Kidney and Bladder Transplantation: Advances, Barriers, and Emerging Solutions
Abstract
1. Introduction
2. Kidney Transplantation: Achievements and Ongoing Challenges
| Domain | Key Achievements | Current Challenges | Potential Solutions | Ref. |
|---|---|---|---|---|
| Surgical Techniques | Standardized implantation in iliac fossa | Limited global access to advanced surgical tools and training | Expand surgical education programs | [34] |
| Adoption of minimally invasive and robotic-assisted procedures | Increase investment in robotic systems in transplant centers | [35] | ||
| Post-Transplant Monitoring | Use of biomarkers, imaging, and AI analytics | Lack of standardized, personalized monitoring protocols | Develop evidence-based monitoring algorithms | [36] |
| Early detection of graft dysfunction | Integrate AI tools in routine follow-up care | [37] | ||
| Immuno- suppressive Therapy | Reduced acute rejection via calcineurin inhibitors, mTOR inhibitors, and biologics | Long-term toxicity: infections, malignancies, metabolic complications | Advance immune tolerance strategies | [38] |
| Rise of pharmacogenomic tailoring | Lifelong dependency | Invest in next-generation, low-toxicity immuno- suppressants | [39] | |
| Organ Preservation | Hypothermic and normothermic perfusion improves graft viability and function | High costs and limited availability in resource-poor settings | Scale up cost-effective machine perfusion systems | [40] |
| Enhance perfusion fluids with therapeutic agents (e.g., anti-inflammatory drugs) | [41] | |||
| Donor–Recipient Matching | Precision genetic and immune profiling | Persistent chronic allograft dysfunction and late rejection | Improve early detection of subclinical rejection | [42] |
| Virtual cross-matching | Research into individualized immunologic risk profiling | [43] | ||
| Immune Tolerance Strategies | Experimental success in chimerism and regulatory T-cell therapy | Lack of clinical validation and scalability | Support clinical trials and translational research | [44] |
| Combine cellular therapies with targeted immunomodulation | [45] | |||
| Alternative Organ Sources | Gene-edited porcine kidneys showing promise | Immunologic risks, zoonoses, technical complexity, and ethical debates | Strengthen ethical/regulatory frameworks | [46] |
| Advances in 3D bioprinting and organ scaffolding | Promote R&D in scaffold engineering, vascularization, and stem cell biology | [47] | ||
| Ethical and Societal Considerations | Growing consensus on ethical organ allocation | Inequities in access and public hesitation toward emerging technologies | Implement equitable allocation policies | [48] |
| Increase public education and donor awareness | [49] | |||
| Economic and Logistical Factors | Efficient transplant systems in high-income countries | High costs of immunosuppression, organ procurement, and post-op care in low-resource areas | Global partnerships and funding mechanisms | [50] |
| Encourage local production of generics and simplified care protocols | [51] |
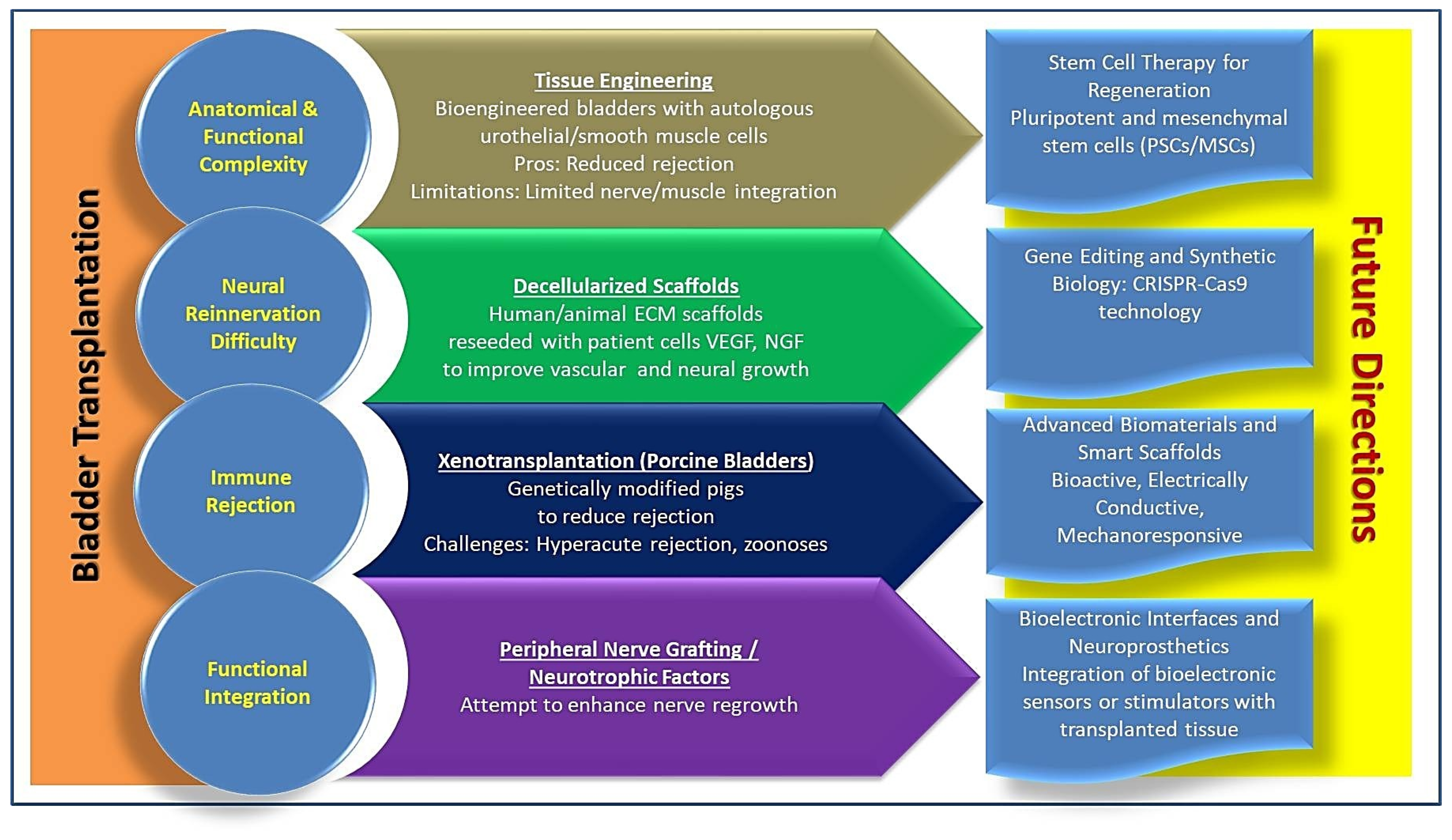
3. Bladder Transplantation: Emerging Prospects
4. Immunological Challenges and Graft Survival
| Aspect | Description | Clinical Implications | Challenges | Solutions/ Innovations | Ref. |
|---|---|---|---|---|---|
| Physiological Environment | Bladder exposed to urine, microbiota, and high urothelial turnover | Increases risk of infection, immune activation, and graft failure | Difficult to maintain immune balance while preserving functional urothelium | Tissue-compatible biomaterials; localized immunosuppression | [3] |
| Acute Rejection | T-cell-mediated inflammation from recognition of alloantigens | Graft damage, inflammation, and possible early transplant loss | Overactivation of the immune response; systemic toxicity from drugs | Calcineurin inhibitors, corticosteroids, CTLA-4 blockers; localized drug delivery | [7] |
| Chronic Rejection | Fibrosis, vascular injury, detrusor dysfunction from prolonged immune response | Long-term graft failure, loss of bladder compliance, incontinence | Hard to detect early; limited reversibility | Costimulation blockers (e.g., belatacept), Treg therapy | [117] |
| Systemic Immuno suppression | Drugs used in kidney transplants are also applied here, but with limitations | Risk of nephrotoxicity, metabolic issues, infections | Side effects undermine long-term safety | Development of bladder-specific regimens; localized delivery platforms | [118] |
| Localized Immuno suppression | Intravesical delivery via nanoparticles, hydrogels | Targets the bladder directly, reduces systemic exposure | Drug penetration and duration: clinical translation | Sustained-release vehicles; targeted immunosuppressants | [119] |
| Gene Editing | CRISPR is used to reduce the immunogenicity of donor cells | Potential for creating hypoimmunogenic bladder tissue | Ethical, safety, and technical issues in editing human grafts | CRISPR/Cas9-modified donor tissues; ongoing safety trials | [120] |
| Biomarkers | Urine cytokines, dd-cfDNA, and proteomics are used for early rejection detection | Enables non-invasive, proactive transplant monitoring | Validation for bladder application; complex interpretation | Integrate with machine learning for predictive modelling | [121] |
| Machine Learning | Analyzes complex biomarker datasets | Enhances early detection, enables personalized immunosuppression | Requires large, high-quality datasets | AI-powered decision tools for transplant monitoring | [122] |
| Regenerative Medicine | Use of stem cells and biocompatible scaffolds to engineer low-immunogenic grafts | Reduces immune response; improves integration | Ensuring muscle function and innervation | Autologous cell seeding, scaffold optimization, and hybrid tissue constructs | [7] |
| Xeno transplantation | Use of porcine bladder tissue modified to reduce immune recognition | May address graft shortages | Hyperacute rejection; zoonotic risk | Knockout of α-Gal; human complement regulators; pathogen screening | [8] |
| Precision Medicine | Tailored regimens based on genetic/biomarker profiles | Safer, more effective immunosuppression for each patient | Complex integration into clinical workflow | Use of pharmacogenomics, personalized dosing algorithms | [123] |
| Health System Factors | Address disparities in access to advanced transplant care | Equity in outcomes and long-term support | Unequal access, lack of education | Policy interventions, public education, and funding access initiatives | [124] |
