Light, Sound, and Melatonin: Investigating Multisensory Pathways for Visual Restoration
Abstract
1. Introduction
1.1. Multisensory Integration in the Brain
1.2. Specialized Sensory Circuits in Peripheral Structures
1.3. Where Integration Occurs
2. The Superior Colliculus
2.1. Laminar Organization: From Sensory Input to Motor Output
2.2. Multisensory Integration in the SC: Principles and Mechanisms
2.3. The SC as a Hub for Orienting and Defensive Behaviors
2.4. Plasticity and Rehabilitation: Lessons from Hemianopia and Neglect
2.5. Clinical Insights: Reflexive vs. Volitional Circuits
3. The Retina’s Hidden Role in Multisensory Perception—Melanopsin, ipRGCS, and Crossmodal Rehabilitation
4. ipRGCs: Bridging Light Detection and Sensory Integration
4.1. Melanopsin and the Discovery of a Non-Classical Photoreceptor
4.2. ipRGCs as Multisensory Modulators
4.3. The ipRGC-Superior Colliculus Pathway: A Crossmodal Link
4.4. Implications for Spatial Neglect and Hemianopia
4.5. Melanopsin Signaling: A Molecular Lever for Rehabilitation
4.5.1. The Melanopsin Phototransduction Cascade
4.5.2. Melatonin’s Dual Role: Retinal and Central Modulation
4.5.3. Spatiotemporal Synergy for Rehabilitation
4.5.4. Crossmodal Integration via ipRGC-SC Pathways
4.5.5. Future Directions and Translational Challenges
5. Therapeutic Potential
5.1. Circadian Interventions: Light and Melatonin Synergy
5.2. Crossmodal Priming for Spatial Neglect
5.3. Optimizing Multisensory Rehabilitation
5.4. Pharmacological Modulation: Opportunities and Challenges
5.5. Quantifying Light Stimuli for Rehabilitation
- Directional Luminance: Characterize spatial properties (e.g., Ganzfeld vs. directional cues) to differentiate local (e.g., SC-targeted) from global (circadian) effects [55].
6. Final Remarks
6.1. Future Directions and Research Gaps
6.1.1. Mechanistic Clarity
6.1.2. Clinical Validation
6.2. Project Proposal
6.3. Limitations and Unanswered Questions
6.4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Full Form |
| AV | Audiovisual |
| BOLD | Blood Oxygen Level Dependent |
| Ca2+ | Calcium Ions |
| CC | Creative Commons (License) |
| CIE | International Commission on Illumination (French: Commission Internationale de l’Éclairage) |
| EDI | Equivalent Daylight Illuminance |
| EEG | Electroencephalogram |
| FEF | Frontal Eye Field |
| fMRI | Functional Magnetic Resonance Imaging |
| Gq | Gq Protein (a type of G-protein) |
| IC | Inferior Colliculus |
| IGL | Intergeniculate Leaflet |
| IP3 | Inositol Trisphosphate |
| ipRGCs | Intrinsically Photosensitive Retinal Ganglion Cells |
| LGN | Lateral Geniculate Nucleus |
| LTP | Long-term Potentiation |
| MT1/MT2 | Melatonin Receptor Type 1/Type 2 |
| NB | Nota Bene (“Note Well”) |
| PET | Positron Emission Tomography |
| PLCβ4 | Phospholipase C beta 4 |
| RGCs | Retinal Ganglion Cells |
| SC | Superior Colliculus |
| SCN | Suprachiasmatic Nucleus |
| SOC | Superior Olivary Complex |
| TRPC6/7 | Transient Receptor Potential Canonical 6/7 (channels) |
| VOCC | Voltage-operated Calcium Channels |
| VR | Virtual Reality |
References
- King, A.J.; Calvert, G.A. Multisensory integration: Perceptual grouping by eye and ear. Curr. Biol. 2001, 11, R322–R325. [Google Scholar] [CrossRef] [PubMed]
- Stein, B.E.; Stanford, T.R. Multisensory integration: Current issues from the perspective of the single neuron. Nat. Rev. Neurosci. 2008, 9, 255–266, Erratum in Nat. Rev. Neurosci. 2008, 9, 406. [Google Scholar] [CrossRef] [PubMed]
- Senkowski, D.; Engel, A.K. Multi-timescale neural dynamics for multisensory integration. Nat. Rev. Neurosci. 2024, 25, 625–642. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.O.; Banks, M.S. Humans integrate visual and haptic information in a statistically optimal fashion. Nature 2002, 415, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Shams, L.; Wozny, D.R.; Kim, R.; Seitz, A. Influences of Multisensory Experience on Subsequent Unisensory Processing. Front. Psychol. 2011, 2, 13064. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kandel, E.R.; Koester, J.D.; Mack, S.H.; Siegelbaum, S.A. (Eds.) Principles of Neural Science, 6e. McGraw Hill. 2021. Available online: https://neurology.mhmedical.com/content.aspx?bookid=3024§ionid=254326756 (accessed on 22 May 2025).
