Serratus Anterior Plane Block for Pain Management After Video-Assisted Thoracoscopic Surgeries: A Narrative Review
Abstract
1. Introduction
2. Methods
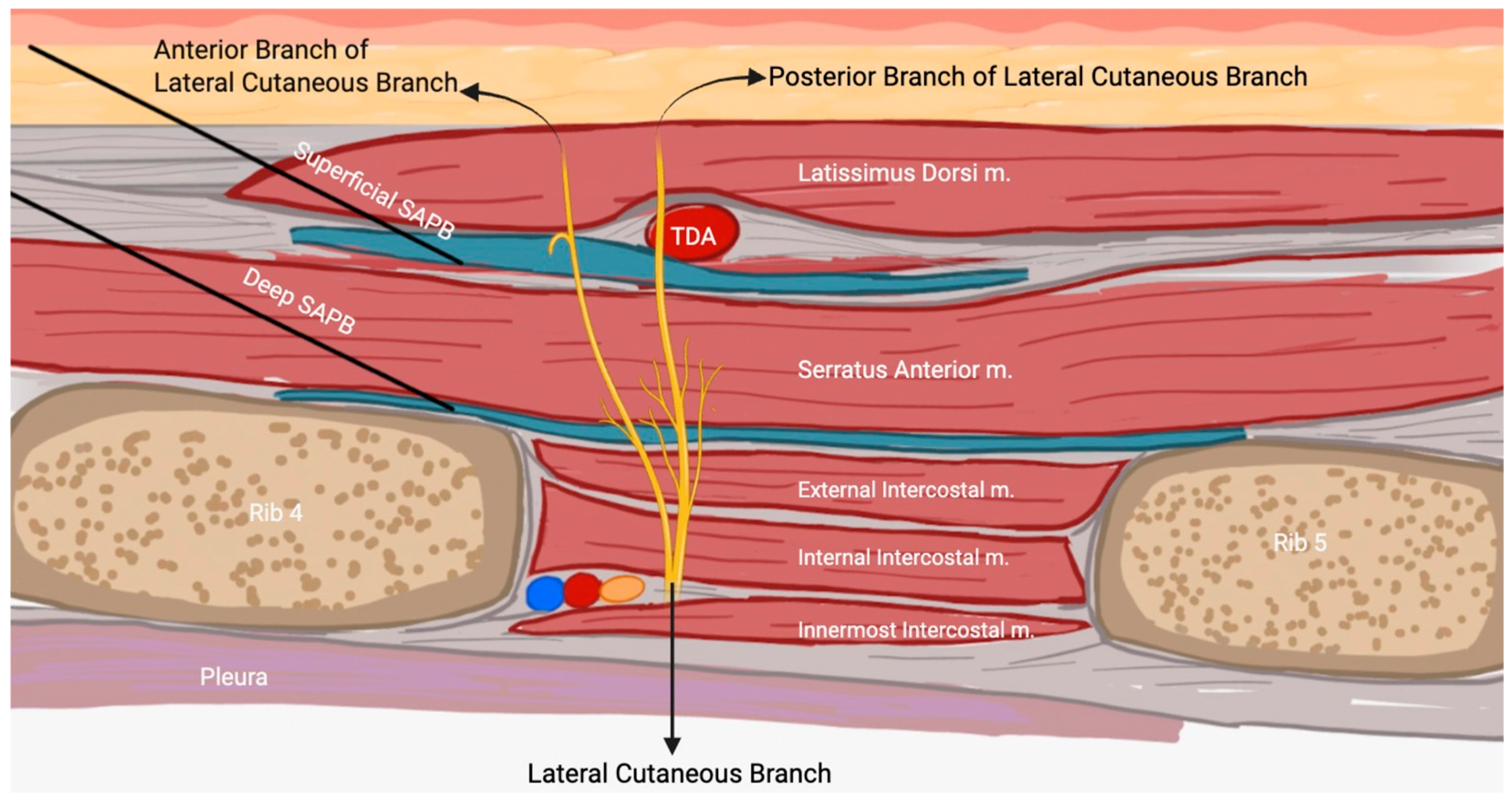
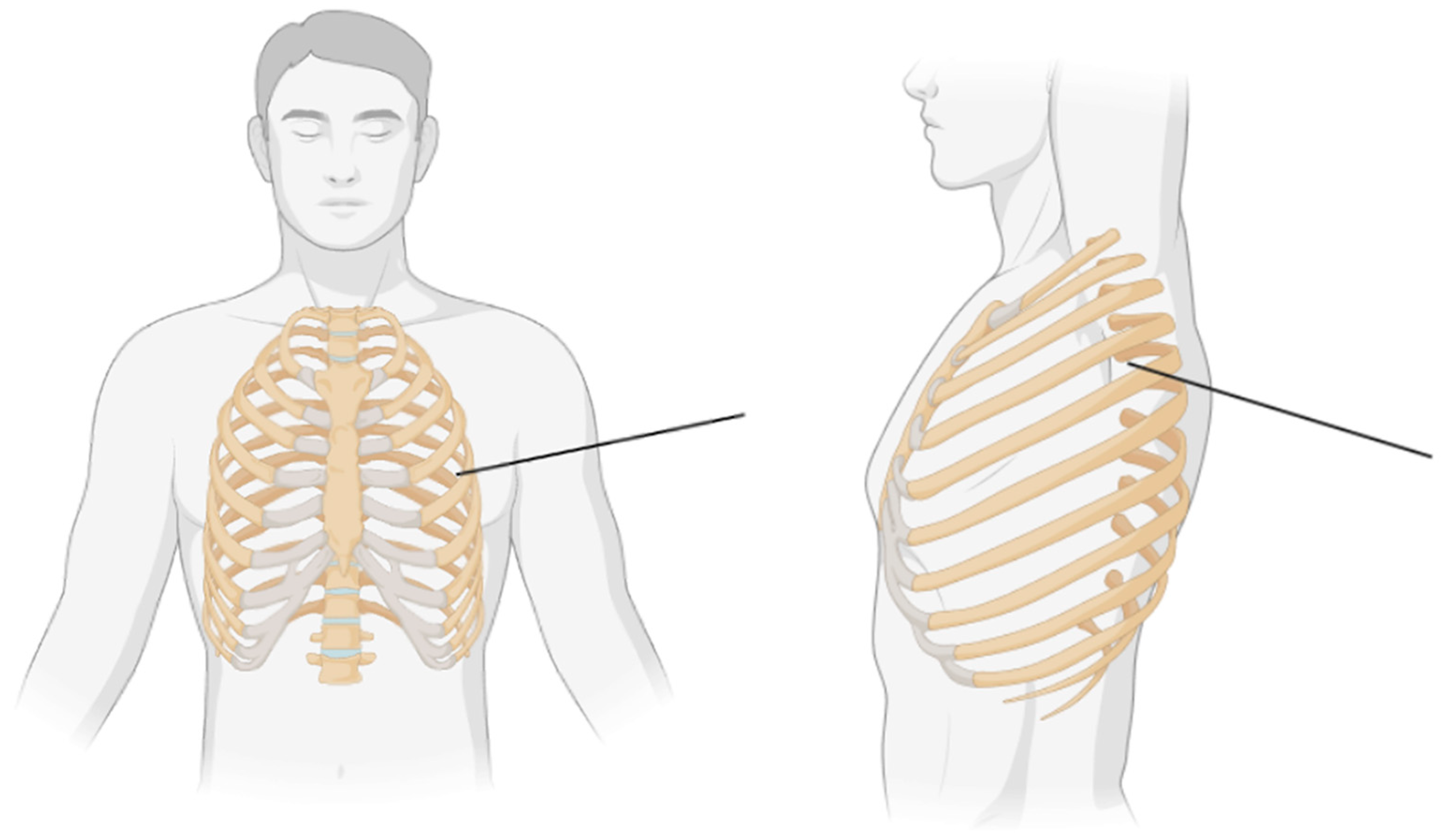
3. Anatomy and Physiology of SAPB
3.1. SAPB Procedure
3.2. SAPB Compared to TEA and TPVB
4. Video-Assisted Thoracic Surgery (VATS)
5. Clinical Studies Evaluating SAPB for VATS
6. Discussion
7. Conclusions
Funding
Conflicts of Interest
References
- Redden, M.D.; Chin, T.Y.; van Driel, M.L. Surgical versus non-surgical management for pleural empyema. In Cochrane Database of Systematic Reviews; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017. [Google Scholar] [CrossRef]
- Video-Assisted Thoracoscopic Surgery—Taking a Step into the Future | European Journal of Cardio-Thoracic Surgery | Oxford Academic. Available online: https://academic.oup.com/ejcts/article/51/4/694/2698411 (accessed on 12 February 2025).
