From Biopsy to Diagnosis: Navigating Aggressive B-Cell Lymphomas in Practice
Abstract
1. Introduction
2. Lymphoma, Carcinoma, Melanoma, or Sarcoma—What Is the Difference?
3. B-Cell Lymphoma, T-Cell Lymphoma, or Hodgkin Lymphoma
4. Histological Characteristics and Developmental Patterns
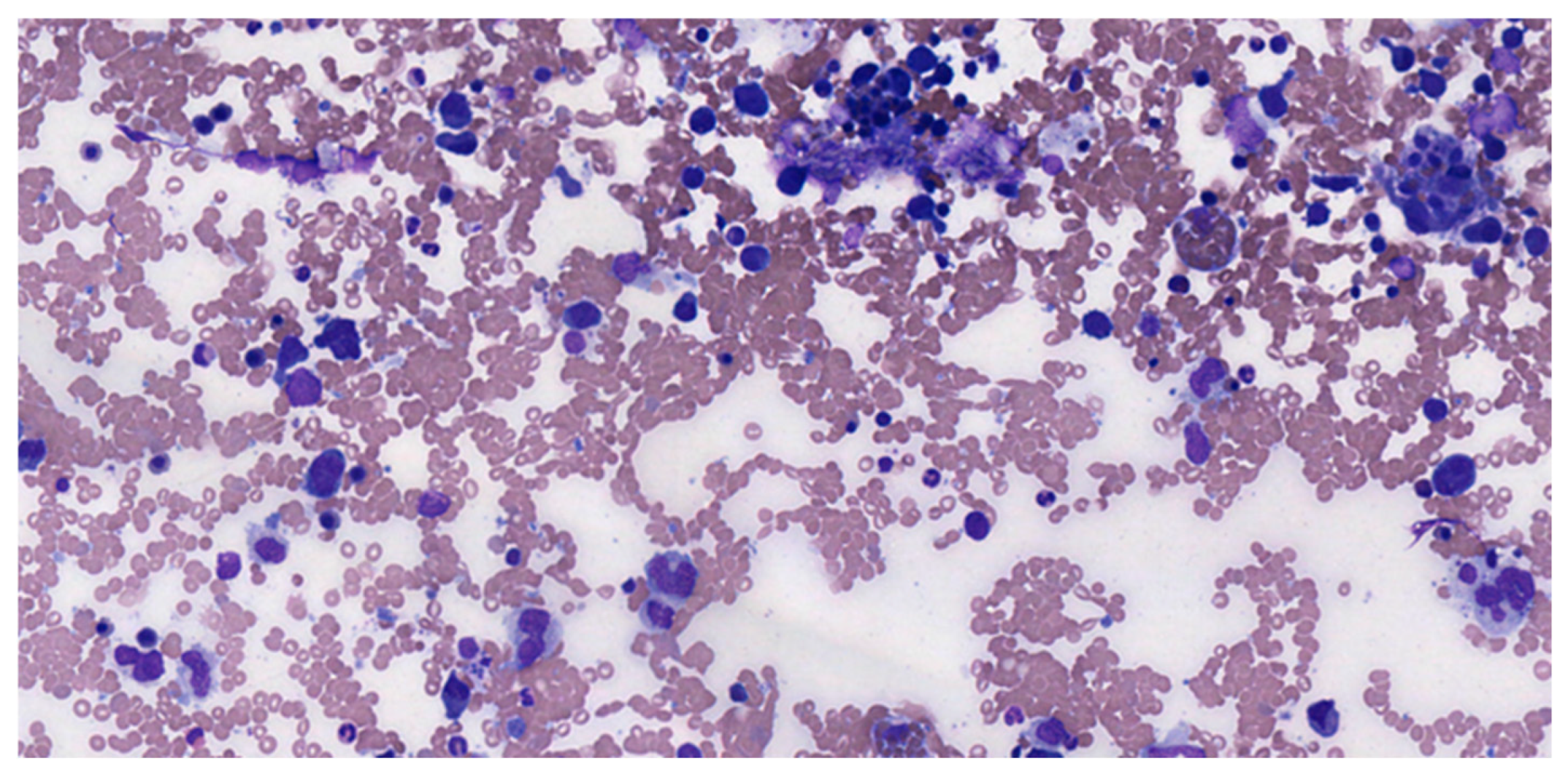
5. Morphological Evaluation
6. Medium-Sized B-Cell Lymphoma with CCND1 Translocation
7. Germinal Center Phenotype
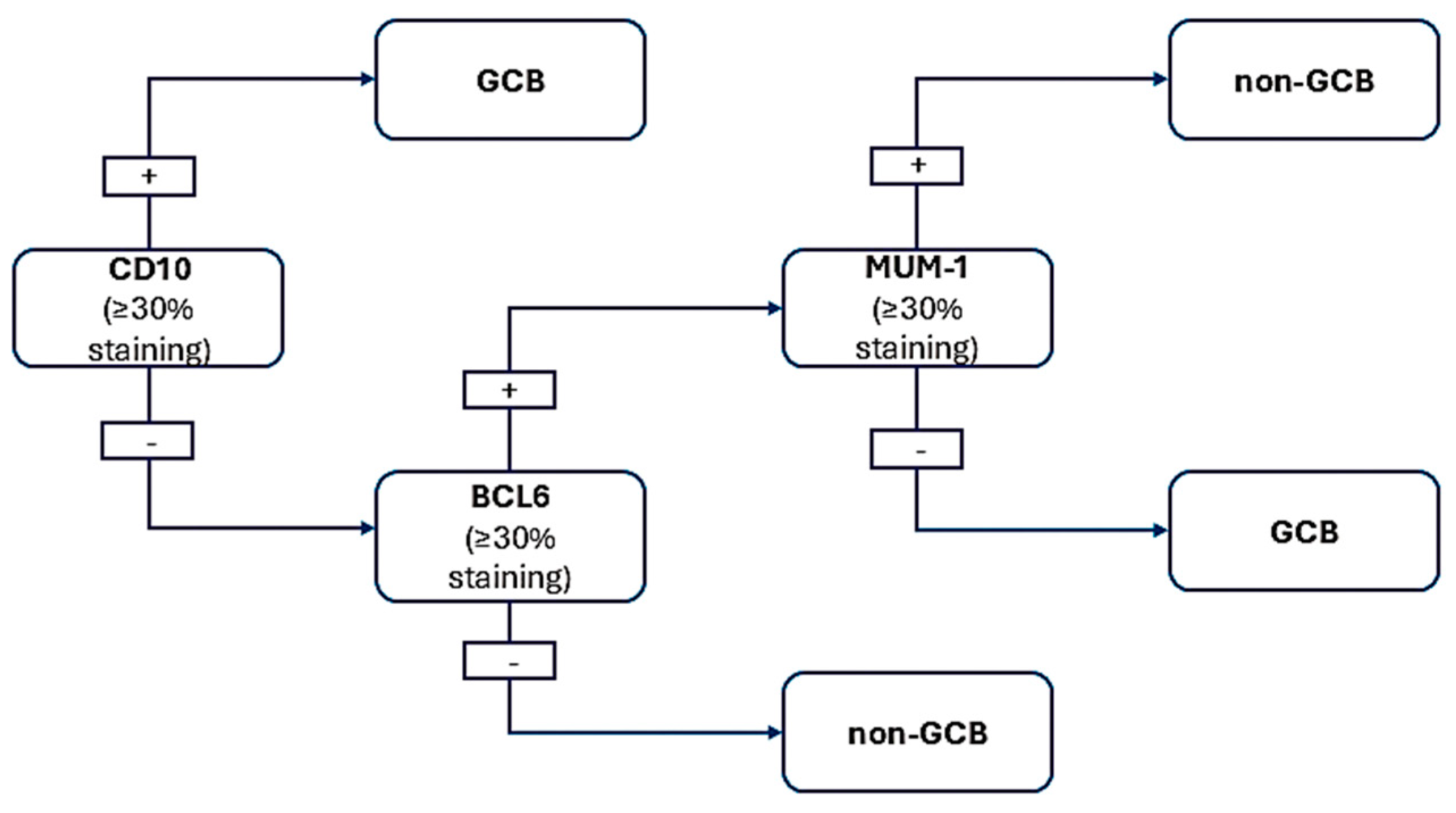
8. Hans Algorithm—For CD10-DLBCL, Request BCL6 and MUM1 IHC
9. Epstein–Barr Virus Infection and Large B-Cell Lymphomas
10. BCL2 and C-Myc Ihc for Double Expresser DLBCL
11. Cytogenetic Testing for Double-Hit/Triple-Hit B-Cell Lymphoma
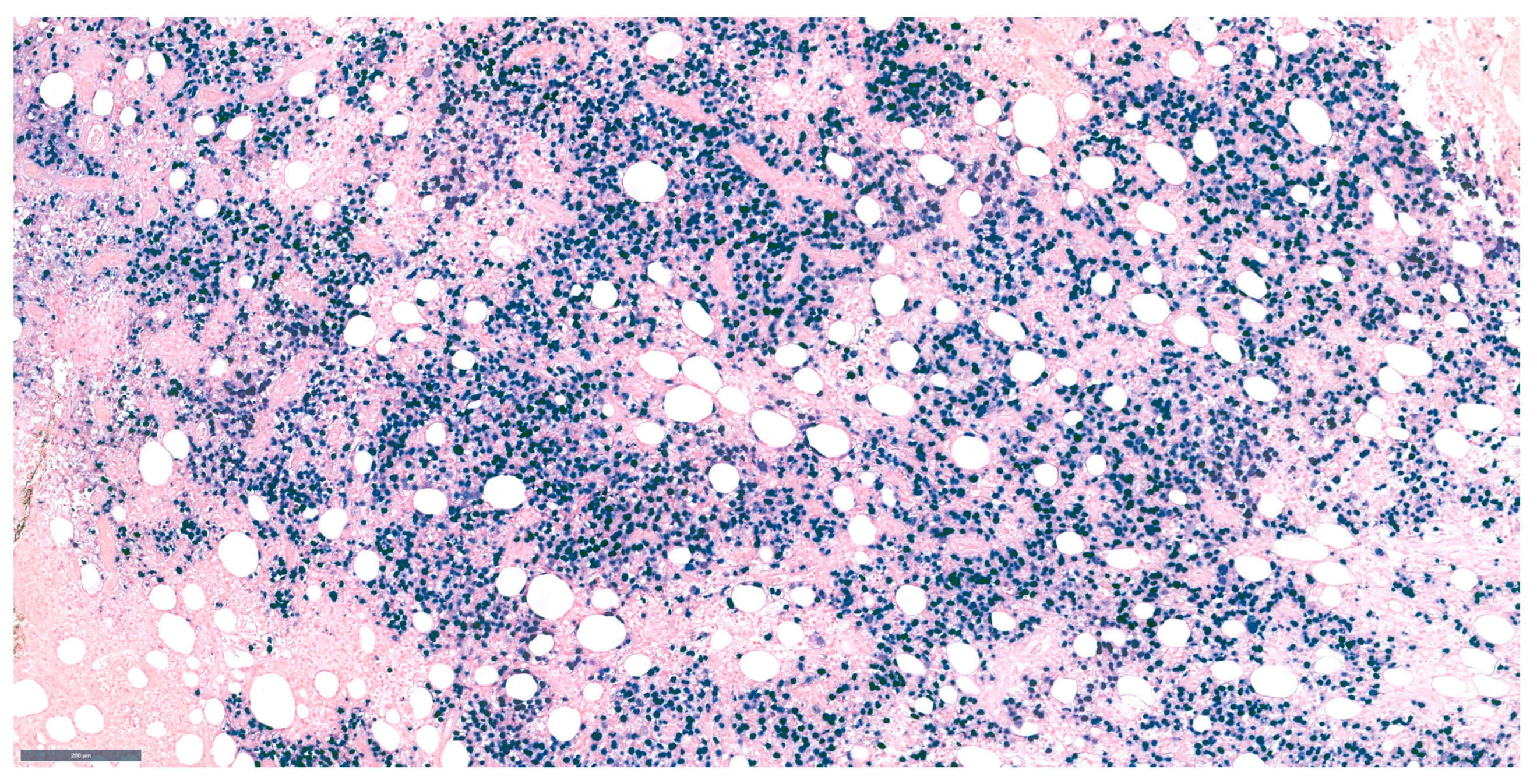
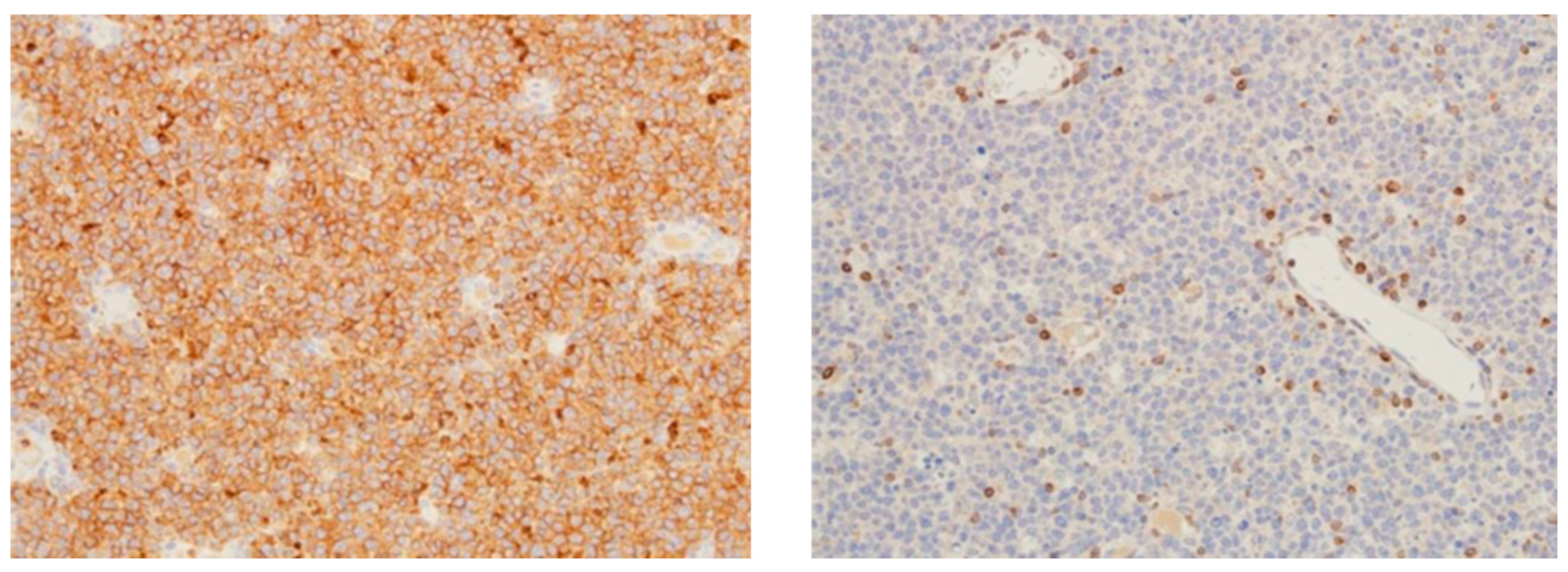
12. DLBCL Proliferation Index
13. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- de Jong, D. Who classification of tumors of hematopoietic and lymphoid tissues update. Int. J. Lab. Hematol. 2023, 45, 48–49. [Google Scholar]
- Quintanilla-Martinez, L. The 2016 updated WHO classification of lymphoid neoplasias. Hematol. Oncol. 2017, 35, 37–45. [Google Scholar] [CrossRef]
- Epperla, N.; Nastoupil, L.J.; Feinberg, B.; Galvin, J.; Pathak, P.; Amoloja, T.; Gentile, D.; Saverno, K. Real-world use of tafasitamab preceding CD19-directed chimeric antigen receptor T-cell therapy for relapsed or refractory diffuse large B-cell lymphoma. Biomark. Res. 2025, 13, 18. [Google Scholar] [CrossRef]
- Takahata, A.; Shimada, T.; Bando, K.; Toyota, S. Epcoritamab-Induced fatal pleural effusion in diffuse large B-Cell lymphoma: A case report and literature review. Ann. Hematol. 2025, 104, 1995–2000. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.M.; Moselhy, S.S.; Mohamad, M.I.; Soliman, A.F.; Hassan, M.N.M.; El-Khazragy, N. Targeting refractory diffuse large B cell lymphoma by CAR-WEE1 T-cells: In vitro evaluation. Ann. Hematol. 2025, 104, 1833–1844. [Google Scholar] [CrossRef]
- Pandey, A.; Roos, K.; Jiang, Y.; Mangoff, K.; Klein, G.; Forward, N.; Stewart, D.; Laneuville, P.; Bence-Bruckler, I.; Mangel, J.; et al. Characteristics of relapsed/refractory diffuse large B-cell lymphoma patients with durable responses to maveropepimut-S, pembrolizumab, and cyclophosphamide: Long-term follow-up from the SPiReL trial. EJHaem 2025, 6, e1062. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.T.; Goloubeva, O.; Rapoport, A.P.; Dahiya, S.; Atanackovic, D.; Hardy, N.; Kocoglu, M.; Lutfi, F.; Alkhaldi, H.; Claiborne, J.P.; et al. CD19 CAR-T with Axicabtagene Ciloleucel in R/R Large B-Cell Lymphoma with/Without Prior Autologous Stem Cell Transplant. Clin. Lymphoma Myeloma Leuk. 2025, in press. [Google Scholar] [CrossRef]