- Purves, D.; Augustine, G.J.; Fitzpatrick, D.; Katz, L.C.; LaMantia, A.S.; McNamara, J.O.; Williams, S.M. (Eds.) Neuroscience, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2001. Available online: https://www.ncbi.nlm.nih.gov/books/NBK10799/ (accessed on 22 May 2025).
- Cuppini, C.; Stein, B.E.; Rowland, B.A. Development of the Mechanisms Governing Midbrain Multisensory Integration. J. Neurosci. 2018, 38, 3453–3465. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kosciessa, J.Q.; Lindenberger, U.; Garrett, D.D. Thalamocortical excitability modulation guides human perception under uncertainty. Nat. Commun. 2021, 12, 2430. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Murray, M.M.; Thelen, A.; Thut, G.; Romei, V.; Martuzzi, R.; Matusz, P.J. The multisensory function of the human primary visual cortex. Neuropsychologia 2016, 83, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Tagliabue, M.; McIntyre, J. A modular theory of multisensory integration for motor control. Front. Comput. Neurosci. 2014, 8, 1. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sookprao, P.; Benjasupawan, K.; Phangwiwat, T.; Chatnuntawech, I.; Lertladaluck, K.; Gutchess, A.; Chunharas, C.; Itthipuripat, S. Conflicting Sensory Information Sharpens the Neural Representations of Early Selective Visuospatial Attention. J. Neurosci. 2024, 44, e2012232024. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rajan, A.; Siegel, S.N.; Liu, Y.; Bengson, J.; Mangun, G.R.; Ding, M. Theta Oscillations Index Frontal Decision-Making and Mediate Reciprocal Frontal–Parietal Interactions in Willed Attention. Cereb. Cortex 2018, 29, 2832–2843. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stein, B.E.; Stanford, T.R.; Rowland, B.A. Development of multisensory integration from the perspective of the individual neuron. Nat. Rev. Neurosci. 2014, 15, 520–535. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ito, T.; Yamamoto, M.; Liu, L.; Saqib, K.A.; Furuyama, T.; Ono, M. Segregated input to thalamic areas that project differently to core and shell auditory cortical fields. iScience 2025, 28, 111721. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fries, P. Rhythms for Cognition: Communication through Coherence. Neuron 2015, 88, 220–235. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Purpura, G.; Cioni, G.; Tinelli, F. Multisensory-Based Rehabilitation Approach: Translational Insights from Animal Models to Early Intervention. Front. Neurosci. 2017, 11, 430. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leo, F.; Bolognini, N.; Passamonti, C.; Stein, B.E.; Làdavas, E. Cross-modal localization in hemianopia: New insights on multisensory integration. Brain 2008, 131, 855–865. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stein, B.E.; Rowland, B.A. Using superior colliculus principles of multisensory integration to reverse hemianopia. Neuropsychologia 2020, 141, 107413. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koelewijn, T.; Bronkhorst, A.; Theeuwes, J. Attention and the multiple stages of multisensory integration: A review of audiovisual studies. Acta Psychol. 2010, 134, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Hoy, J.L.; Farrow, K. The superior colliculus. Curr. Biol. 2025, 35, R164–R168. [Google Scholar] [CrossRef] [PubMed]
- Meredith, M.A.; Stein, B.E. Visual, auditory, and somatosensory convergence on cells in superior colliculus results in multisensory integration. J. Neurophysiol. 1986, 56, 640–662. [Google Scholar] [CrossRef] [PubMed]
- May, P.J. The mammalian superior colliculus: Laminar structure and connections. Prog. Brain Res. 2006, 151, 321–378. [Google Scholar] [CrossRef] [PubMed]