5. Simultaneous Bladder–Kidney Transplantation
6. Ethical and Social Implications
| Domain | Ethical/Social Dimensions | Critical Reflections | Solutions | Ref. |
|---|---|---|---|---|
| Transplant Ethics | Scarcity, consent, equity, legitimacy | Ethics must co-evolve with biotechnology to reconcile innovation with distributive justice | Develop adaptive, ethically responsive policy frameworks and continuous ethical oversight | [131,132] |
| Kidney Transplantation | Allocation ethics, structural disparities | Normative acceptance masks systemic inequities. | Refine allocation algorithms to incorporate equity metrics; enhance donor recruitment campaigns | [46,133] |
| Living Donation | Autonomy vs. coercion, commodification | Voluntariness is often compromised by relational or economic pressures | Enforce rigorous consent protocols, prohibit financial inducements, and strengthen donor protection laws | [125,134] |
| Bladder Transplantation | Non-vitality, experimental status, consent burden | Risk–benefit calculus is ethically complex in non-life-saving interventions | Prioritize transparency; establish a specialized ethics review for quality-of-life transplant trials | [135,136] |
| Cultural Reception | Identity, embodiment, and acceptability | Deeply embedded symbolic meanings affect social legitimacy | Initiate culturally sensitive public engagement; include anthropologists and sociologists in policy design | [48,137] |
| Religious Paradigms | Purity, dignity, moral authority | Religious resistance may impede the implementation | Foster interfaith dialogues; incorporate theological perspectives into bioethical consultation | [134,138] |
| Global Health Justice | Access asymmetry, systemic bias | Biomedical innovation risks entrenching global disparities | Create equitable access policies; support international transplant cooperation and capacity-building | [139,140] |
| Emerging Frontiers | Xenotransplantation, gene editing, and synthetic biology | These modalities destabilize classical bioethical categories | Establish anticipatory ethics frameworks; integrate risk ethics and public deliberation mechanisms | [131,141] |
| Strategic Imperative | Normative adaptability, stakeholder inclusion | Ethical governance must remain reflexive and pluralistic | Institutionalize interdisciplinary ethics committees; maintain open, iterative dialogue across sectors | [142,143] |
7. Challenges and Future Prospects
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, J.K.; Dos Santos, J.; Rickard, M.; Lorenzo, A.J. Review—Renal transplantation for congenital urological diseases. J. Pediatr. Urol. 2024, 20, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, A.; Jackman, S.V.; Costa, G.; Davies, B.; Wu, T.; Bond, G.; Abu-Elmagd, K. Urogenital Disorders Associated With Gut Failure and Intestinal Transplantation. J. Urol. 2007, 178, 2067–2072. [Google Scholar] [CrossRef] [PubMed]
- Piovesan, A.C.; Andraus, W.; Pellanda, A.B.; David, E.; D’albuquerque, L.C.; Nahas, W.C. Bladder Transplantation: The New Frontier in Abdominal Organ Transplantation. ABCD Arq. Bras. Cir. Dig. (São Paulo) 2024, 37, e1808. [Google Scholar] [CrossRef] [PubMed]
- Al-Qudimat, A.R.; Altahtamoun, S.B.; Kilic, F.; Al-Zoubi, R.M.; Al Zoubi, M.S. The risk of solid organ tumors in patients with chronic kidney disease: A narrative review of literature. Heliyon 2024, 10, e32822. [Google Scholar] [CrossRef]
- Tian, Y.; Frischknecht, L.; Mallone, A.; Rössler, F.; Schachtner, T.; Nilsson, J. Evaluation of de novo donor specific antibodies after kidney transplantation in the era of donor-derived cell-free DNA. Front. Immunol. 2025, 15, 1530065. [Google Scholar] [CrossRef]
- Mahler, C.F.; Friedl, F.; Nusshag, C.; Speer, C.; Benning, L.; Göth, D.; Schaier, M.; Sommerer, C.; Mieth, M.; Mehrabi, A.; et al. Evaluation of deceased-donor kidney offers: Development and validation of novel data driven and expert based prediction models for early transplant outcomes. Front. Immunol. 2025, 15, 1511368. [Google Scholar] [CrossRef]
- Czarnogórski, M.C.; Koper, K.; Petrasz, P.; Vetterlein, M.W.; Pokrywczyńska, M.; Juszczak, K.; Drewa, T.; Adamowicz, J. Urinary bladder transplantation in humans—Current status and future perspectives. Nat. Rev. Urol. 2024, 22, 175–186. [Google Scholar] [CrossRef]
- Zeng, X.X.; Wu, Y. Strategies of Bladder Reconstruction after Partial or Radical Cystectomy for Bladder Cancer. Mol. Biotechnol. 2024, 67, 1735–1751. [Google Scholar] [CrossRef]
- Kuttymuratov, G.; Aynakulov, A.; Ayaganov, A.; Mirmanov, A.; Bikhanov, N.; Tuleuzhan, A.; Uderbayev, N.; Oshakbayev, K. Bladder and Kidney Transplantation En Bloc: Review of Experience and Prospects for Development. Exp. Clin. Transplant. 2024, 22, 579–585. [Google Scholar]
- Parkinson, E.; Robb, A.; McCarthy, L. Long-term Survival of Bladder Augmentation is Influenced by its Shape and Mucosal Lining. J. Pediatr. Surg. 2024, 60, 162051. [Google Scholar] [CrossRef]
- Tang, Z.; Feng, C.; Li, Y.; Li, T.; Zhang, H.; Zeng, Y.; Peng, L.; Xie, X.; Peng, F.; Dai, H. Mouse kidney transplantation model: Three novel methods. J. Cent. South Univ. Med. Sci. 2024, 49, 220–235. [Google Scholar] [CrossRef]
- Akhlaghpasand, M.; Tavanaei, R.; Allameh, F.; Hosseinpoor, M.; Toreyhi, H.; Golmohammadi, M.; Hajarizadeh, A.; Alikhani, A.; Hafizi, M.; Oraee-Yazdani, M.; et al. Improvement of Neurogenic Bladder Dysfunction Following Combined Cell Therapy with Mesenchymal Stem Cell and Schwann Cell in Spinal Cord Injury: A Randomized, Open-Label, Phase II Clinical Trial. World Neurosurg. 2024, 194, 123402. [Google Scholar] [CrossRef] [PubMed]
- Macêdo, C.T.; Souza, B.S.d.F.; Villarreal, C.F.; Silva, D.N.; da Silva, K.N.; Souza, C.L.e.M.d.; Paixão, D.d.S.; Bezerra, M.d.R.; Costa, A.O.d.S.M.; Brazão, E.S.; et al. Transplantation of autologous mesenchymal stromal cells in complete cervical spinal cord injury: A pilot study. Front. Med. 2024, 11, 1451297. [Google Scholar] [CrossRef] [PubMed]
- Abbaszadeh, S.; Nosrati-Siahmazgi, V.; Musaie, K.; Rezaei, S.; Qahremani, M.; Xiao, B.; Santos, H.A.; Shahbazi, M.-A. Emerging strategies to bypass transplant rejection via biomaterial-assisted immunoengineering: Insights from islets and beyond. Adv. Drug Deliv. Rev. 2023, 200, 115050. [Google Scholar] [CrossRef]
- Zhang, Q.-Q.; Xie, Y.-L.; Cao, S.; Chen, S.; Zhang, W.-J.; Chang, S. A Lesson of Immunosuppression in Renal Transplant: Retreat or Hold? Yale J. Biol. Med. 2023, 96, 57–77. [Google Scholar] [CrossRef]