- Ljungqvist, O.; Scott, M.; Fearon, K.C. Enhanced Recovery After Surgery: A Review. JAMA Surg. 2017, 152, 292–298. [Google Scholar] [CrossRef]
- Lobectomy by Video-Assisted Thoracic Surgery (VATS) Versus Thoracotomy for Lung Cancer—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0022522309004826?via%3Dihub (accessed on 14 February 2025).
- Loop, T. Fast track in thoracic surgery and anaesthesia: Update of concepts. Curr. Opin. Anesthesiol. 2016, 29, 20. [Google Scholar] [CrossRef] [PubMed]
- Essential Elements of Multimodal Analgesia in Enhanced Recovery After Surgery (ERAS) Guidelines—ClinicalKey. Available online: https://www.clinicalkey.com/#!/content/playContent/1-s2.0-S193222751730023X?returnurl=https:%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS193222751730023X%3Fshowall%3Dtrue&referrer=https:%2F%2Fpubmed.ncbi.nlm.nih.gov%2F (accessed on 14 February 2025).
- Elmore, B.; Nguyen, V.; Blank, R.; Yount, K.; Lau, C. Pain Management Following Thoracic Surgery. Thorac. Surg. Clin. 2015, 25, 393–409. [Google Scholar] [CrossRef]
- Lee, H.J.; Choi, S.; Yoon, S.; Yoon, S.; Bahk, J.H. Effect of an intravenous acetaminophen/ibuprofen fixed-dose combination on postoperative opioid consumption and pain after video-assisted thoracic surgery: A double-blind randomized controlled trial. Surg. Endosc. 2024, 38, 3061–3069. [Google Scholar] [CrossRef]
- Piccioni, F.; Segat, M.; Falini, S.; Umari, M.; Putina, O.; Cavaliere, L.; Ragazzi, R.; Massullo, D.; Taurchini, M.; Del Naja, C.; et al. Enhanced recovery pathways in thoracic surgery from Italian VATS Group: Peri-operative analgesia protocols. J. Thorac. Dis. 2018, 10 (Suppl. 4), S555. [Google Scholar] [CrossRef] [PubMed]
- Hanley, C.; Wall, T.; Bukowska, I.; Redmond, K.; Eaton, D.; Ní Mhuircheartaigh, R.; Hearty, C. Ultrasound-guided continuous deep serratus anterior plane block versus continuous thoracic paravertebral block for perioperative analgesia in videoscopic-assisted thoracic surgery. Eur. J. Pain 2020, 24, 828–838. [Google Scholar] [CrossRef]
- Chen, J.Q.; Yang, X.L.; Gu, H.; Chai, X.Q.; Wang, D. The Role of Serratus Anterior Plane Block During in Video-Assisted Thoracoscopic Surgery. Pain Ther. 2021, 10, 1051–1066. [Google Scholar] [CrossRef]
- Anatomy, Thorax, Serratus Anterior Muscles—StatPearls—NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK531457/ (accessed on 28 January 2025).
- Ultrasound-Guided Serratus Plane Block for ED Rib Fracture Pain Control—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0735675716304156?via%3Dihub (accessed on 28 January 2025).
- Zocca, J.A.; Chen, G.H.; Puttanniah, V.G.; Hung, J.C.; Gulati, A. Ultrasound-Guided Serratus Plane Block for Treatment of Postmastectomy Pain Syndromes in Breast Cancer Patients: A Case Series. Pain Pract. 2017, 17, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Blanco, R.; Parras, T.; McDonnell, J.G.; Prats-Galino, A. Serratus plane block: A novel ultrasound-guided thoracic wall nerve block. Anaesthesia 2013, 68, 1107–1113. [Google Scholar] [CrossRef]
- Khalil, A.E.; Abdallah, N.M.; Bashandy, G.M.; Kaddah, T.A.H. Ultrasound-Guided Serratus Anterior Plane Block Versus Thoracic Epidural Analgesia for Thoracotomy Pain. J. Cardiothorac. Vasc. Anesth. 2017, 31, 152–158. [Google Scholar] [CrossRef]
- Southgate, S.J.; River, G.F.; Herbst, M.K. Ultrasound-Guided Serratus Anterior Blocks. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK538476/ (accessed on 28 January 2025).