- King, A.J.; Palmer, A.R. Integration of visual and auditory information in bimodal neurones in the guinea-pig superior colliculus. Exp. Brain Res. 1985, 60, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Zingg, B.; Chou, X.-L.; Zhang, Z.-G.; Mesik, L.; Liang, F.; Tao, H.W.; Zhang, L.I. AAV-Mediated Anterograde Transsynaptic Tagging: Mapping Corticocollicular Input-Defined Neural Pathways for Defense Behaviors. Neuron 2017, 93, 33–47. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stein, B.E.; Jiang, W.; Wallace, M.T.; Stanford, T.R. Nonvisual influences on visual-information processing in the superior colliculus. Prog. Brain Res. 2001, 134, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Benavidez, N.L.; Bienkowski, M.S.; Zhu, M.; Garcia, L.H.; Fayzullina, M.; Gao, L.; Bowman, I.; Gou, L.; Khanjani, N.; Cotter, K.R.; et al. Organization of the inputs and outputs of the mouse superior colliculus. Nat. Commun. 2021, 12, 4004. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Passamonti, C.; Bertini, C.; Làdavas, E. Audio-visual stimulation improves oculomotor patterns in patients with hemianopia. Neuropsychologia 2009, 47, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Karnath, H.-O.; Rorden, C. The anatomy of spatial neglect. Neuropsychologia 2012, 50, 1010–1017. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Takahashi, M.; Sugiuchi, Y.; Na, J.; Shinoda, Y. Brainstem Circuits Triggering Saccades and Fixation. J. Neurosci. 2021, 42, 789–803. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bolognini, N.; Vallar, G. Hemianopia, spatial neglect, and their multisensory rehabilitation. In Multisensory Perception: From Laboratory to Clinic; Academic Press: Cambridge, MA, USA, 2020; pp. 423–447. [Google Scholar] [CrossRef]
- Ecker, J.L.; Dumitrescu, O.N.; Wong, K.Y.; Alam, N.M.; Chen, S.-K.; LeGates, T.; Renna, J.M.; Prusky, G.T.; Berson, D.M.; Hattar, S. Melanopsin-Expressing Retinal Ganglion-Cell Photoreceptors: Cellular Diversity and Role in Pattern Vision. Neuron 2010, 67, 49–60. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sonoda, T.; Lee, S.K.; Birnbaumer, L.; Schmidt, T.M. Melanopsin Phototransduction Is Repurposed by ipRGC Subtypes to Shape the Function of Distinct Visual Circuits. Neuron 2018, 99, 754–767.e4. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- D’Souza, S.; Lang, R.A. Retinal ganglion cell interactions shape the developing mammalian visual system. Development 2020, 147, dev196535. [Google Scholar] [CrossRef] [PubMed]
- Berson, D.M.; Dunn, F.A.; Takao, M. Phototransduction by Retinal Ganglion Cells That Set the Circadian Clock. Science 2002, 295, 1070–1073. [Google Scholar] [CrossRef] [PubMed]
- Barnard, A.R.; Hattar, S.; Hankins, M.W.; Lucas, R.J. Melanopsin Regulates Visual Processing in the Mouse Retina. Curr. Biol. 2006, 16, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Ma, J.; Yu, Y.-Q.; Gao, X.-F.; Bai, Y.; Sun, Y.; Liu, J.; Liu, X.; Barry, D.M.; Wilhelm, S.; et al. A non-canonical retina-ipRGCs-SCN-PVT visual pathway for mediating contagious itch behavior. Cell Rep. 2022, 41, 111444. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Harrison, K.R.; Chervenak, A.P.; Resnick, S.M.; Reifler, A.N.; Wong, K.Y. Amacrine Cells Forming Gap Junctions with Intrinsically Photosensitive Retinal Ganglion Cells: ipRGC Types, Neuromodulator Contents, and Connexin Isoform. Investig. Opthalmol. Vis. Sci. 2021, 62, 10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kinane, C.; Calligaro, H.; Jandot, A.; Coutanson, C.; Haddjeri, N.; Bennis, M.; Dkhissi-Benyahya, O. Dopamine modulates the retinal clock through melanopsin-dependent regulation of cholinergic waves during development. BMC Biol. 2023, 21, 146. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lacoste, B.; Angeloni, D.; Dominguez-Lopez, S.; Calderoni, S.; Mauro, A.; Fraschini, F.; Descarries, L.; Gobbi, G. Anatomical and cellular localization of melatonin MT1 and MT2 receptors in the adult rat brain. J. Pineal Res. 2015, 58, 397–417. [Google Scholar] [CrossRef] [PubMed]