- Hurst, D.J.; Cooper, D.K.C. The importance of public engagement in clinical xenotransplantation. Health Care Sci. 2024, 3, 124–130. [Google Scholar] [CrossRef]
- George, A.J. Ethics, virtues and xenotransplantation. Perfusion 2024, 39, 334–343. [Google Scholar] [CrossRef]
- Padilla, L.A.; Hurst, D.J.; Zink, A.; Parent, B.; Kimberly, L.L. Public attitudes to xenotransplantation: A national survey in the United States. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2024, 24, 2066–2079. [Google Scholar] [CrossRef]
- Anastasopoulos, N.-A.; Papalois, V. Environmentally sustainable kidney care through transplantation: Current status and future challenges. Surg. 2024, 22, 233–235. [Google Scholar] [CrossRef]
- Basile, G.; Pecoraro, A.; Gallioli, A.; Territo, A.; Berquin, C.; Robalino, J.; Bravo, A.; Huguet, J.; Rodriguez-Faba, Ó.; Gavrilov, P.; et al. Robotic kidney transplantation. Nat. Rev. Urol. 2024, 21, 521–533. [Google Scholar] [CrossRef]
- Elamin, M.; Alabbasi, B.; Aloufi, M. Growth in Children After a Kidney Transplant: A Retrospective, Observational Single-Center Study. Cureus 2024, 16, e69003. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.S.; Van Kuiken, M.E. Urodynamics in the Transplant Population. Curr. Bladder Dysfunct. Rep. 2024, 19, 62–69. [Google Scholar] [CrossRef]
- Roth, J.D.; Bowen, D.; Fuchs, M.E.; Gargollo, P.C.; Gottlich, H.; Hains, D.S.; Strine, A.C.; Szymanski, K.M. End-stage and chronic kidney disease in classic bladder exstrophy: A retrospective muti-institutional cohort study. J. Pediatr. Urol. 2024, 21, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Rautanen, T.; Ahopelto, K.; Niinikoski, H.; Karppinen, S.; Lempinen, M.; Ortiz, F.; Helanterä, I. Outcomes of kidney transplantation in patients with lysinuric protein intolerance. Clin. Kidney J. 2024, 18, sfae373. [Google Scholar] [CrossRef] [PubMed]
- Khairallah, P.; Lorenz, E.C.; Waterman, A.; Aggarwal, N.; Pai, A.; Winkelmayer, W.C.; Niu, J. Trends in Kidney Allograft Failure Among First-Time Transplant Recipients in the United States. Am. J. Kidney Dis. 2024, 85, 273–283.e1. [Google Scholar] [CrossRef]
- Prakash, J.V.S.; Thiruvarul, P.V.; Natarajan, V.; Vetrichandar, S.; Arasi, K.V.; Paranjothi, A.K.; Dhineshkumar, P. A Study on Early Surgical Complications in Renal Transplant Recipients. Indian J. Transplant. 2024, 18, 27–37. [Google Scholar] [CrossRef]
- Deshpande, R.; Augustine, T. Smart transplants: Emerging role of nanotechnology and big data in kidney and islet transplantation, a frontier in precision medicine. Front. Immunol. 2025, 16, 1567685. [Google Scholar] [CrossRef]
- Ramalhete, L.; Almeida, P.; Ferreira, R.; Abade, O.; Teixeira, C.; Araújo, R. Revolutionizing Kidney Transplantation: Connecting Machine Learning and Artificial Intelligence with Next-Generation Healthcare—From Algorithms to Allografts. BioMedInformatics 2024, 4, 673–689. [Google Scholar] [CrossRef]
- Semenova, Y.; Bayanova, M.; Rakhimzhanova, S.; Altynova, S.; Sailybayeva, A.; Asanova, A.; Pya, Y. Understanding Pediatric Kidney Transplant Rejection: Its Pathophysiology, Biomarkers, and Management Strategies. Curr. Med. Chem. 2025, 32, 3571–3590. [Google Scholar] [CrossRef]
- Smirnova, V.V.; Shmarina, N.V.; Dmitriev, I.V.; Balkarov, A.G.; Zagorodnikova, N.V.; Vinogradov, V.E.; Minina, M.G. Early and long-term outcomes of deceased-donor kidney transplant in recipients 70 years of age and older. Russ. J. Transplantology Artif. Organs 2024, 26, 111–116. [Google Scholar] [CrossRef]
- Erdal, K.; Karazeybek, E. Impact of text message reminders on immunosuppressive medication adherence among kidney transplant recipients: A randomized controlled study. J. Eval. Clin. Pr. 2024, 31, e14178. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.-Y.; Dai, L.-R.; Yu, C.-Z.; Chen, R.-J.; Yu, F.-H.; Chen, S.; Chang, S.; Zhang, W.-J. Immunosuppressant nonadherence profile in kidney transplant recipients and the impact of medication adherence on transplant outcomes. Front. Pharmacol. 2024, 15, 1493166. [Google Scholar]
- Belardi, R.; Pacifici, F.; Baldetti, M.; Velocci, S.; Minieri, M.; Pieri, M.; Campione, E.; Della-Morte, D.; Tisone, G.; Anselmo, A.; et al. Trends in Precision Medicine and Pharmacogenetics as an Adjuvant in Establishing a Correct Immunosuppressive Therapy for Kidney Transplant: An Up-to-Date Historical Overview. Int. J. Mol. Sci. 2025, 26, 1960. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Kang, S.; Rhew, S.A.; Yoon, C.E.; Moon, H.W.; Park, Y.H.; Cho, H.J. Robot-assisted ureteral reconstruction for managing kidney transplant patients with ureteric complications. Investig. Clin. Urol. 2025, 66, 18–26. [Google Scholar] [CrossRef]
- Heidenberg, D.J.; Choudry, M.M.; Briggs, L.G.; Ahmadieh, K.; Abdul-Muhsin, H.M.; Katariya, N.N.; Cheney, S.M. Robotic-assisted Laparoscopic Repair of Kidney Transplant Ureteral Strictures. Urology 2024, 193, 186–191. [Google Scholar] [CrossRef]
- Avramidou, E.; Srinivasan, D.; Todorov, D.; Tsoulfas, G.; Papalois, V. Diagnostic and Prognostic Value of Machine Perfusion Biomarkers in Kidney Graft Evaluation. Transplant. Proc. 2024, 56, 1308–1318. [Google Scholar] [CrossRef]
- Valencia, O.A.G.; Thongprayoon, C.; Jadlowiec, C.C.; Mao, S.A.; Miao, J.; Leeaphorn, N.; Suppadungsuk, S.; Csongradi, E.; Budhiraja, P.; Khoury, N.; et al. Navigating pancreas transplant perceptions: Assessing public sentiment and strategies using AI-driven analysis. Front. Digit. Heal. 2024, 6, 1453341. [Google Scholar] [CrossRef]
- Béland, M.A.; Lapointe, I.; Côté, I.; Lesage, J.; Houde, I.; Wagner, E.; Riopel, J.; Latulippe, E.; Désy, O.; De Serres, S.A. HLA class, calcineurin inhibitor levels, and the risk of graft failure in kidney recipients with donor-specific antibodies. Front. Immunol. 2024, 15, 1493878. [Google Scholar] [CrossRef]
- Jawdeh, B.G.A.; Me, H.M. Immunosuppression in Kidney Transplant Recipients: An Update for the General Nephrologist. Adv. Kidney Dis. Health 2024, 31, 408–415. [Google Scholar] [CrossRef]
- Tingle, S.J.; Thompson, E.R.; Figueiredo, R.S.; Moir, J.A.; Goodfellow, M.; Talbot, D.; Wilson, C.H. Normothermic and hypothermic machine perfusion preservation versus static cold storage for deceased donor kidney transplantation. Cochrane Database Syst. Rev. 2024, 2024, CD011671. [Google Scholar] [CrossRef]
- Kirste, G. Cold but not too cold: Advances in hypothermic and normothermic organ perfusion. Korean J. Transplant. 2022, 36, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, S. Enhancing kidney transplantation through multi-agent kidney exchange programs: A comprehensive review and optimization models. Int. J. Ind. Eng. Comput. 2025, 16, 483–498. [Google Scholar] [CrossRef]