- Attygalle, A.D.; Chan, J.K.C.; Coupland, S.E.; Du, M.Q.; Ferry, J.A.; Jong, D.; Gratzinger, D.; Lim, M.S.; Naresh, K.N.; Nicolae, A.; et al. The 5th edition of the World Health Organization Classification of mature lymphoid and stromal tumors—An overview and update. Leuk. Lymphoma 2024, 65, 413–429. [Google Scholar] [CrossRef]
- Ponnatt, T.; Bradley, K.; Li, S.Y.; Barclift, D.P. Updates on Non-B-Lymphoid Neoplasms: A Review of the 5th Edition of the WHO Classification of Hematolymphoid Tumors. J. Clin. Pract. Res. 2024, 46, 113–121. [Google Scholar] [CrossRef]
- Jaffe, E.S.; Carbone, A. B- and T-/NK-Cell Lymphomas in the 2022 International Consensus Classification of Mature Lymphoid Neoplasms and Comparison with the WHO Fifth Edition. Hemato 2024, 5, 157–170. [Google Scholar] [CrossRef]
- Medeiros, L.J.; Chadburn, A.; Natkunam, Y.; Naresh, K.N. Fifth Edition of the World Health Classification of Tumors of the Hematopoietic and Lymphoid Tissues: B-cell Neoplasms. Mod. Pathol. 2024, 37, 100441. [Google Scholar] [CrossRef]
- Kotkaranta, P.; Chan, M.; Vuolio, T.; Miinalainen, I.; Kuitunen, H.; Turpeenniemi-Hujanen, T.; Teppo, H.R.; Kuittinen, O.; Kuusisto, M.E.L. DLBCL cells with ferroptosis morphology can be detected with a deep convolutional neural network. Biomed. Pharmacother. 2025, 182, 117785. [Google Scholar] [CrossRef]
- Sotiriou, S.; Koletsa, T.; Kostopoulos, I. Diffuse Large B-Cell Lymphoma with Clear Cell Morphology. Int. J. Surg. Pathol. 2019, 27, 649–651. [Google Scholar] [CrossRef]
- Paulli, M.; Lucioni, M.; Maffi, A.; Croci, G.A.; Nicola, M.; Berti, E. Primary cutaneous diffuse large B-cell lymphoma (PCDLBCL), leg-type and other: An update on morphology and treatment. G. Ital. Dermatol. Venereol. 2012, 147, 589–601. [Google Scholar]
- Cherian, S.; Fromm, J.R. Evaluation of primary mediastinal large B cell lymphoma by flow cytometry. Cytom. Part B-Clin. Cytom. 2018, 94, 459–467. [Google Scholar] [CrossRef]
- Marcondes, N.A.; Fernandes, F.B.; Faulhaber, G.A.M. Ki-67 Expression in Mature B-Cell Neoplasms: A Flow Cytometry Study. Rev. Da Assoc. Médica Bras. 2018, 64, 525–529. [Google Scholar] [CrossRef]
- AbdullGaffar, B.; Seliem, R.M. De Novo Unclassifiable CD20-Negative Diffuse Large B-Cell Lymphoma: A Diagnostic and Therapeutic Challenge. Int. J. Surg. Pathol. 2017, 26, 266–270. [Google Scholar] [CrossRef]