- LeGates, T.A.; Fernandez, D.C.; Hattar, S. Light as a central modulator of circadian rhythms, sleep and affect. Nat. Rev. Neurosci. 2014, 15, 443–454. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Provencio, I.; Rodriguez, I.R.; Jiang, G.; Hayes, W.P.; Moreira, E.F.; Rollag, M.D. A Novel Human Opsin in the Inner Retina. J. Neurosci. 2000, 20, 600–605. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, X.; Stafford, B.K.; Godin, A.L.; King, W.M.; Wong, K.Y. Photoresponse diversity among the five types of intrinsically photosensitive retinal ganglion cells. J. Physiol. 2014, 592, 1619–1636. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cao, D.; Barrionuevo, P.A. The importance of intrinsically photosensitive retinal ganglion cells and implications for lighting design. J. Solid State Light 2015, 2, 10. [Google Scholar] [CrossRef]
- Maruani, J.; Geoffroy, P.A. Multi-Level Processes and Retina–Brain Pathways of Photic Regulation of Mood. J. Clin. Med. 2022, 11, 448. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mrosovsky, N. Locomotor Activity and Non-Photic Influences on Circadian Clocks. Biol. Rev. 1996, 71, 343–372. [Google Scholar] [CrossRef] [PubMed]
- Morin, L.P. Neuroanatomy of the extended circadian rhythm system. Exp. Neurol. 2013, 243, 4–20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hattar, S.; Kumar, M.; Park, A.; Tong, P.; Tung, J.; Yau, K.; Berson, D.M. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J. Comp. Neurol. 2006, 497, 326–349. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Duda, M.; Domagalik, A.; Orlowska-Feuer, P.; Krzysztynska-Kuleta, O.; Beldzik, E.; Smyk, M.K.; Stachurska, A.; Oginska, H.; Jeczmien-Lazur, J.S.; Fafrowicz, M.; et al. Melanopsin: From a small molecule to brain functions. Neurosci. Biobehav. Rev. 2020, 113, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Ursino, M.; Cuppini, C.; Magosso, E. Neurocomputational approaches to modelling multisensory integration in the brain: A review. Neural Netw. 2014, 60, 141–165. [Google Scholar] [CrossRef] [PubMed]
- Magosso, E.; Cuppini, C.; Bertini, C. Audiovisual Rehabilitation in Hemianopia: A Model-Based Theoretical Investigation. Front. Comput. Neurosci. 2017, 11, 113. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mahoney, H.L.; Schmidt, T.M. The cognitive impact of light: Illuminating ipRGC circuit mechanisms. Nat. Rev. Neurosci. 2024, 25, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Lucas, R.J.; Peirson, S.N.; Berson, D.M.; Brown, T.M.; Cooper, H.M.; Czeisler, C.A.; Figueiro, M.G.; Gamlin, P.D.; Lockley, S.W.; O’hagan, J.B.; et al. Measuring and using light in the melanopsin age. Trends Neurosci. 2014, 37, 1–9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schenke, N.; Eling, P.; Duning, T.; Hildebrandt, H. Monocular eye patching modulates ipsilesional reactive saccades and smooth pursuit in patients with left hemispatial neglect. Brain Cogn. 2023, 173, 106101. [Google Scholar] [CrossRef] [PubMed]
- Amorim-De-Sousa, A.; Chakraborty, R.; Collins, M.J.; Fernandes, P.; González-Méijome, J.; Hannibal, J.; Hoseini-Yazdi, H.; Read, S.A.; Ellrich, J.; Schilling, T. Blue light stimulation of the blind spot in human: From melanopsin to clinically relevant biomarkers of myopia. Bioelectron. Med. 2024, 10, 26. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fan, Y.-T.; Wu, C.-Y.; Tsai, W.-C.; Lin, K.-C. Effects of lateralized light flash and color on unilateral neglect. Disabil. Rehabil. 2015, 37, 2400–2406. [Google Scholar] [CrossRef] [PubMed]
- Müller-Oehring, E.M.; Kasten, E.; Poggel, D.A.; Schulte, T.; Strasburger, H.; Sabel, B.A. Neglect and Hemianopia Superimposed. J. Clin. Exp. Neuropsychol. 2003, 25, 1154–1168. [Google Scholar] [CrossRef] [PubMed]
- Tinelli, F.; Purpura, G.; Cioni, G. Audio-Visual Stimulation Improves Visual Search Abilities in Hemianopia due to Childhood Acquired Brain Lesions. Multisensory Res. 2015, 28, 153–171. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.; Hankins, M.W.; Foster, R.G.; Peirson, S.N. Melanopsin phototransduction: Slowly emerging from the dark. Prog. Brain Res. 2012, 199, 19–40. [Google Scholar] [CrossRef] [PubMed]