- Park, M.H.; Kim, J.A.; Ahn, H.J.; Yang, M.K.; Son, H.J.; Seong, B.G. A randomised trial of serratus anterior plane block for analgesia after thoracoscopic surgery. Anaesthesia 2018, 73, 1260–1264. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.P.; Álvarez, S.L.; Fuentes, A.F.; Lorefice, F.M.; Bartolomé, C.B.; de Zárate, J.G. Quality of postoperative recovery after breast surgery. General anaesthesia combined with paravertebral versus serratus-intercostal block. Rev. Española Anestesiol. Y Reanim. 2016, 63, 564–571. [Google Scholar] [CrossRef]
- Khemka, R.; Chakraborty, A.; Ahmed, R.; Datta, T.; Agarwal, S. Ultrasound-Guided Serratus Anterior Plane Block in Breast Reconstruction Surgery. AA Pract. 2016, 6, 280. [Google Scholar] [CrossRef] [PubMed]
- Kunhabdulla, N.; Agarwal, A.; Gaur, A.; Gautam, S.; Gupta, R.; Agarwal, A. Serratus Anterior Plane Block for Multiple Rib Fractures. Available online: https://www.painphysicianjournal.com/linkout?issn=&vol=17&page=E553 (accessed on 28 January 2025).
- Fu, P.; Weyker, P.D.; Webb, C.A.J. Case Report of Serratus Plane Catheter for Pain Management in a Patient With Multiple Rib Fractures and an Inferior Scapular Fracture. AA Pract. 2017, 8, 132. [Google Scholar] [CrossRef]
- Reyad, R.M.; Shaker, E.H.; Ghobrial, H.Z.; Abbas, D.N.; Reyad, E.M.; Abd Alrahman, A.A.M.; AL-Demery, A.; Issak, E.R. The impact of ultrasound-guided continuous serratus anterior plane block versus intravenous patient-controlled analgesia on the incidence and severity of post-thoracotomy pain syndrome: A randomized, controlled study. Eur. J. Pain 2020, 24, 159–170. [Google Scholar] [CrossRef]
- Gautam, S.K. Serratus Anterior Plane Block: A New AnalgesicTechnique for Post-Thoracotomy Pain. Pain Phys. 2015, 18, E421–E424. [Google Scholar] [CrossRef]
- Vorobeichik, L.; Brull, R.; Bowry, R.; Laffey, J.G.; Abdallah, F.W. Should continuous rather than single-injection interscalene block be routinely offered for major shoulder surgery? A meta-analysis of the analgesic and side-effects profiles. Br. J. Anaesth. 2018, 120, 679–692. [Google Scholar] [CrossRef]
- Chou, R.; Gordon, D.B.; de Leon-Casasola, O.A.; Rosenberg, J.M.; Bickler, S.; Brennan, T.; Carter, T.; Cassidy, C.L.; Chittenden, E.H.; Degenhardt, E.; et al. Management of Postoperative Pain: A Clinical Practice Guideline From the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J. Pain 2016, 17, 131–157. [Google Scholar] [CrossRef]
- Takimot, K. Serratus Plane Block for Persistent Painafter Partial Mastectomy and Axillary NodeDissection. Pain Phys. 2016, 19, E481–E486. [Google Scholar] [CrossRef]
- Gottschalk, A.; Cohen, S.P.; Yang, S.; Ochroch, E.A.; Warltier, D.C. Preventing and Treating Pain after Thoracic Surgery. Anesthesiology 2006, 104, 594. [Google Scholar] [CrossRef]
- Chu, G.M.; Jarvis, G.C. Serratus Anterior Plane Block to Address Postthoracotomy and Chest Tube-Related Pain: A Report on 3 Cases. AA Pract. 2017, 8, 322. [Google Scholar] [CrossRef] [PubMed]
- Norum, H.M.; Breivik, H. A systematic review of comparative studies indicates that paravertebral block is neither superior nor safer than epidural analgesia for pain after thoracotomy. Scand. J. Pain 2010, 1, 12–23. [Google Scholar] [CrossRef]
- Kadomatsu, Y.; Mori, S.; Ueno, H.; Uchiyama, M.; Wakai, K. Comparison of the analgesic effects of modified continuous intercostal block and paravertebral block under surgeon’s direct vision after video-assisted thoracic surgery: A randomized clinical trial. Gen. Thorac. Cardiovasc. Surg. 2018, 66, 425–431. [Google Scholar] [CrossRef]
- Gulati, S.; Housman, B.; Flores, R. Approaching lobectomy in a VIOLET tinted world: Video-assisted thoracoscopic surgery (VATS) vs. open thoracotomy for lobectomy. Video-Assist. Thorac. Surg. 2024, 9. [Google Scholar] [CrossRef]
- Mehrotra, M.; D’Cruz, J.R.; Bishop, M.A.; Arthur, M.E. Video-Assisted Thoracoscopy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK532952/ (accessed on 5 March 2025).