- Hadžisejdić, I.; Klarica, L.; Babarović, E.; Marijić, B.; Valković, T.; Jonjić, N. Primary Nodal Unclassifiable CD20 Negative Diffuse Large B-Cell Lymphoma with Dual IgK and TCR Gene Rearrangement: A Diagnostic Challenge. Clin. Pathol. 2023, 16, 2632010x221149978. [Google Scholar] [CrossRef]
- Starr, A.; Kwon, D.H.; Kallakury, B. Epstein-Barr Virus-Positive CD20-and CD45-Negative Diffuse Large B-Cell Lymphoma: A Diagnostic Challenge. Int. J. Surg. Pathol. 2019, 27, 98–101. [Google Scholar] [CrossRef]
- Yin, L.; Xu, J.; Li, M.; Reddy, V.; Zhou, Q.; Liu, H.; Chu, P.; Zhang, Q.; Huang, Q.; Gao, Z.; et al. Oct2 and Bob1 Are Sensitive and Specific Markers in Lineage Determination of B Cell Lymphomas with No Expression of Conventional B Cell Markers. Histopathology 2016, 69, 775–783. [Google Scholar] [CrossRef]
- Ye, X.; Xu, G.; Li, X.; Wei, J.; Zhang, X.; Zhang, X.; Zhu, Y.; Lv, Y.; Xiao, F.; Yang, C.; et al. Clinical Characteristics and Prognosis of Patients with Follicular Lymphoma Grade 3A: A Real-World Study in a Single Centre. Preprint 2024. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, H.; Hu, S.M.; Miranda, R.N.; Xu, X.J.; Bayerl, M.G.; Artymiuk, C.J.; Berg, H.; King, R.L.; Shi, M.; et al. Follicular lymphoma and diffuse large B-cell lymphoma with BCL2 and IRF4 rearrangements in adult patients. Hum. Pathol. 2023, 141, 22–29. [Google Scholar] [CrossRef]
- Chisholm, K.M.; Mohlman, J.; Liew, M.; Termuhlen, A.; Cairo, M.S.; Gross, T.G.; Perkins, S.L.; Miles, R.R. IRF4 translocation status in pediatric follicular and diffuse large B-cell lymphoma patients enrolled in Children’s Oncology Group trials. Pediatr. Blood Cancer 2019, 66, e27770. [Google Scholar] [CrossRef]
- Bukhari, U.; Lateef, F.; Jamal, S. Frequency of Subgroups of Diffuse Large B-Cell Lymphoma by Immunohistochemistry. J. Liaquat Univ. Med. Health Sci. 2015, 14, 78–82. [Google Scholar]
- Reber, R.; Banz, Y.; Garamvolgyi, E.; Perren, A.; Novak, U. Determination of the molecular subtypes of diffuse large B-cell lymphomas using immunohistochemistry: A case series from the Inselspital, Bern, and a critical appraisal of this determination in Switzerland. Swiss Med. Wkly. 2013, 143, w13748. [Google Scholar] [CrossRef]
- Jablonska, J.; Jesionek-Kupnicka, D. Usefulness of immunohistochemistry in identification of prognostically important subgroups (GCB and ABC) in a heterogeneous group of diffuse large B-cell lymphomas—A review article. Pol. J. Pathol. 2008, 59, 121–127. [Google Scholar]
- Xie, Y.; Pittaluga, S.; Jaffe, E.S. The Histological Classification of Diffuse Large B-cell Lymphomas. Semin. Hematol. 2015, 52, 57–66. [Google Scholar] [CrossRef]
- Hartmann, S. From a pathologist’s point of view: Histiocytic cells in Hodgkin lymphoma and T cell/histiocyte rich large B cell lymphoma. Pathol. Res. Pract. 2015, 211, 901–904. [Google Scholar] [CrossRef]