- Alkozi, H. Melatonin and melanopsin in the eye: Friends or foes? An. Real Acad. Nac. Farm. 2019, 85, 49–59. [Google Scholar]
- Bailes, H.J.; Lucas, R.J. Melanopsin and inner retinal photoreception. Cell Mol. Life Sci. 2009, 67, 99–111. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wescott, D.L.; Hasler, B.P.; Franzen, P.L.; Taylor, M.L.; Klevens, A.M.; Gamlin, P.; Siegle, G.J.; A Roecklein, K. Circadian photoentrainment varies by season and depressed state: Associations between light sensitivity and sleep and circadian timing. Sleep 2024, 47, zsae066. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pack, W.; Hill, D.; Wong, K. Melatonin modulates M4-type ganglion-cell photoreceptors. Neuroscience 2015, 303, 178–188. [Google Scholar] [CrossRef]
- Jilg, A.; Bechstein, P.; Saade, A.; Dick, M.; Li, T.X.; Tosini, G.; Rami, A.; Zemmar, A.; Stehle, J.H. Melatonin modulates daytime-dependent synaptic plasticity and learning efficiency. J. Pineal Res. 2019, 66, e12553. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.M.; Wong, K.Y. Melanopsin-expressing, Intrinsically Photosensitive Retinal Ganglion Cells (ipRGCs). 2008 Aug 1 [Updated 2016 Nov 2]. In Webvision: The Organization of the Retina and Visual System; Kolb, H., Fernandez, E., Nelson, R., Eds.; University of Utah Health Sciences Center. Available online: https://webvision.med.utah.edu/book/part-ii-anatomy-and-physiology-of-the-retina/melanopsin-expressing-intrinsically-photosensitive-retinal-ganglion-cells/ (accessed on 22 May 2025).
- Gooley, J.J.; Mien, I.H.; Hilaire, M.A.S.; Yeo, S.-C.; Chua, E.C.-P.; van Reen, E.; Hanley, C.J.; Hull, J.T.; Czeisler, C.A.; Lockley, S.W. Melanopsin and Rod–Cone Photoreceptors Play Different Roles in Mediating Pupillary Light Responses during Exposure to Continuous Light in Humans. J. Neurosci. 2012, 32, 14242–14253. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Federici, A.; Bernardi, G.; Senna, I.; Fantoni, M.; Ernst, M.O.; Ricciardi, E.; Bottari, D. Crossmodal plasticity following short-term monocular deprivation. NeuroImage 2023, 274, 120141. [Google Scholar] [CrossRef] [PubMed]
- Alwashmi, K.; Meyer, G.; Rowe, F.; Ward, R. Enhancing learning outcomes through multisensory integration: A fMRI study of audio-visual training in virtual reality. NeuroImage 2023, 285, 120483. [Google Scholar] [CrossRef] [PubMed]
- Alwashmi, K.; Meyer, G.; Rowe, F.J. Audio-visual stimulation for visual compensatory functions in stroke survivors with visual field defect: A systematic review. Neurol. Sci. 2022, 43, 2299–2321. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mazziotta, J.C.; Phelps, M.E.; Halgren, E. Local cerebral glucose metabolic response to audiovisual stimulation and deprivation: Studies in human subjects with positron CT. Hum. Neurobiol. 1983, 2, 11–23. [Google Scholar] [PubMed]
- Grasso, P.A.; Làdavas, E.; Bertini, C. Compensatory Recovery after Multisensory Stimulation in Hemianopic Patients: Behavioral and Neurophysiological Components. Front. Syst. Neurosci. 2016, 10, 45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pan, D.; Wang, Z.; Chen, Y.; Cao, J. Melanopsin-mediated optical entrainment regulates circadian rhythms in vertebrates. Commun. Biol. 2023, 6, 1054. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jones, K.A.; Hatori, M.; Mure, L.S.; Bramley, J.R.; Artymyshyn, R.; Hong, S.P.; Marzabadi, M.; Zhong, H.; Sprouse, J.; Zhu, Q.; et al. Small-molecule antagonists of melanopsin-mediated phototransduction. Nat. Chem. Biol. 2013, 9, 630–635. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- CIE S 026:2018; CIE System for Metrology of Optical Radiation for ipRGC-Influenced Responses to Light. Commission Internationale de l’Éclairage (CIE): Vienna, Austria, 2018.