- Fourdrain, A.; De Dominicis, F.; Iquille, J.; Lafitte, S.; Merlusca, G.; Witte-Pfister, A.; Meynier, J.; Bagan, P.; Berna, P. Intraoperative conversion during video-assisted thoracoscopy does not constitute a treatment failure†. Eur. J. Cardio-Thorac. Surg. 2019, 55, 660–665. [Google Scholar] [CrossRef]
- Mitchell, J.D. Techniques of VATS lobectomy. J. Thorac. Dis. 2013, 5 (Suppl. 3), S177–S181. [Google Scholar] [CrossRef] [PubMed]
- Sihoe, A.D.L. Transition from multiportal video-assisted thoracic surgery to uniportal video-assisted thoracic surgery… and evolution to uniportal robotic-assisted thoracic surgery? Ann. Cardiothorac. Surg. 2023, 12, 820–890. [Google Scholar] [CrossRef]
- Gerner, P. Post-thoracotomy Pain Management Problems. Anesthesiol. Clin. 2008, 26, 355–367. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Wang, Y.; Zhang, W. Analgesic effectiveness of serratus anterior plane block in patients undergoing video-assisted thoracoscopic surgery: A systematic review and updated meta-analysis of randomized controlled trials. BMC Anesthesiol. 2023, 23, 235. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, M.; Huang, S.; He, X.; Gu, X.; Ma, Z. Ultrasound-guided serratus anterior plane block versus paravertebral block on postoperation analgesia and safety following the video-assisted thoracic surgery: A prospective, randomized, double-blinded non-inferiority clinical trial. Asian J. Surg. 2023, 46, 4215–4221. [Google Scholar] [CrossRef]
- Ülger, G.; Zengin, M.; Küçük, O.; Baldemir, R.; Kaybal, O.; Tunç, M.; Sazak, H.; Alagöz, A. Comparison of combined deep and superficial serratus anterior block with thoracic paravertebral block for postoperative pain in patients undergoing video-assisted thoracoscopic surgery. Turk. J. Med. Sci. 2024, 54, 1021–1032. [Google Scholar] [CrossRef]
- Lusianawati Suhardi, C.J.; Sumartono, C.; Kencono Wungu, C.D. Efficacy and safety of the serratus anterior block compared to thoracic epidural analgesia in surgery: Systematic review and meta-analysis. Tzu Chi Med. J. 2023, 35, 329–337. [Google Scholar] [CrossRef]
- Xiang, Y.; Chen, L.; Jia, J.; Yili, F.; Changwei, W. The association of regional block with intraoperative opioid consumption in patients undergoing video-assisted thoracoscopic surgery: A single-center, retrospective study. J. Cardiothorac. Surg. 2024, 19, 124. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, Z.; Fang, T.; Wang, K.; Liu, Z.; Li, H.; Jiang, W.; Cao, X. A comparison of the analgesic efficacy of serratus anterior plane block vs. paravertebral nerve block for video-assisted thoracic surgery: A randomized controlled trial. Videosurg. Other Miniinvasive Tech. 2022, 17, 134–142. [Google Scholar] [CrossRef]
- Gao, W.; Yang, X.L.; Hu, J.C.; Gu, H.; Wu, X.N.; Hu, S.S.; Wang, S.; Chai, X.Q.; Wang, D. Continuous Serratus Anterior Plane Block Improved Early Pulmonary Function After Lung Cancer Surgical Procedure. Ann. Thorac. Surg. 2022, 113, 436–443. [Google Scholar] [CrossRef]
- Baytar, M.S.; Yılmaz, C.; Karasu, D.; Baytar, Ç. Comparison of ultrasonography guided serratus anterior plane block and thoracic paravertebral block in video-assisted thoracoscopic surgery: A prospective randomized double-blind study. Korean J. Pain 2021, 34, 234–240. [Google Scholar] [CrossRef]
| Author (Year) | Study Design | Findings | Conclusions |
|---|---|---|---|
| Hanley et al. (2020) [10] | Single-center, double-blind randomized controlled non-inferiority trial comparing ultrasound-guided continuous deep SAPB with surgically placed continuous TPVB in patients undergoing VATS. Evaluated whether SAPB is non-inferior to TPVB in reducing forty-eight-hour postoperative opioid consumption, improving pain scores and functional recovery metrics. |
| SAPB is an effective, opioid-sparing alternative to TPVB for perioperative analgesia in VATS, offering comparable pain control with enhanced functional recovery. |