- Huttl, K.S.; Staiger, A.M.; Richter, J.; Ott, M.M.; Kalmbach, S.; Klapper, W.; Biesdorf, A.S.; Trumper, L.; Rosenwald, A.; Ziepert, M.; et al. The “Burkitt-like” immunophenotype and genotype is rarely encountered in diffuse large B cell lymphoma and high-grade B cell lymphoma, NOS. Virchows Arch. 2021, 479, 575–583. [Google Scholar] [CrossRef]
- El Dana, F.; Narvaez, S.A.G.; El-Mallawany, N.K.; Agrusa, J.E.; Dreyer, Z.E.; Marcogliese, A.N.; Elghetany, M.T.; Punia, J.N.; Ok, C.Y.; Patel, K.P.; et al. Childhood and Adolescent Relapsed/Refractory Aggressive B-Cell Lymphomas with t(8;14) and BCL2 Expression, Burkitt Lymphoma Versus Diffuse Large B-Cell Lymphoma: A Diagnostic Challenge. Pediatr. Dev. Pathol. 2024, 27, 348–353. [Google Scholar] [CrossRef]
- Alsharif, R.; Dunleavy, K. Burkitt Lymphoma and Other High-Grade B-Cell Lymphomas with or without MYC, BCL2, and/or BCL6 Rearrangements. Hematol. Oncol. Clin. N. Am. 2019, 33, 587–596. [Google Scholar] [CrossRef]
- Kushwaha, P.; Singh, M.; Mallya, V.; Jain, S.; Aggarwal, S.; Singh, K. Transformation of diffuse large B-cell lymphoma to lymphoblastic lymphoma. J. Cancer Res. Ther. 2022, 18, S475–S477. [Google Scholar] [CrossRef]
- Ok, C.Y.; Medeiros, L.J.; Thakral, B.; Tang, G.; Jain, N.; Jabbour, E.; Pierce, S.A.; Konoplev, S. High-grade B-cell lymphomas with TdT expression: A diagnostic and classification dilemma. Mod. Pathol. 2019, 32, 48–58. [Google Scholar] [CrossRef]
- Kallen, M.E.; Rao, N.P.; Kulkarni, S.K.; Pullarkat, S.T.; Said, J.; Tirado, C.A.; Ahmed, R.S.; Miller, J.M.; Chung, P.Y.; Kahn, D.G.; et al. B-lymphoblastic transformation of mantle cell lymphoma/leukemia with “double hit” changes. J. Hematop. 2015, 8, 31–36. [Google Scholar] [CrossRef]
- Daniel, F.; Assi, H.; Karaoui, W.R.; Cheikh, J.E.; Bannoura, S.; Nassif, S. A Single Mass Forming Colonic Primary Mantle Cell Lymphoma. Case Rep. Gastrointest. Med. 2016, 2016, 2561507. [Google Scholar] [CrossRef]
- Fernández, V.; Salamero, O.; Espinet, B.; Solé, F.; Royo, C.; Navarro, A.; Camacho, F.I.; Beà, S.l.; Hartmann, E.; Amador, V.; et al. Genomic and Gene Expression Profiling Defines Indolent Forms of Mantle Cell Lymphoma. Cancer Res. 2010, 70, 1408–1418. [Google Scholar] [CrossRef]
- Matsuo, T.; Ichimura, K.; Kubonishi, S. Mantle Cell Lymphoma Diagnosed by Conjunctival Salmon-Pink Lesion Biopsy. J. Clin. Exp. Hematop. 2014, 54, 143–147. [Google Scholar] [CrossRef]