- Mattoo, A.; Jaffe, I.S.; Keating, B.; Montgomery, R.A.; Mangiola, M. Improving long-term kidney allograft survival by rethinking HLA compatibility: From molecular matching to non-HLA genes. Front. Genet. 2024, 15, 1442018. [Google Scholar] [CrossRef] [PubMed]
- Owen, M.C.M.; Kopecky, B.J. Targeting Macrophages in Organ Transplantation: A Step Toward Personalized Medicine. Transplantation 2024, 108, 2045–2056. [Google Scholar] [CrossRef]
- Knoedler, L.; Dean, J.; Diatta, F.; Thompson, N.; Knoedler, S.; Rhys, R.; Sherwani, K.; Ettl, T.; Mayer, S.; Falkner, F.; et al. Immune modulation in transplant medicine: A comprehensive review of cell therapy applications and future directions. Front. Immunol. 2024, 15, 1372862. [Google Scholar] [CrossRef]
- Martinelli, V.; Lumer, E.L.L.; Chiappedi, M.; Politi, P.; Gregorini, M.; Rampino, T.; Peri, A.; Pietrabissa, A.; Fusar-Poli, L. Ethical Issues in Living Donor Kidney Transplantation: An Update from a Psychosocial Perspective. Healthcare 2024, 12, 1832. [Google Scholar] [CrossRef]
- Pisheh, H.R.; Haghdel, M.; Jahangir, M.; Hoseinian, M.S.; Yasuj, S.R.; Roodbari, A.S. Effective and new technologies in kidney tissue engineering. Front. Bioeng. Biotechnol. 2024, 12, 1476510. [Google Scholar] [CrossRef]
- Iwai, Y.; Blanks, J.C.; Raghunathan, S.; Wright, S.T.; Behne, F.M.; Long, J.M.; Brinkley-Rubinstein, L. A Scoping Review of Organ Transplantation in Populations Experiencing Incarceration. J. Correct. Heal. Care 2024, 30, 311–319. [Google Scholar] [CrossRef]
- Ambagtsheer, F.; Bunnik, E.; Pengel, L.H.M.; Reinders, M.E.; Elias, J.J.; Lacetera, N.; Macis, M. Public Opinions on Removing Disincentives and Introducing Incentives for Organ Donation: Proposing a European Research Agenda. Transpl. Int. 2024, 37, 12483. [Google Scholar] [CrossRef]
- Da Silva, A.M.; Benites, P.T.; Zulin, M.E.G.; Júnior, M.A.F.; de Queiroz Cardoso, A.I.; Cury, E.R.J. Global legislation regulating the donation, procurement and distribution processes of organs and tissues from deceased donors for transplants: A scoping review. Heliyon 2024, 10, e26313. [Google Scholar] [CrossRef]
- Finocchietti, M.; Marino, M.L.; Rosa, A.; Bellini, A.; Masiero, L.; Cardillo, M.; Massari, M.; Alegiani, S.S.; Pierobon, S.; Ferroni, E.; et al. Immunosuppression with Generics in Liver and Kidney Transplantation: A Real-World Evidence Study. Drug Des. Dev. Ther. 2024, 18, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Faria, I.; Canizares, S.; Viana, P.; Kueht, M. Navigating the changing landscape of transplant research: Trends, topics, and gender disparities. Am. J. Surg. 2024, 239, 116003. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sun, C.; Mo, S.; Hu, C.; Ning, Y.; Liang, H.; Liu, Z.; Fan, X.; Wang, Y. Salvaging donated kidneys from prolonged warm ischemia during ex vivo hypothermic oxygenated perfusion. Kidney Int. 2024, 106, 273–290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, I.W.; Lurje, I.; Lurje, G.; Knosalla, C.; Schoenrath, F.; Tacke, F.; Engelmann, C. Combined Organ Transplantation in Patients with Advanced Liver Disease. Semin. Liver Dis. 2024, 44, 369–382. [Google Scholar] [CrossRef]
- Ali, A.; Kurome, M.; Kessler, B.; Kemter, E.; Wolf, E. What Genetic Modifications of Source Pigs Are Essential and Sufficient for Cell, Tissue, and Organ Xenotransplantation? Transpl. Int. 2024, 37, 13681. [Google Scholar] [CrossRef]
- Barbachowska, A.; Gozdowska, J.; Durlik, M. Kidney Transplantation in Older Recipients Regarding Surgical and Clinical Complications, Outcomes, and Survival: A Literature Review. Geriatrics 2024, 9, 151. [Google Scholar] [CrossRef]
- Fichtner, A.; Gauché, L.; Süsal, C.; Tran, T.H.; Waldherr, R.; Krupka, K.; Guzzo, I.; Carraro, A.; Oh, J.; Zirngibl, M.; et al. Incidence, risk factors, management strategies, and outcomes of antibody-mediated rejection in pediatric kidney transplant recipients-a multicenter analysis of the Cooperative European Paediatric Renal Transplant Initiative (CERTAIN). Pediatr. Nephrol. 2025, 40, 491–503. [Google Scholar] [CrossRef]
- Von Ahrens, D.; Santeusanio, A.D.; Weinberg, A.D.; Moon, J.; Iyer, K.R. Risk factors for renal dysfunction after isolated intestinal transplantation. Clin. Transplant. 2024, 38, e15228. [Google Scholar] [CrossRef]
- Pham, J.A.; Coronel, M.M. Unlocking Transplant Tolerance with Biomaterials. Adv. Heal. Mater. 2024, 14, e2400965. [Google Scholar] [CrossRef]
- Little, C.J.; Kim, S.C.; Fechner, J.H.; Post, J.; Coonen, J.; Chlebeck, P.; Winslow, M.; Kobuzi, D.; Strober, S.; Kaufman, D.B. Early allogeneic immune modulation after establishment of donor hematopoietic cell-induced mixed chimerism in a nonhuman primate kidney transplant model. Front. Immunol. 2024, 15, 1343616. [Google Scholar] [CrossRef]
- Shah, K.; Leandro, M.; Cragg, M.; Kollert, F.; Schuler, F.; Klein, C.; Reddy, V. Disrupting B and T-cell collaboration in autoimmune disease: T-cell engagers versus CAR T-cell therapy? Clin. Exp. Immunol. 2024, 217, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, D.; Laurent, C.; Lemoine, M.; Lebourg, L.; Hanoy, M.; Le Roy, F.; Nezam, D.; Pruteanu, D.; Grange, S.; De Nattes, T.; et al. Evaluation of T Cell Response to SARS-CoV-2 in Kidney Transplant Recipients Receiving Monoclonal Antibody Prophylaxis and the Utility of a Bivalent mRNA Vaccine Booster Dose. Microorganisms 2024, 12, 722. [Google Scholar] [CrossRef] [PubMed]
- Riella, L.V. Xenotransplantation: The Future Is Here. J. Am. Soc. Nephrol. 2024, 36, 305–307. [Google Scholar] [CrossRef] [PubMed]
- Patry, C.; Webb, N.J.A.; Meier, M.; Pape, L.; Fichtner, A.; Höcker, B.; Tönshoff, B. Kidney Transplantation in Children and Adolescents With C3 Glomerulopathy or Immune Complex Membranoproliferative Glomerulonephritis: An International Survey of Current Practice. Pediatr. Transplant. 2025, 29, e70048. [Google Scholar] [CrossRef]
- Onyeaghala, G.; Dorr, C.R.; Israni, A.K. Opportunities and challenges for immunosuppression in the context of pig-to-human xenotransplantation. Med 2024, 5, 842–844. [Google Scholar] [CrossRef]
- Porrett, P.M.; Orandi, B.J.; Kumar, V.; Houp, J.; Anderson, D.; Killian, A.C.; Hauptfeld-Dolejsek, V.; Martin, D.E.; Macedon, S.; Budd, N.; et al. First clinical-grade porcine kidney xenotransplant using a human decedent model. Am. J. Transplant. 2022, 22, 1037–1053. [Google Scholar] [CrossRef]