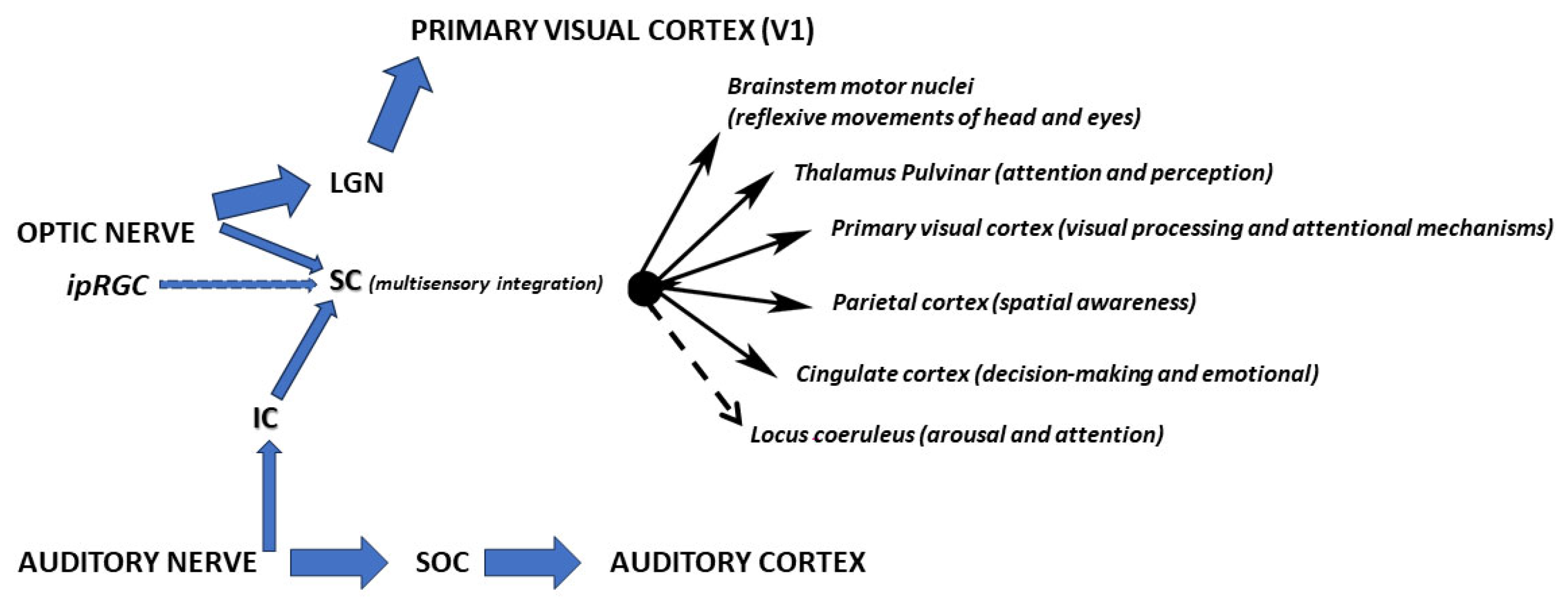

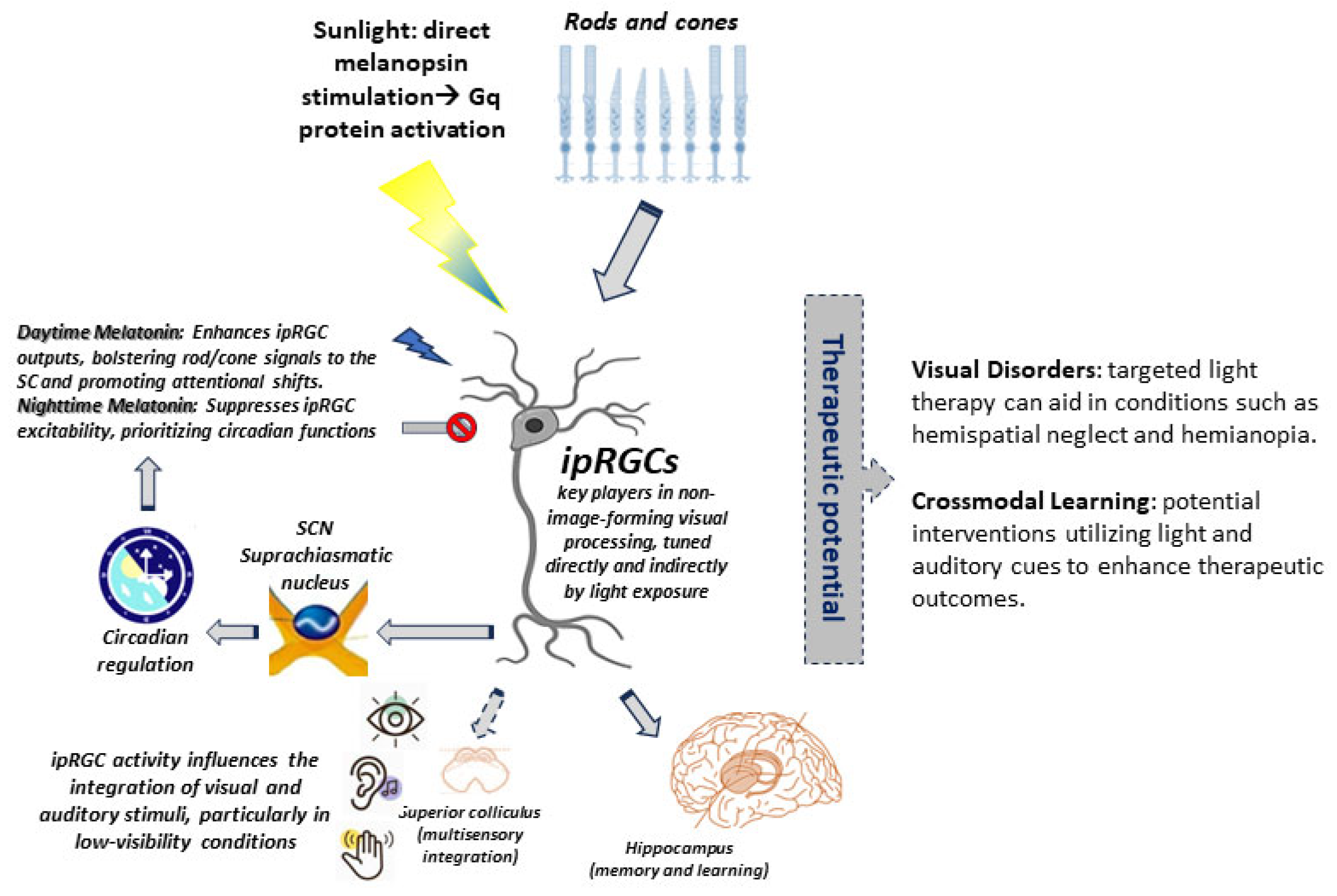
| Optic Nerve | Pathways | Targets | Fibers | Function |
| Image Forming (conscious vision) | Lateral Geniculate Nucleus (LGN) → Primary Visual Cortex (V1) | ~90% of RGC axons (mostly from rods/cones). | High-acuity, conscious visual perception (shape, color, motion). | |
| Non-Image Forming (Reflexive/Circadian) | Superior Colliculus (SC) | ~10% of RGCs (including ipRGCs and motion-sensitive RGCs) | Orienting reflexes, multisensory integration, and spatial attention | |
| Pretectal Nuclei | Subset of RGCs (mainly ipRGCs and ON cells) | Pupillary light reflex and accommodation | ||
| Suprachiasmatic Nucleus (SCN) | Dedicated ipRGCs (melanopsin-expressing) | Circadian rhythm entrainment | ||
| Ventral Lateral Geniculate (vLGN) & Intergeniculate Leaflet (IGL) | Non-image-forming RGCs | Modulation of circadian rhythms by non-photic cues (e.g., activity) |
| Type | Description | References |
|---|---|---|
| Visual Responsive Neurons | These neurons respond to visual stimuli. The superior colliculus receives direct input from the retina and is involved in processing visual information related to the location and movement of objects in the visual field | [23] |
| Auditory Responsive Neurons | Neurons in the superior colliculus respond to auditory stimuli. These neurons contribute to the localization of sound sources in the environment. | [24,25] |