- Katono, K.; Shirasawa, M.; Harada, S.; Niwa, H.; Nakahara, Y.; Igawa, S.; Yokoba, M.; Kubota, M.; Masuda, N. Endobronchial Involvement of Mantle Cell Lymphoma: A Case Report. Respir. Med. Case Rep. 2016, 19, 77–79. [Google Scholar] [CrossRef][Green Version]
- Bhattacharyya, I.; Chehal, H.; Cohen, D.M.; Al-Quran, S.Z. Primary Diffuse Large B-Cell Lymphoma of the Oral Cavity: Germinal Center Classification. Head. Neck Pathol. 2010, 4, 181–191. [Google Scholar] [CrossRef]
- Sadeghipour, A.; Taha, S.R.; Zadeh, M.S.; Kosari, F.; Babaheidarian, P.; Fattahi, F.; Abdi, N.; Tajik, F. Expression and Clinical Significance of Ki-67, CD10, BCL6, MUM1, C-Myc, and EBV in Diffuse Large B Cell Lymphoma Patients. Appl. Immunohistochem. Mol. Morphol. 2024, 32, 309–321. [Google Scholar] [CrossRef]
- Peters, T.L.; Li, L.; Tula-Sanchez, A.A.; Pongtornpipat, P.; Schatz, J.H. Control of Translational Activation by PIM Kinase in Activated B-Cell Diffuse Large B-Cell Lymphoma Confers Sensitivity to Inhibition by PIM447. Oncotarget 2016, 7, 63362–63373. [Google Scholar] [CrossRef]
- Hu, S.; Xu-Monette, Z.Y.; Tzankov, A.; Green, T.; Wu, L.; Balasubramanyam, A.; Liu, W.-m.; Visco, C.; Li, Y.; Miranda, R.N.; et al. MYC/BCL2 Protein Coexpression Contributes to the Inferior Survival of Activated B-Cell Subtype of Diffuse Large B-Cell Lymphoma and Demonstrates High-Risk Gene Expression Signatures: A Report From the International DLBCL Rituximab-Chop Consortium Program. Blood 2013, 121, 4021–4031. [Google Scholar] [CrossRef]
- Baptista, M.J.; Tapia, G.; Muñoz-Mármol, A.M.; Muncunill, J.; García, O.; Montoto, S.; Gribben, J.G.; Calaminici, M.; Martinez, A.; Veloza, L.; et al. Genetic and Phenotypic Characterisation of HIV-associated Aggressive B-cell non-Hodgkin Lymphomas, Which Do Not Occur Specifically in This Population: Diagnostic and Prognostic Implications. Histopathology 2022, 81, 826–840. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Koo, D.H.; Suh, C.; Kim, S.; Park, B.H.; Kang, J.W.; Huh, J. Prognostic Value of Immunohistochemical Biomarkers at Different Cut-Off Values in Patients with Diffuse Large B-Cell Lymphoma Treated with CHOP Chemotherapy. J. Korean Med. Sci. 2011, 26, 1556. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, M.; Hollander, P.; Pandzic, T.; Mansouri, L.; Ednersson, S.B.; Andersson, P.O.; Hultdin, M.; Fors, M.; Erlanson, M.; Degerman, S.; et al. Cell-of-origin Determined by Both Gene Expression Profiling and Immunohistochemistry Is the Strongest Predictor of Survival in Patients with Diffuse Large B-cell Lymphoma. Am. J. Hematol. 2019, 95, 57–67. [Google Scholar] [CrossRef]