- Montgomery, R.A.; Stern, J.M.; Lonze, B.E.; Tatapudi, V.S.; Mangiola, M.; Wu, M.; Weldon, E.; Lawson, N.; Deterville, C.; Dieter, R.A.; et al. Results of Two Cases of Pig-to-Human Kidney Xenotransplantation. N. Engl. J. Med. 2022, 386, 1889–1898. [Google Scholar] [CrossRef]
- Judd, E.; Kumar, V.; Porrett, P.M.; Hyndman, K.A.; Anderson, D.J.; Jones-Carr, M.E.; Shunk, A.; Epstein, D.R.; Fatima, H.; Katsurada, A.; et al. Physiologic homeostasis after pig-to-human kidney xenotransplantation. Kidney Int. 2024, 105, 971–979. [Google Scholar] [CrossRef]
- Firl, D.J.; Lassiter, G.; Hirose, T.; Policastro, R.; D’attilio, A.; Markmann, J.F.; Kawai, T.; Hall, K.C. Clinical and molecular correlation defines activity of physiological pathways in life-sustaining kidney xenotransplantation. Nat. Commun. 2023, 14, 3022. [Google Scholar] [CrossRef]
- Rodger, D.; Cooper, D.K.C. Kidney xenotransplantation: Future clinical reality or science fiction? Nurs. Health Sci. 2022, 25, 161–170. [Google Scholar] [CrossRef]
- Yang, C.; Wei, Y.; Li, X.; Xu, K.; Huo, X.; Chen, G.; Zhao, H.; Wang, J.; Wei, T.; Qing, Y.; et al. Production of Four-Gene (GTKO/hCD55/hTBM/hCD39)-Edited Donor Pigs and Kidney Xenotransplantation. Xenotransplantation 2024, 31, e12881. [Google Scholar] [CrossRef] [PubMed]
- Gajsak, L.R.; Zibar, L. Non-compliance as ethical dilemma for kidney transplantation. Eur. Psychiatry 2024, 67, S577. [Google Scholar] [CrossRef]
- Loukelis, K.; Koutsomarkos, N.; Mikos, A.G.; Chatzinikolaidou, M. Advances in 3D bioprinting for regenerative medicine applications. Regen. Biomater. 2024, 11, rbae033. [Google Scholar] [CrossRef] [PubMed]
- Böhmig, G.A.; Diebold, M.; Budde, K. Opinions on the Future of Clinical Pig Kidney Xenotransplantation. Transpl. Int. 2024, 37, 13475. [Google Scholar] [CrossRef]
- Zhao, W. Pig organs in humans: A forum on xenotransplantation. Natl. Sci. Rev. 2024, 11, nwae208. [Google Scholar] [CrossRef]
- Zhang, J.Q.J.; Cavazzoni, E.; Durkan, A.M.; Hahn, D.; McCarthy, H.; Alexander, S.; Thomas, G.; Kennedy, S.E.; Kermond, R.; Skowno, J.; et al. Effect of perioperative management on early graft function in living donor paediatric kidney transplantation. Pediatr. Nephrol. 2024, 40, 231–242. [Google Scholar] [CrossRef]
- Sever, M.S.; Van Biesen, W.; Vanholder, R.; Mallick, N.; London, G.; Schena, F.P.; Nagy, J.; Buturovic-Ponikvar, J.; Heering, P.; Maggiore, U.; et al. Ethical and medical dilemmas in paid living kidney donor transplantation. Transplant. Rev. 2022, 36, 100726. [Google Scholar] [CrossRef]
- Martin, D.E.; Fadhil, R.A.; Więcek, A. Ethical Aspects of Kidney Donation and Transplantation for Migrants. Semin. Nephrol. 2022, 42, 151271. [Google Scholar] [CrossRef]
- Bayliss, G. Practical ethical concerns in allocation of pig kidneys to humans. Clin. Kidney J. 2022, 15, 2161–2168. [Google Scholar] [CrossRef]
- Uvelius, B.; Andersson, K.E. Can Urinary Bladder Innervation Be Restored After Outlet Obstruction and Denervation? Int. Neurourol. J. 2024, 28, 75–82. [Google Scholar] [CrossRef]
- Crapo, P.M.; Medberry, C.J.; Reing, J.E.; Tottey, S.; van der Merwe, Y.; Jones, K.E.; Badylak, S.F. Biologic scaffolds composed of central nervous system extracellular matrix. Biomaterials 2012, 33, 3539–3547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ding, F.; Han, J.; Wang, Z.; Tian, W. Recent advances in innovative biomaterials for promoting bladder regeneration: Processing and functionalization. Front. Bioeng. Biotechnol. 2025, 12, 1528658. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.H.; Dunn, A.J.; Talovic, M.; Haas, G.J.; Marcinczyk, M.; Elmashhady, H.; Kalaf, E.G.; Sell, S.A.; Garg, K. Aligned nanofibers of decellularized muscle ECM support myogenic activity in primary satellite cells in vitro. Biomed. Mater. 2019, 14, 035010. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, C.; Ponsaerts, P.; Wyndaele, J.J. Cell-Based Therapies in Lower Urinary Tract Disorders. Cell Transplant. 2015, 24, 1679–1686. [Google Scholar] [CrossRef]
- Dhandapani, V.; Vermette, P. Decellularized bladder as scaffold to support proliferation and functionality of insulin-secreting pancreatic cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2023, 111, 1890–1902. [Google Scholar] [CrossRef]
- Sabetkish, S.; Sabetkish, N.; Kajbafzadeh, A.-M. Regeneration of muscular wall of the bladder using a ureter matrix graft as a scaffold. Biotech. Histochem. 2021, 97, 207–214. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Imamura, T.; Kitahara, R.; Inoue, Y.; Saito, T.; Ueno, M.; Minagawa, T.; Ogawa, T.; Ishizuka, O. Bi-layered Adipose Mesenchymal Cell Sheets Improve Bladder Compliance in Spinal Cord-Injured Rats. Tissue Eng. Part A 2024, 31, 409–418. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, H.; Ling, Z.; An, Z.; Xiao, S.; Wang, P.; Fu, Z.; Shao, J.; Sun, Y.; Fu, W. A bilayer bioengineered patch with sequential dual-growth factor release to promote vascularization in bladder reconstruction. Regen. Biomater. 2024, 11, rbae083. [Google Scholar] [CrossRef]
- Sommerfeld, S.D.; Zhou, X.; Mejías, J.C.; Oh, B.C.; Maestas, D.R.; Furtmüller, G.J.; Laffont, P.A.; Elisseeff, J.H.; Brandacher, G. Biomaterials-based immunomodulation enhances survival of murine vascularized composite allografts. Biomater. Sci. 2023, 11, 4022–4031. [Google Scholar] [CrossRef]
- Matsui, K.; Kinoshita, Y.; Inage, Y.; Matsumoto, N.; Morimoto, K.; Saito, Y.; Takamura, T.; Matsunari, H.; Yamanaka, S.; Nagashima, H.; et al. Cryopreservation of Fetal Porcine Kidneys for Xenogeneic Regenerative Medicine. J. Clin. Med. 2023, 12, 2293. [Google Scholar] [CrossRef]
- Sykes, M.; Sachs, D.H. Progress in xenotransplantation: Overcoming immune barriers. Nat. Rev. Nephrol. 2022, 18, 745–761. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, M.; Wei, H.; Zhang, B.; Peng, J.; Shang, P.; Sun, S. Research prospects for kidney xenotransplantation: A bibliometric analysis. Ren. Fail. 2024, 46, 2301681. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, S. Generation of chimeric kidneys using progenitor cell replacement: Oshima Award Address 2021. Clin. Exp. Nephrol. 2022, 26, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, J.; Moris, D.; Hayes, B.; Abraham, S.N.; Cendales, L.C. Introducing a novel experimental model of bladder transplantation in mice. Am. J. Transplant. 2020, 20, 3558–3566. [Google Scholar] [CrossRef]
- Pokrywczynska, M.; Jundzill, A.; Kloskowski, T.; Bodnar, M.; Marszalek, A.; Drewa, T. A new heterotropic vascularized model of total urinary bladder transplantation in a rat model. Sci. Rep. 2021, 11, 3775. [Google Scholar] [CrossRef]