| Somatosensory Responsive Neurons | Some neurons in the superior colliculus are responsive to somatosensory stimuli, such as touch or proprioception. This input helps in integrating tactile and proprioceptive information with visual and auditory signals. | [22,26] |
| Multisensory Neurons | Multisensory neurons are those that integrate information from more than one sensory modality. These neurons play a critical role in combining signals from different senses to create a more accurate and robust representation of the external world. | [2,10,22] |
| Feature | Bottom-Up (ipRGC-Driven Pathways) | Top-Down (Cortical Feedback Pathways) |
|---|---|---|
| Stimulus Origin | Light (480 nm blue light), auditory/somatosensory cues | Cognitive demands (attention, predictions), learned spatial statistics |
| Key Structures |
|
|
| Neural Pathways | ipRGCs → SC (direct melanopsin projections) + IC/somatosensory → SC convergence | FEF/parietal cortex → thalamus → SC (corticotectal feedback) |
| Mechanisms |
|
|
| Temporal Dynamics | Tonic (slow, sustained) | Phasic (fast, task-dependent) |
| Functional Outcomes |
|
|
| Clinical Targets | Hemianopia, low-light rehabilitation | Spatial neglect, attentional deficits |
| Therapeutic Levers |
|
|
| Strategy | Actuation | References |
|---|---|---|
| Circadian Optimization | Light therapy targeting melanopsin stabilizes sleep patterns in stroke patients, improving cognitive recovery | [66] |
| Melatonin supplementation complements the above: nighttime doses consolidate sleep, while daytime administration enhances learning-dependent plasticity via MT1 receptors in ipRGCs and hippocampus | [63,64] | |
