosis surgery. Moving forward, prospective multicenter randomized controlled trials are warranted to confirm these results and to establish evidence-based guidelines for antibiotic selection, timing, and duration. Additionally, the integration of patient-specific factors such as ASA score, age, underlying etiology, and potentially preoperative skin microbiota analysis could further help in refining prophylactic strategies and optimizing surgical outcomes [
46,
47].
These data may serve as a foundation for standardized protocols aimed at minimizing SSIs across diverse pediatric scoliosis populations. Furthermore, the observed safety profile of amikacin strengthens its potential use in pediatric orthopedic settings, potentially influencing broader perioperative antibiotic policies. Importantly, this approach could contribute to reducing unnecessary prolonged antibiotic use and limiting antimicrobial resistance.
5. Strengths and Limitations
The study’s strengths are the cumulative sample size of the study consisting of 296 patients, including 222 affected by AIS, which makes the findings regarding the effectiveness of dual antibiotic prophylaxis in preventing SSIs relatively reliable, and, since the study was conducted in a single-center setting, it allowed for a standardized approach to surgical procedures and perioperative management for all patients, thereby minimizing the biases typically associated with multicenter studies.
Nevertheless, this study has several limitations that must be acknowledged. First, its retrospective design could have introduced selection, information, confounding, and observer bias, affecting the generalizability of the results. Secondly, the absence of a control group receiving only cefazolin prevents a clear comparison of postoperative infection rates between patients treated with dual antibiotic prophylaxis and those receiving the standard antibiotic prophylaxis. Thirdly, the three patient groups analyzed differ significantly in size, which may contribute to some inconsistencies in the comparative results. Additionally, the univariate regression analysis may not have identified significant risk factors due to the relatively low number of postoperative SSIs observed across the three groups.