- Nassiri, N.; Cacciamani, G.; Gill, I.S. Robotic Bladder Autotransplantation: Preclinical Studies in Preparation for First-in-human Bladder Transplant. J. Urol. 2023, 210, 600–610. [Google Scholar] [CrossRef]
- Gargollo, P.C.; Ahmed, M.E.; Prieto, M.; Butaney, M.; Cramer, C.H.; Joshi, V.; Heimbach, J.K.; Granberg, C.F. Feasibility Study of Vascularized Composite Urinary Bladder Allograft Transplantation in a Cadaver Model. J. Urol. 2021, 206, 115–123. [Google Scholar] [CrossRef]
- Shen, J.-D.; Chen, S.-J.; Chen, H.-Y.; Chiu, K.-Y.; Chen, Y.-H.; Chen, W.-C. Review of Animal Models to Study Urinary Bladder Function. Biology 2021, 10, 1316. [Google Scholar] [CrossRef]
- Schmidt-Lauber, C.; Günster, C.; Huber, T.B.; Spoden, M.; Grahammer, F. Collateral Effects and Mortality of Kidney Transplant Recipients during the COVID-19 Pandemic. Kidney360 2022, 3, 325–336. [Google Scholar] [CrossRef]
- Parga-Vidal, L.; van Aalderen, M.C.; Stark, R.; van Gisbergen, K.P.J.M. Tissue-resident memory T cells in the urogenital tract. Nat. Rev. Nephrol. 2022, 18, 209–223. [Google Scholar] [CrossRef]
- Al-Talib, M.; Skaria, A.; Griffin, S. Cellular Immunity Against BK Polyomavirus in Kidney Transplant Recipients: A Comprehensive Review. Transpl. Infect. Dis. 2024, 27, e14401. [Google Scholar] [CrossRef] [PubMed]
- Unsal, Y.; Baltu, D.; Gulhan, B.; Okur, F.V.; Ozaltın, F.; Düzova, A.; Topaloğlu, R.; Ozon, Z.A.; Gonç, E.N. Calcineurin inhibitor-related hyperkalemia is caused by hyporeninemic hypoaldosteronism and fludrocortisone is an effective treatment: Report of a case series and review of the literature. Pediatr. Transplant. 2024, 28, e14778. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, B.; Tripathi, S.; Nikiforow, S.; Chandraker, A. Adoptive Immune Effector Cell Therapies in Cancer and Solid Organ Transplantation: A Review. Semin. Nephrol. 2024, 44, 151498. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.W.; Wheless, W.; Vrochides, D. Management of long-term complications from immunosuppression. Liver Transplant. 2024, 30, 647–658. [Google Scholar] [CrossRef]
- Sánchez, M.J.T.; Fuentes, M.C.R.; García, E.C.; Chica, N.R.; Muñoz, K.E.; Huete, M.J.E.; Molina, M.C.; Osuna, A.; Wangensteen, R. Hydroxyproline in Urine Microvesicles as a Biomarker of Fibrosis in the Renal Transplant Patient. Biomedicines 2024, 12, 2836. [Google Scholar] [CrossRef]
- Clos-Sansalvador, M.; Taco, O.; Rodríguez-Martínez, P.; Garcia, S.G.; Font-Morón, M.; Bover, J.; Vila-Santandreu, A.; Franquesa, M.; Juega, J.; Borràs, F.E. Towards clinical translation of urinary vitronectin for non-invasive detection and monitoring of renal fibrosis in kidney transplant patients. J. Transl. Med. 2024, 22, 1030. [Google Scholar] [CrossRef]
- Martin-Martin, C.; Suarez-Alvarez, B.; González, M.; Torres, I.B.; Bestard, O.; Martín, J.E.; Barceló-Coblijn, G.; Moreso, F.; Aransay, A.M.; Lopez-Larrea, C.; et al. Exploring kidney allograft rejection: A proof-of-concept study using spatial transcriptomics. Am. J. Transplant. 2024, 24, 1161–1171. [Google Scholar] [CrossRef]
- Boshart, A.; Petrovic, S.; Abovsky, M.; Pastrello, C.; Farkona, S.; Manion, K.; Neupane, S.; Allen, M.; Jurisica, I.; Konvalinka, A. Molecular landscape of kidney allograft tissues data integration portal (NephroDIP): A curated database to improve integration of high-throughput kidney transplant datasets. Front. Immunol. 2024, 15, 1469500. [Google Scholar] [CrossRef]
- Naaz, A.; Turnquist, H.R.; Gorantla, V.S.; Little, S.R. Drug delivery strategies for local immunomodulation in transplantation: Bridging the translational gap. Adv. Drug Deliv. Rev. 2024, 213, 115429. [Google Scholar] [CrossRef]
- Xu, W.; Boer, K.; Hesselink, D.A.; Baan, C.C. Extracellular Vesicles and Immune Activation in Solid Organ Transplantation: The Impact of Immunosuppression. BioDrugs 2025, 39, 445–459. [Google Scholar] [CrossRef]
- Sarfraz, M.; Qamar, S.; Rehman, M.U.; Tahir, M.A.; Ijaz, M.; Ahsan, A.; Asim, M.H.; Nazir, I. Nano-Formulation Based Intravesical Drug Delivery Systems: An Overview of Versatile Approaches to Improve Urinary Bladder Diseases. Pharmaceutics 2022, 14, 1909. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.Y.; Yang, H.M.; Kim, C.H.; Goo, Y.T.; Kang, M.J.; Lee, S.; Choi, Y.W. Current status of the development of intravesical drug delivery systems for the treatment of bladder cancer. Expert Opin. Drug Deliv. 2020, 17, 1555–1572. [Google Scholar] [CrossRef] [PubMed]
- Bromberg, J.; Demko, Z.P.; Kaur, N.; Marshall, K.; Armer-Cabral, M.; Tabriziani, H.; Bhorade, S.; Gauthier, P.; Samaniego-Picota, M. Increases in donor-derived cell-free DNA prior to biopsy proven rejection in kidney transplant. Transplantation 2024, 108. [Google Scholar] [CrossRef]
- Kataria, A.; Athreya, A.; Gupta, G. Biomarkers in Kidney Transplantation. Adv. Kidney Dis. Health 2024, 31, 427–435. [Google Scholar] [CrossRef]
- Cho, A.; Han, A.; Lee, J.; Park, J.B.; Jung, C.W.; Kim, Y.C.; Park, S.; Min, S.; Ha, J. Donor-derived cell-free DNA identifies subclinical antibody-mediated rejection in de novo donor-specific antibody-positive kidney transplant recipients. Transplantation 2024, 108, 339. [Google Scholar] [CrossRef]
- Kim, H.D.; Bae, H.; Kang, H.; Lee, H.; Eum, S.H.; Yang, C.W.; Choi, Y.J.; Chung, B.H.; Oh, E.-J. Donor-derived cell-free DNA predicted allograft rejection and severe microvascular inflammation in kidney transplant recipients. Front. Immunol. 2024, 15, 1433918. [Google Scholar] [CrossRef]
- Scurt, F.G.; Hammoud, B.; Bose, K.; Mertens, P.R.; Chatzikyrkou, C. Short-Term, Mid-Term, and Long-Term Outcomes after Deceased Donor Kidney Transplantation in Patients with AKI. Kidney360 2024, 5, 1012–1031. [Google Scholar] [CrossRef]
- Zicarelli, M.; Errante, A.; Andreucci, M.; Coppolino, G.; Bolignano, D. Immunosuppressive Therapy, Puberty and Growth Outcomes in Pediatric Kidney Transplant Recipients: A Pragmatic Review. Pediatr. Transplant. 2024, 28, e14878. [Google Scholar] [CrossRef]
- Corr, M.; Walker, A.; Maxwell, A.P.; McKay, G.J. Non-adherence to immunosuppressive medications in kidney transplant recipients- a systematic scoping review. Transplant. Rev. 2024, 39, 100900. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, P.; Wuren, T. Narrative Review of Mesenchymal Stem Cell Therapy in Renal Diseases: Mechanisms, Clinical Applications, and Future Directions. Stem Cells Int. 2024, 2024, 8658246. [Google Scholar] [CrossRef]