- Zhang, F.; Yan, L.X.; Lin, S.-X.; Ye, Z.-Y.; Zhuang, H.-G.; Yun, J.P.; Lin, H.-L.; Luo, D.; Xu, F.-P.; Luo, X.-L.; et al. Immunophenotypic Features and T(14;18) (Q32;q21) Translocation of Chinese Follicular Lymphomas Helps to Distinguish Subgroups. Diagn. Pathol. 2013, 8, 154. [Google Scholar] [CrossRef][Green Version]
- Shold, J.; Jukic, L.; Farrell, D.; Cui, W.; Zhang, D. EBV Positive Diffuse Large B Cell Lymphoma with Negative Pan-B Cell Markers, Case Report, and Literature Review. Case Rep. Hematol. 2024, 2024, 4803071. [Google Scholar] [CrossRef]
- Huang, W.M.; Wei, X.L.; Wei, Y.Q.; Feng, R. CD30 Promotes Proliferation Via ERK 1/2 and p38 MAPK Signal Pathways in ABC-Diffuse Large B Cell Lymphoma. Blood 2021, 138, 4337. [Google Scholar] [CrossRef]
- Maracaja, D.L.V.; Puthenpura, V.; Pels, S.G.; O’Malley, D.P.; Sklar, J.L.; Finberg, K.E.; Xu, M.L. EBV-Positive Primary Large B-Cell Lymphoma: The Role of Immunohistochemistry and XPO1 in the Diagnosis of Mediastinal Lymphomas. Appl. Immunohistochem. Mol. Morphol. 2020, 28, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Badri, N.; Ngamdu, K.; Torabi, A.; Guar, S. Brentuximab vedotin demonstrates an objective response in a patient with refractory CD30+primary mediastinal B-cell lymphoma. J. Cancer Res. Ther. 2020, 16, 183–185. [Google Scholar] [CrossRef]
- Afrantou, T.; Natsis, K.S.; Papadopoulos, G.; Lagoudaki, R.; Poulios, C.; Mamouli, D.; Kostopoulos, I.; Grigoriadis, N. A case of CD30+ALK1-anaplastic large cell lymphoma resembling acute disseminated encephalomyelitis. Mult. Scler. Relat. Disord. 2017, 13, 119–121. [Google Scholar] [CrossRef]
- Nakamura, S.; Kagawa, K.; Sumitani, R.; Uemura, M.; Takahashi, M.; Iwasa, M.; Fujii, S.; Miki, H.; Abe, M. Primary CD30-positive Diffuse Large B-cell Lymphoma in the Superior Vena Cava. Intern. Med. 2017, 56, 2043–2047. [Google Scholar] [CrossRef][Green Version]
- Mehta, A.; Verma, A.; Gupta, G.; Tripathi, R.; Sharma, A. Double Hit and Double Expresser Diffuse Large B Cell Lymphoma Subtypes: Discrete Subtypes and Major Predictors of Overall Survival. Indian. J. Hematol. Blood Transfus. 2020, 36, 627–634. [Google Scholar] [CrossRef]
- Riedell, P.A.; Smith, S.M. Double hit and double expressors in lymphoma: Definition and treatment. Cancer 2018, 124, 4622–4632. [Google Scholar] [CrossRef]
- Rosenthal, A.C.; Younes, A. High Grade B-Cell Lymphoma with Rearrangements of MYC and BCL2 and/or BCL6: Double Hit and Triple Hit Lymphomas and Double Expressing Lymphoma. Blood Rev. 2017, 31, 37–42. [Google Scholar] [CrossRef]