| Ülger et al. (2024) [40] | Prospective, randomized, double-blind equivalence study compared the efficacy of combined deep and superficial SAPB (C-SAPB) with TPVB for postoperative pain management in sixty patients undergoing VATS. Variables tested were pain scores, opioid consumption, side effects, and patient satisfaction over forty-eight hours postoperatively. |
| C-SAPB is an effective and safe alternative to TPVB for postoperative pain management in VATS, offering comparable pain relief with a potentially simpler and faster application process. |
| Lusianawati et al. (2023) [41] | Systematic review and meta-analysis including six randomized controlled trials comparing SAPB and TEA for thoracic and breast surgeries. Aimed to compare the efficacy and safety of SAPB and TEA, evaluating postoperative pain, incidence of hypotension, and PONV. |
| SAPB is a safe and equally effective alternative to TEA for pain management in thoracic and breast surgeries, with a lower incidence of hypotension. Larger studies are needed to confirm these findings. |
| Xiang et al. (2024) [42] | Single-center, retrospective cohort study including 2159 patients undergoing VATS, divided into four groups based on regional block type: GA, TEA, TPVB, and SAPB, with matching for age, sex, ASA physical status, and surgery duration. Evaluated intraoperative opioid-sparing effects of TEA, TPVB, and SAPB compared to general anesthesia alone in VATS patients. |
| TEA and TPVB significantly reduced intraoperative opioid consumption, making them part of an optimal analgesic strategy for VATS. SAPB did not show significant opioid-sparing effects. Further studies are needed to explore opioid-free anesthesia options for VATS. |
| Zhang et al. (2022) [43] | Randomized controlled trial including seventy-four patients undergoing VATS, divided into three groups: deep serratus plane block (DSPB), superficial serratus anterior plane block (SSPB), and paravertebral nerve block (TPVB). Compared analgesic efficacy of DSPB, SSPB, and TPVB for postoperative pain management in VATS via opioid consumption, pain scores, and patient satisfaction. |
| All three techniques provided effective postoperative analgesia, with PVB offering better intraoperative pain control. DSPB and SSPB are easier to perform and could be viable alternatives to PVB, but further research on continuous infusion techniques is needed. |
| Baytar et al. (2021) [45] | A prospective, randomized, double-blind study compared ultrasound-guided SAPB and TPVB in VATS patients. Determined whether SAPB is as effective as TPVB for pain control in VATS. Secondary objectives included assessing satisfaction, block application time, analgesic requirement, postoperative complications, and hospital stay length. |
| SAPB is not inferior to TPVB for postoperative pain control in VATS, offering a faster block application time and serving as a viable alternative to TPVB. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmadzadeh, S.; Serio, M.A.; Nguyen, A.; Dethloff, D.R.; Robichaux, C.; Mosieri, C.N.; Shekoohi, S.; Kaye, A.D. Serratus Anterior Plane Block for Pain Management After Video-Assisted Thoracoscopic Surgeries: A Narrative Review. Medicina 2025, 61, 1010. https://doi.org/10.3390/medicina61061010
Ahmadzadeh S, Serio MA, Nguyen A, Dethloff DR, Robichaux C, Mosieri CN, Shekoohi S, Kaye AD. Serratus Anterior Plane Block for Pain Management After Video-Assisted Thoracoscopic Surgeries: A Narrative Review. Medicina. 2025; 61(6):1010. https://doi.org/10.3390/medicina61061010
Chicago/Turabian StyleAhmadzadeh, Shahab, Macie A. Serio, Angela Nguyen, Drew R. Dethloff, Camille Robichaux, Chizoba N. Mosieri, Sahar Shekoohi, and Alan D. Kaye. 2025. "Serratus Anterior Plane Block for Pain Management After Video-Assisted Thoracoscopic Surgeries: A Narrative Review" Medicina 61, no. 6: 1010. https://doi.org/10.3390/medicina61061010
APA StyleAhmadzadeh, S., Serio, M. A., Nguyen, A., Dethloff, D. R., Robichaux, C., Mosieri, C. N., Shekoohi, S., & Kaye, A. D. (2025). Serratus Anterior Plane Block for Pain Management After Video-Assisted Thoracoscopic Surgeries: A Narrative Review. Medicina, 61(6), 1010. https://doi.org/10.3390/medicina61061010