- Janfeshan, S.; Afshari, A.; Yaghobi, R.; Roozbeh, J. Urinary CXCL-10, a prognostic biomarker for kidney graft injuries: A systematic review and meta-analysis. BMC Nephrol. 2024, 25, 292. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.; Divard, G.; Raynaud, M.; Cohen, A.; Mone, T.D.; Rosenthal, J.T.; Bentall, A.J.; Stegall, M.D.; Naesens, M.; Zhang, H.; et al. A Machine Learning-Driven Virtual Biopsy System For Kidney Transplant Patients. Nat. Commun. 2024, 15, 554. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.M.; Elefant, N.; Tedesco, M.; Bogyo, K.; Vena, N.; Murthy, S.K.; Bheda, S.A.; Yang, S.; Tomar, N.; Zhang, J.Y.; et al. Developing a genetic testing panel for evaluation of morbidities in kidney transplant recipients. Kidney Int. 2024, 106, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.E.; Capron, A.M.; Fadhil, R.A.; Forsythe, J.L.; Padilla, B.; Pérez-Blanco, A.; Van Assche, K.; Bengochea, M.; Cervantes, L.; Forsberg, A.; et al. Supporting Financial Neutrality in Donation of Organs, Cells, and Tissues. Transplantation 2024, 109, 48–59. [Google Scholar] [CrossRef]
- Ashwin, A.; Cherukuri, S.; Rammohan, A. The psychology, legality, ethics and medical aspects of organ donation by minors. Transplant. Rev. 2024, 38, 100832. [Google Scholar] [CrossRef]
- Al-Thnaibat, M.H.; Balaw, M.K.; Al-Aquily, M.K.; Ghannam, R.A.; Mohd, O.B.; Alabidi, F.; Alabidi, S.; Hussein, F.; Rawashdeh, B. Addressing Kidney Transplant Shortage: The Potential of Kidney Paired Exchanges in Jordan. J. Transplant. 2024, 2024, 4538034. [Google Scholar] [CrossRef]
- Arvanitis, P.; Davis, M.R.; London, A.; Farmakiotis, D. Medical malpractice in organ transplantation: Public allegations and key legal outcomes. Front. Heal. Serv. 2024, 4, 1408934. [Google Scholar] [CrossRef]
- Salybekov, A.A.; Yerkos, A.; Sedlmayr, M.; Wolfien, M. Ethics and Algorithms to Navigate AI’s Emerging Role in Organ Transplantation. J. Clin. Med. 2025, 14, 2775. [Google Scholar] [CrossRef]
- Garg, N.; Thiessen, C.; Garonzik-Wang, J.; Mezrich, J.; Mandelbrot, D.A. Navigating challenges in recipient selection for end-chain kidneys. Am. J. Transplant. 2024, 25, 471–475. [Google Scholar] [CrossRef]
- Feil, G.; Daum, L.; Amend, B.; Maurer, S.; Renninger, M.; Vaegler, M.; Seibold, J.; Stenzl, A.; Sievert, K.-D. From tissue engineering to regenerative medicine in urology—The potential and the pitfalls. Adv. Drug Deliv. Rev. 2011, 63, 375–378. [Google Scholar] [CrossRef]
- Hawthorne, W.J. Ethical and legislative advances in xenotransplantation for clinical translation: Focusing on cardiac, kidney and islet cell xenotransplantation. Front. Immunol. 2024, 15, 1355609. [Google Scholar] [CrossRef] [PubMed]
- Alcorn, J. Continuously Balancing the Ethics of Organ Allocation. Curr. Transplant. Rep. 2024, 11, 7–14. [Google Scholar] [CrossRef]
- Tungsanga, S.; Ghimire, A.; Hariramani, V.K.; Abdulrahman, A.; Khan, A.S.; Ye, F.; Kung, J.Y.; Klarenbach, S.; Thompson, S.; Collister, D.; et al. Global trends in chronic kidney disease-related mortality: A systematic review protocol. BMJ Open 2024, 14, e078485. [Google Scholar] [CrossRef] [PubMed]
- Mor, E. Letter to the Editor Living organ donation in polarized societies. Am. J. Transplant. 2024, 24, 1514–1515. [Google Scholar] [CrossRef]
- Lopez, C.D.; Girard, A.O.; Redett, R.J. Expanding indications for urogenital transplantation: Congenital and oncologic defects, and gender affirmation. Curr. Opin. Organ Transplant. 2023, 28, 425–430. [Google Scholar] [CrossRef]
- Martin, D.E.; Capron, A.M.; Fadhil, R.A.; Forsythe, J.L.; Padilla, B.; Pérez-Blanco, A.; Van Assche, K.; Bengochea, M.; Cervantes, L.; Forsberg, A.; et al. Prevention of Trafficking in Organs, Tissues, and Cells. Transplantation 2025, 109, 88–97. [Google Scholar] [CrossRef]
- Albertsen, A. Unjust organ markets and why it is irrelevant that selling a kidney is the best option. J. Med. Ethics 2024, 51, 263–267. [Google Scholar] [CrossRef]
- Alshehri, M.; Tawhari, I.; Alqahtani, T.S.; Alqahtani, A.Y.; Al Jallal, M.S.; Asiri, G.B.; Alshahrani, M.A.; Majrashi, M.A.; Khuzayyim, A.A.; Albishri, F.D.; et al. Knowledge and willingness to donate kidney for transplantation among general population in Saudi Arabia. BMC Public Health 2024, 24, 2277. [Google Scholar] [CrossRef]
- Pionnier, Y.; Darius, T.; Penaloza, A.; Steenebruggen, F.; Dupriez, F.; Neyrinck, A.; Genbrugge, C. Solid organ transplantation originating from uncontrolled donation after circulatory death in Europe: A narrative review. Scand. J. Trauma Resusc. Emerg. Med. 2024, 32, 130. [Google Scholar] [CrossRef]
- Birtan, D.; Akpınar, A. Ethical challenges in organ transplantation for Syrian refugees in Türkiye. BMC Med. Ethics 2024, 25, 124. [Google Scholar] [CrossRef]
- Klapholz, J.; Eickel, G.; Reeb, M.; Jaffe, I.; Klitenic, S.; Alejo, J.; Lonze, B.; Levan, M. Emerging Logistic Challenges, Health Disparities, and Bioethical Concerns in Kidney Xenotransplantation: A Literature Review. Curr. Transplant. Rep. 2024, 11, 160–168. [Google Scholar] [CrossRef]
- Birtan, D.; Akpinar, A. Ethical challenges in organ transplants for refugees in a healthcare system. Nurs. Ethics 2024, 32, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Courtwright, A.M.; Erler, K.S.; Bandini, J.I.; Zwirner, M.; Cremens, M.C.; McCoy, T.H.; Robinson, E.M.; Rubin, E. Ethics Consultation for Adult Solid Organ Transplantation Candidates and Recipients: A Single Centre Experience. J. Bioethical Inq. 2021, 18, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Padilla, L.A.; Rhodes, L.; Sorabella, R.A.; Hurst, D.J.; Cleveland, D.C.; Dabal, R.J.; Cooper, D.K.; Paris, W.; Carlo, W.F. Attitudes toward xenotransplantation: A survey of parents and pediatric cardiac providers. Pediatr. Transplant. 2020, 25, e13851. [Google Scholar] [CrossRef]
- Hurst, D.J.P.; Padilla, L.M.; Merlocco, A.M.; Rodger, D.M.; Bobier, C.; Gray, W.H.; Sorabella, R.; Cooper, D.K.C.; Pierson, R.N. Pediatric Cardiac Xenotransplantation: Recommendations for the Ethical Design of Clinical Trials. Transplantation 2024, 108, e292–e300. [Google Scholar] [CrossRef]
- DeLaura, I.; Anwar, I.J.; Ladowski, J.; Patino, A.; Cantrell, S.; Sanoff, S. Attitudes of patients with renal disease on xenotransplantation: A systematic review. Xenotransplantation 2023, 30, e12794. [Google Scholar] [CrossRef]
- Yahav, D.; Rozen-Zvi, B.; Mashraki, T.; Atamna, A.; Ben-Zvi, H.; Bar-Haim, E.; Rahamimov, R. Immunosuppression reduction when administering a booster dose of the BNT162b2 mRNA SARS-CoV-2 vaccine in kidney transplant recipients without adequate humoral response following two vaccine doses: Protocol for a randomised controlled trial (BECAME study). BMJ Open 2021, 11, e055611. [Google Scholar] [CrossRef]