- Zeremski, V.; McPhail, E.D.; Habermann, T.M.; Schieppati, F.; Gebauer, N.; Vassilakopoulos, T.P.; Mougiakakos, D. Treatment Intensification Might Not Improve Survival in High-grade B-cell Lymphoma with a Concurrent MYC and BCL2 and/or BCL6 Rearrangement: A Retrospective, Multicenter, Pooled Analysis. Hematol. Oncol. 2023, 41, 776–780. [Google Scholar] [CrossRef]
- Miyaoka, M.; Kikuti, Y.Y.; Carreras, J.; Ikoma, H.; Hiraiwa, S.; Ichiki, A.; Kojima, M.; Ando, K.; Yokose, T.; Sakai, R.; et al. Clinicopathological and Genomic Analysis of Double-Hit Follicular Lymphoma: Comparison with High-Grade B-Cell Lymphoma with MYC and BCL2 and/or BCL6 Rearrangements. Mod. Pathol. 2018, 31, 313–326. [Google Scholar] [CrossRef]
- Almeida, R.; Abrantes, A.M.; Gigliano, D.; Oliveira, R.C.; Teixeira, P.; Viegas, M.; Rodrigues, Â.B.; Julião, M.J. Clinical and Pathological Features of Double-Hit and Triple-Hit High-Grade B-Cell Lymphomas: A Retrospective Study From Three Portuguese Tertiary Centers. Int. J. Hematol.-Oncol. Stem Cell Res. 2022, 16, 94–102. [Google Scholar] [CrossRef]
- Chong, Y.; Kim, T.E.; Cho, U.; Jin, M.-S.; Yim, K.; Thakur, N.; Kim, J.O.; Cho, I.; Park, G. Comparison of Ki-67 Labeling Index Patterns of Diffuse Large B-Cell Lymphomas and Burkitt Lymphomas Using Image Analysis: A Multicenter Study. Diagnostics 2021, 11, 343. [Google Scholar] [CrossRef]
- Mimery, A.; Jabbour, J.; Sykes, B.A.; MacDermid, E.; Al-Askari, M.; Clercq, S.D. Burkitt Leukemia Presenting as Acute Appendicitis: A Case Report and Literature Review. Am. J. Case Rep. 2020, 21, e921568. [Google Scholar] [CrossRef]
- Yamashita, T.; Vollbrecht, C.; Hirsch, B.; Kleo, K.; Anagnostopoulos, I.; Hummel, M. Integrative Genomic Analysis Focused on Cell Cycle Genes for MYC-driven Aggressive Mature B-Cell Lymphoma. J. Clin. Exp. Hematop. 2020, 60, 87–96. [Google Scholar] [CrossRef]
- Yang, G.; Deisch, J.; Tavares, M.; Hai-xia, Q.; Cobb, C.J.; Raza, A. Primary B-cell Lymphoma of the Uterine Cervix: Presentation in Pap-test Slide and Cervical Biopsy. Diagn. Cytopathol. 2016, 45, 235–238. [Google Scholar] [CrossRef]
- Kushwaha, P.; Singh, M.; Vindal, A.; Verma, N.; Jain, S. Primary ALK-Positive Large B Cell Lymphoma of Pancreas. J. Gastrointest. Cancer 2022, 53, 830–833. [Google Scholar] [CrossRef]
- Castillo, J.J.; Bibas, M.; Miranda, R.N. The Biology and Treatment of Plasmablastic Lymphoma. Blood 2015, 125, 2323–2330. [Google Scholar] [CrossRef]
- Takiar, R.; Phillips, T. Durable Responses with ALK Inhibitors for Primary Refractory Anaplastic Lymphoma Kinase-Positive Large B-Cell Lymphoma. Blood Adv. 2023, 7, 2912–2916. [Google Scholar] [CrossRef]
- Chowdhury, Z. Anaplastic Lymphoma Kinase Positive Large B-Cell Lymphoma: Diagnostic Perils and Pitfalls, an Underrecognized Entity. Cureus J. Med. Sci. 2021, 13, e16882. [Google Scholar] [CrossRef]