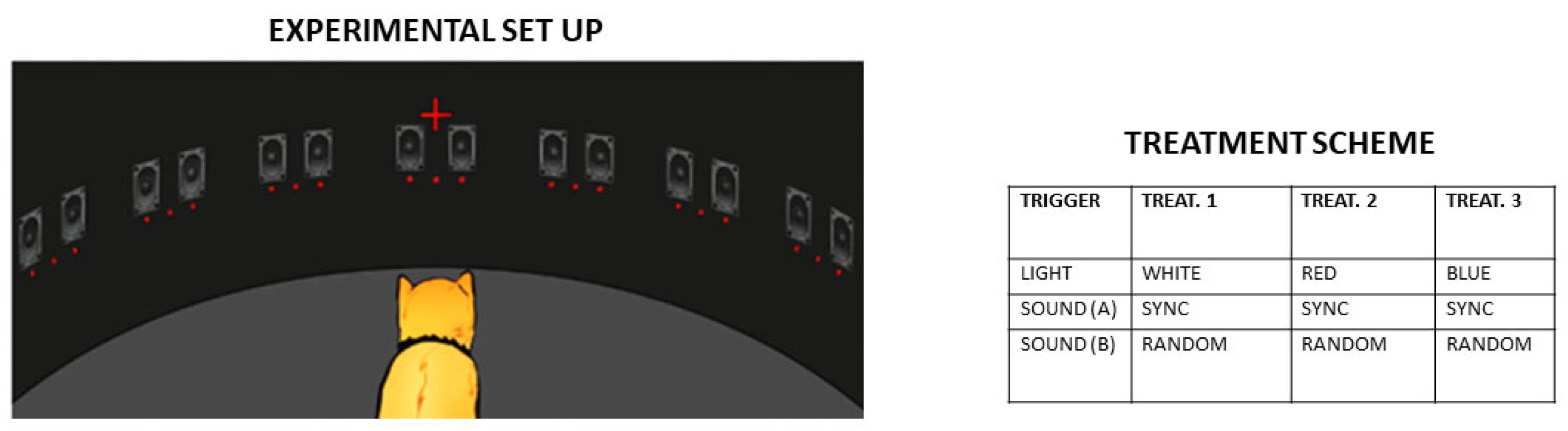
| Crossmodal Priming | In hemispatial neglect, pairing blue light (activating ipRGC-SC pathways) with prism adaptation therapy may amplify attentional shifts to the neglected field. | [31] |
| Federici and colleagues demonstrate that auditory crossmodal plasticity can compensate for visual deprivation, suggesting synergistic potential with light/melatonin timing. | [67] | |
| Pharmacological Modulation | Melanopsin antagonists (e.g., opsinamides) which selectively inhibit melanopsin-mediated light responses highlights the potential for targeted interventions in circadian disorders, photophobia, and other light-dependent behaviors. | [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusciano, D. Light, Sound, and Melatonin: Investigating Multisensory Pathways for Visual Restoration. Medicina 2025, 61, 1009. https://doi.org/10.3390/medicina61061009
Rusciano D. Light, Sound, and Melatonin: Investigating Multisensory Pathways for Visual Restoration. Medicina. 2025; 61(6):1009. https://doi.org/10.3390/medicina61061009
Chicago/Turabian StyleRusciano, Dario. 2025. "Light, Sound, and Melatonin: Investigating Multisensory Pathways for Visual Restoration" Medicina 61, no. 6: 1009. https://doi.org/10.3390/medicina61061009
APA StyleRusciano, D. (2025). Light, Sound, and Melatonin: Investigating Multisensory Pathways for Visual Restoration. Medicina, 61(6), 1009. https://doi.org/10.3390/medicina61061009