- Güzel, H.; Ovayolu, Ö.; Ovayolu, N.; Ilter, S.M. The relationship between compliance with immunosuppressive therapy and religious attitudes of kidney transplant patients. Transpl. Immunol. 2024, 85, 102080. [Google Scholar] [CrossRef]
- Cao, M.-L.; Han, R.-Y.; Chen, S.-D.; Zhao, D.-Y.; Shi, M.-Y.; Zou, J.-H.; Li, L.; Jiang, H.-K. Gene Editing: An Effective Tool for the Future Treatment of Kidney Disease. J. Inflamm. Res. 2025, 18, 4001–4018. [Google Scholar] [CrossRef]
- Kuokkanen, S.; Zhu, L.; Pollard, J.W. Xenografted tissue models for the study of human endometrial biology. Differentiation 2017, 98, 62–69. [Google Scholar] [CrossRef]
- Lau, R.W.K.; Wang, B.; Ricardo, S.D. Gene editing of stem cells for kidney disease modelling and therapeutic intervention. Nephrology 2018, 23, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Güell, M. Genome-Wide PERV Inactivation in Pigs Using CRISPR/Cas9. In Xenotransplantation: Methods and Protocols, 2nd ed.; Humana: New York, NY, USA, 2020; Volume 2110, pp. 139–149. [Google Scholar]
- Eisenson, D.L.; Hisadome, Y.; Yamada, K. Progress in Xenotransplantation: Immunologic Barriers, Advances in Gene Editing, and Successful Tolerance Induction Strategies in Pig-To-Primate Transplantation. Front. Immunol. 2022, 13, 899657. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, D.; Singh, Y.P.; Yeo, M.; Deng, G.; Lai, J.; Chen, F.; Ozbolat, I.T.; Yu, Y. Progress in Organ Bioprinting for Regenerative Medicine. Engineering 2024, 42, 121–142. [Google Scholar] [CrossRef]
- Schlidt, K.; Asgardoon, M.; Febre-Alemañy, D.A.; El-Mallah, J.C.; Waldron, O.; Dawes, J.; Agrawal, S.; Landmesser, M.E.; Ravnic, D.J. Surgical Bioengineering of the Microvasculature and Challenges in Clinical Translation. Tissue Eng. Part B Rev. 2025. [Google Scholar] [CrossRef]
- Ibi, Y.; Nishinakamura, R. Kidney Bioengineering for Transplantation. Transplantation 2023, 107, 1883–1894. [Google Scholar] [CrossRef]
- Xu, K.; Han, Y.; Huang, Y.; Wei, P.; Yin, J.; Jiang, J. The application of 3D bioprinting in urological diseases. Mater. Today Bio 2022, 16, 100388. [Google Scholar] [CrossRef]
- Wang, P.; Li, C.; Peng, T.; Ruan, L.; Wu, A.; Zhu, J.; Shi, W.; Chen, M.; Zhang, T. Tolerogenic CD11c+dendritic cells regulate CD4+Tregs in replacing delayed ischemic preconditioning to alleviate ischemia-reperfusion acute kidney injury. Faseb J. 2024, 38, e23575. [Google Scholar] [CrossRef]
- Ho, Q.Y.; Hester, J.; Issa, F. Regulatory cell therapy for kidney transplantation and autoimmune kidney diseases. Pediatr. Nephrol. 2024, 40, 39–52. [Google Scholar] [CrossRef]
- Loupy, A.; Certain, A.; Tangprasertchai, N.S.; Racapé, M.; Ursule-Dufait, C.; Benbadi, K.; Raynaud, M.; Vaskova, E.; Marchis, C.; Casas, S.; et al. Evaluation of a Decentralized Donor-Derived Cell-Free DNA Assay for Kidney Allograft Rejection Monitoring. Transpl. Int. 2024, 37, 13919. [Google Scholar] [CrossRef]
- Parajuli, S.; Garg, N.; Dodin, B.; Breyer, I.; Zona, E.; Patel, S.; Pinney, K.; Mandelbrot, D. Changes in Donor-Derived Cell-Free DNA Before and After Rejection and De Novo DSA Detection in Primary and Repeat Kidney Transplant Recipients. Clin. Transplant. 2024, 38, e70019. [Google Scholar] [CrossRef]
- Yatim, K.; Ribas, G.T.; Elton, D.C.; Rockenbach, M.A.; Al Jurdi, A.; Pickhardt, P.J.; Garrett, J.W.; Dreyer, K.J.; Bizzo, B.C.; Riella, L.V. Applying Artificial Intelligence to Quantify Body Composition on Abdominal CTs and Better Predict Kidney Transplantation Wait-List Mortality. J. Am. Coll. Radiol. 2025, 22, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Raina, R.; Doshi, K.; Aggarwal, P.; Kim, P.; Sasse, J.; Sethi, S.; Abitbol, C.; Abu-Arja, R.; Kashani, K. Application of artificial intelligence and machine learning for risk stratification acute kidney injury among hematopoietic stem cell transplantation patients: PCRRT ICONIC AI Initiative Group Meeting Proceedings. Clin. Nephrol. 2025, 103, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Wellekens, K.; Koshy, P.; Naesens, M. Challenges in standardizing preimplantation kidney biopsy assessments and the potential of AI-Driven solutions. Curr. Opin. Nephrol. Hypertens. 2025, 34, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Mizera, J.; Pondel, M.; Kepinska, M.; Jerzak, P.; Banasik, M. Advancements in Artificial Intelligence for Kidney Transplantology: A Comprehensive Review of Current Applications and Predictive Models. J. Clin. Med. 2025, 14, 975. [Google Scholar] [CrossRef]
- Xi, J.; Zheng, W.; Chen, M.; Zou, Q.; Tang, C.; Zhou, X. Genetically engineered pigs for xenotransplantation: Hopes and challenges. Front. Cell Dev. Biol. 2023, 10, 1093534. [Google Scholar] [CrossRef]
- Negri, A.; Wilson, L. Future Systems of Xenotransplantation: Melding Historical and Bioethical Methodology. Cell Transplant. 2023, 32, 09636897231170510. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Z.-M. Ethics and governance of trustworthy medical artificial intelligence. BMC Med. Inform. Decis. Mak. 2023, 23, 7. [Google Scholar] [CrossRef]
- Gong, M.; Jiang, Y.; Sun, Y.; Liao, R.; Liu, Y.; Yan, Z.; He, A.; Zhou, M.; Yang, J.; Wu, Y.; et al. Knowledge domain and frontier trends of artificial intelligence applied in solid organ transplantation: A visualization analysis. Int. J. Med. Inform. 2025, 195, 105782. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuttymuratov, G.; Saliev, T.; Ainakulov, A.; Ayaganov, A.; Oshakbayev, K.; Zharassov, D.; Tuleuzhan, A.; Uderbayev, N. Kidney and Bladder Transplantation: Advances, Barriers, and Emerging Solutions. Medicina 2025, 61, 1045. https://doi.org/10.3390/medicina61061045
Kuttymuratov G, Saliev T, Ainakulov A, Ayaganov A, Oshakbayev K, Zharassov D, Tuleuzhan A, Uderbayev N. Kidney and Bladder Transplantation: Advances, Barriers, and Emerging Solutions. Medicina. 2025; 61(6):1045. https://doi.org/10.3390/medicina61061045
Chicago/Turabian StyleKuttymuratov, Gani, Timur Saliev, Ardak Ainakulov, Askar Ayaganov, Kuat Oshakbayev, Daulet Zharassov, Abdurakhman Tuleuzhan, and Nurlybek Uderbayev. 2025. "Kidney and Bladder Transplantation: Advances, Barriers, and Emerging Solutions" Medicina 61, no. 6: 1045. https://doi.org/10.3390/medicina61061045
APA StyleKuttymuratov, G., Saliev, T., Ainakulov, A., Ayaganov, A., Oshakbayev, K., Zharassov, D., Tuleuzhan, A., & Uderbayev, N. (2025). Kidney and Bladder Transplantation: Advances, Barriers, and Emerging Solutions. Medicina, 61(6), 1045. https://doi.org/10.3390/medicina61061045