- Castillo, J.J.; Chavez, J.C.; Hernandez-Ilizaliturri, F.J.; Montes-Moreno, S. CD20-negative diffuse large B-cell lymphomas: Biology and emerging therapeutic options. Expert Rev. Hematol. 2015, 8, 343–354. [Google Scholar] [CrossRef]
- Xiong, H.; Liu, S.; Yang, Y.; Tan, X.; Lü, X.; Peng, J.; Xiong, Z.; Chen, H.; Chen, J.; Li, Z.; et al. An Unusual Case of Anaplastic Lymphoma Kinase-Positive Large B-Cell Lymphoma in an Elderly Patient: A Case Report and Discussion. Exp. Ther. Med. 2016, 11, 1799–1802. [Google Scholar] [CrossRef]
- Ahn, J.; Okal, R.; Vos, J.A.; Smolkin, M.B.; Kanate, A.S.; Rosado, F. Plasmablastic Lymphoma Versus Plasmablastic Myeloma: An Ongoing Diagnostic Dilemma. J. Clin. Pathol. 2017, 70, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Nassiri, M. Lymphoproliferative Neoplasms with Plasmablastic Morphology. Arch. Pathol. Lab. Med. 2021, 146, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Bharathi, V. Epidemiological Trends and Survival Analysis of HHV8-Positive Diffuse Large B-Cell Lymphoma (HDN) in Multicentric Castleman Disease (CD) Patients: A SEER Database Investigation. Clin. Lymphoma Myeloma Leuk. 2024, 24, S469. [Google Scholar] [CrossRef]
- Lorenzi, L.; Fisogni, S.; Pellegrini, W.; Gazzola, A.; Vermi, W.; Agostinelli, C.; Massarelli, G.; Pileri, S.A.; Facchetti, F. EBV+ HHV8- Germinotropic Large B Cell Lymphoma: A Lymphoproliferative Disorder with Intermediate Features between EBV+ Large B Cell Lymphomas and Classical Hodgkin Lymphoma. Mod. Pathol. 2012, 25, 353A. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halcu, G.; Evsei-Seceleanu, A.; Cerbu, M.; Bara, M.A.; Turbatu, A.; Ceausu, M.C. From Biopsy to Diagnosis: Navigating Aggressive B-Cell Lymphomas in Practice. Medicina 2025, 61, 842. https://doi.org/10.3390/medicina61050842
Halcu G, Evsei-Seceleanu A, Cerbu M, Bara MA, Turbatu A, Ceausu MC. From Biopsy to Diagnosis: Navigating Aggressive B-Cell Lymphomas in Practice. Medicina. 2025; 61(5):842. https://doi.org/10.3390/medicina61050842
Chicago/Turabian StyleHalcu, Georgian, Anca Evsei-Seceleanu, Mihai Cerbu, Marina Alina Bara, Andrei Turbatu, and Mihail Constantin Ceausu. 2025. "From Biopsy to Diagnosis: Navigating Aggressive B-Cell Lymphomas in Practice" Medicina 61, no. 5: 842. https://doi.org/10.3390/medicina61050842
APA StyleHalcu, G., Evsei-Seceleanu, A., Cerbu, M., Bara, M. A., Turbatu, A., & Ceausu, M. C. (2025). From Biopsy to Diagnosis: Navigating Aggressive B-Cell Lymphomas in Practice. Medicina, 61(5), 842. https://doi.org/10.3390/medicina61050842





