A Meta-Analysis Examining the Impact of Consuming Nitrogen-Free Analogs of Essential Amino Acids on the Progression of Chronic Renal Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources
- The research was a randomized controlled trial, a prospective study, or a retrospective study.
- The goal populace comprised persons with CKD.
- The intervention consisted of a diet featuring NFAs of EAAs.
- The study involved comparisons between very-LPDs that were supplemented with NFA and standard LPDs.
- Studies that failed to assess the effect of the diet involving NFAs of EAAs on the deterioration of CKD.
- Studies involving subjects receiving interventions other than a diet of NFAs of EAAs.
- Studies that did not emphasize the impact of comparative results.
2.3. Search Strategy
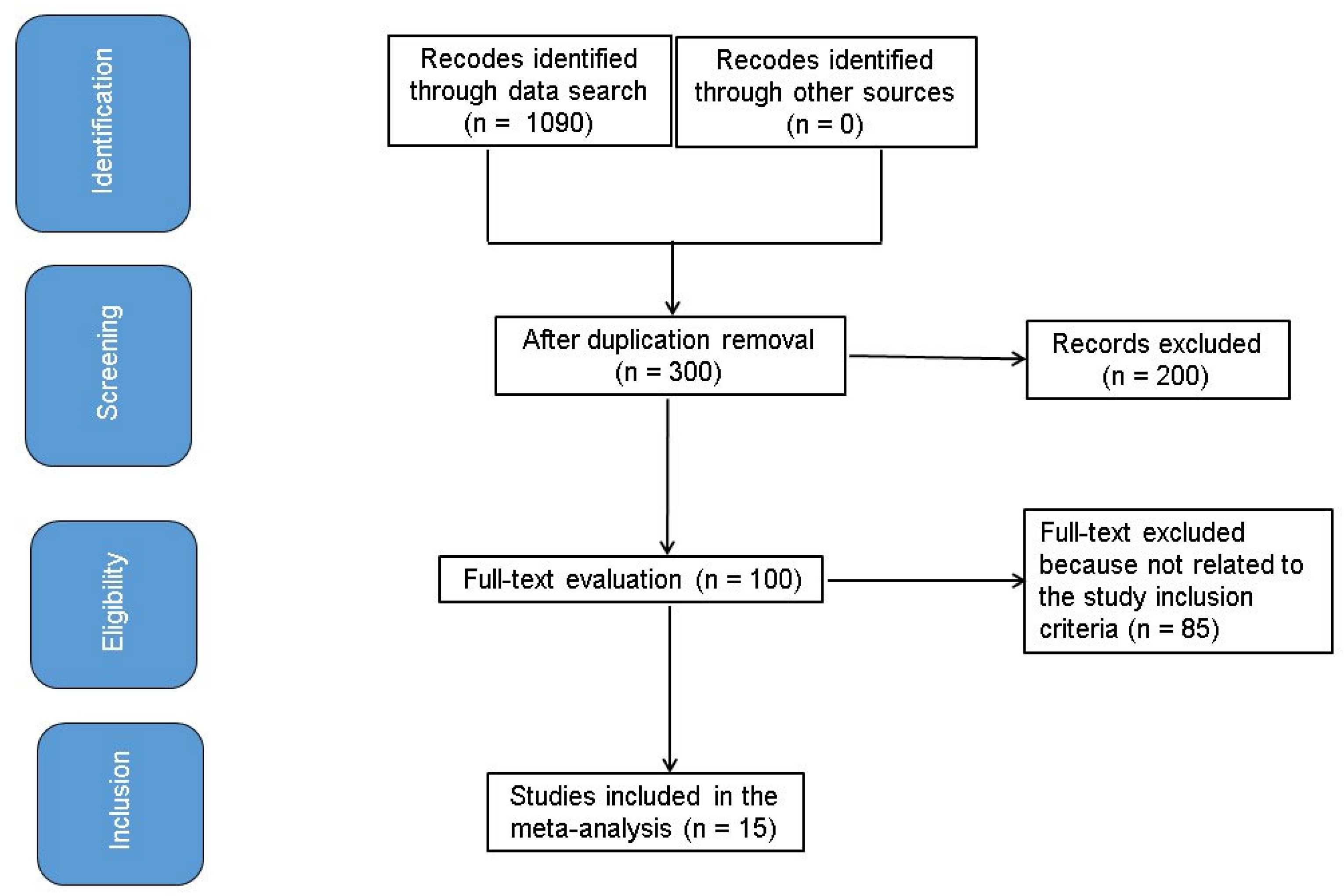
2.4. Selection Process
2.5. Data Collection Process
2.6. Data Items
2.7. Study Risk of Bias Assessment
2.8. Effect Measures
2.9. Synthesis Methods
2.10. Reporting Bias Assessment
2.11. Certainty Assessment
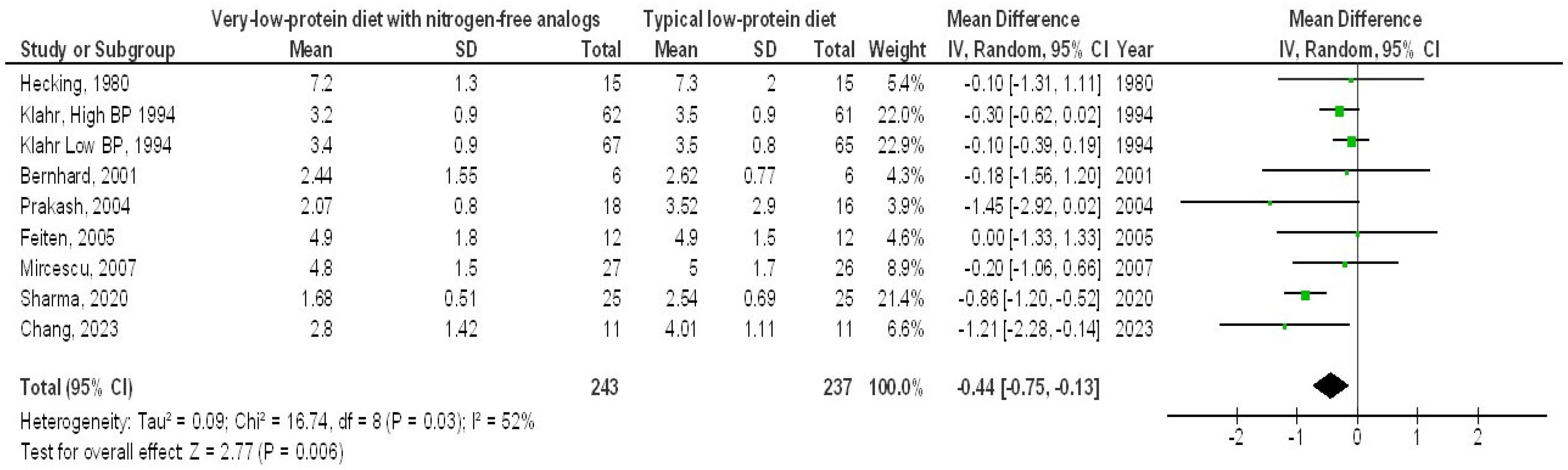
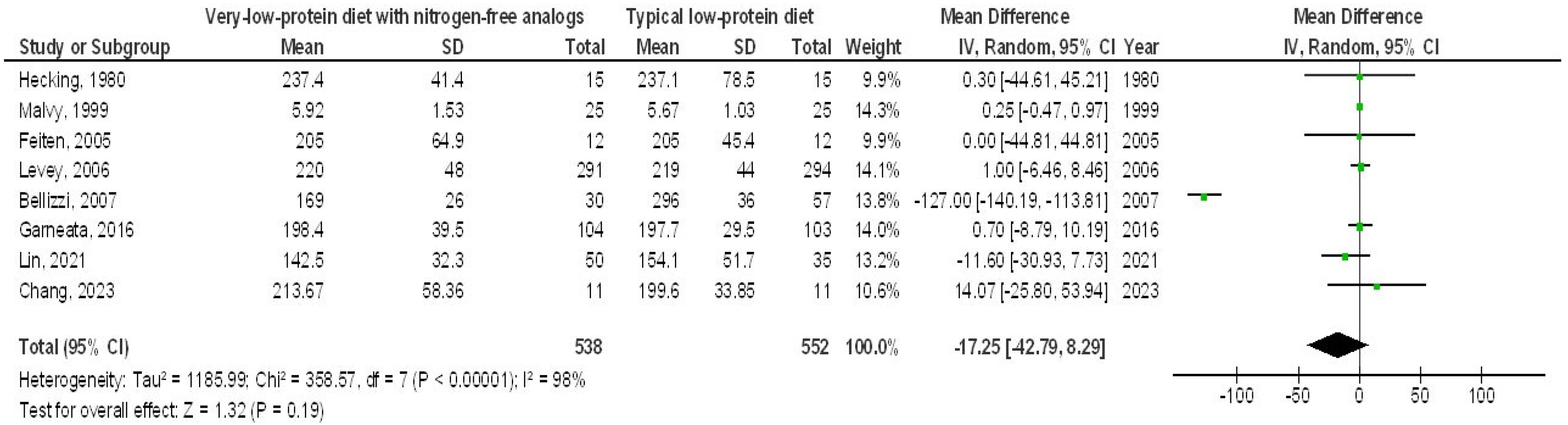
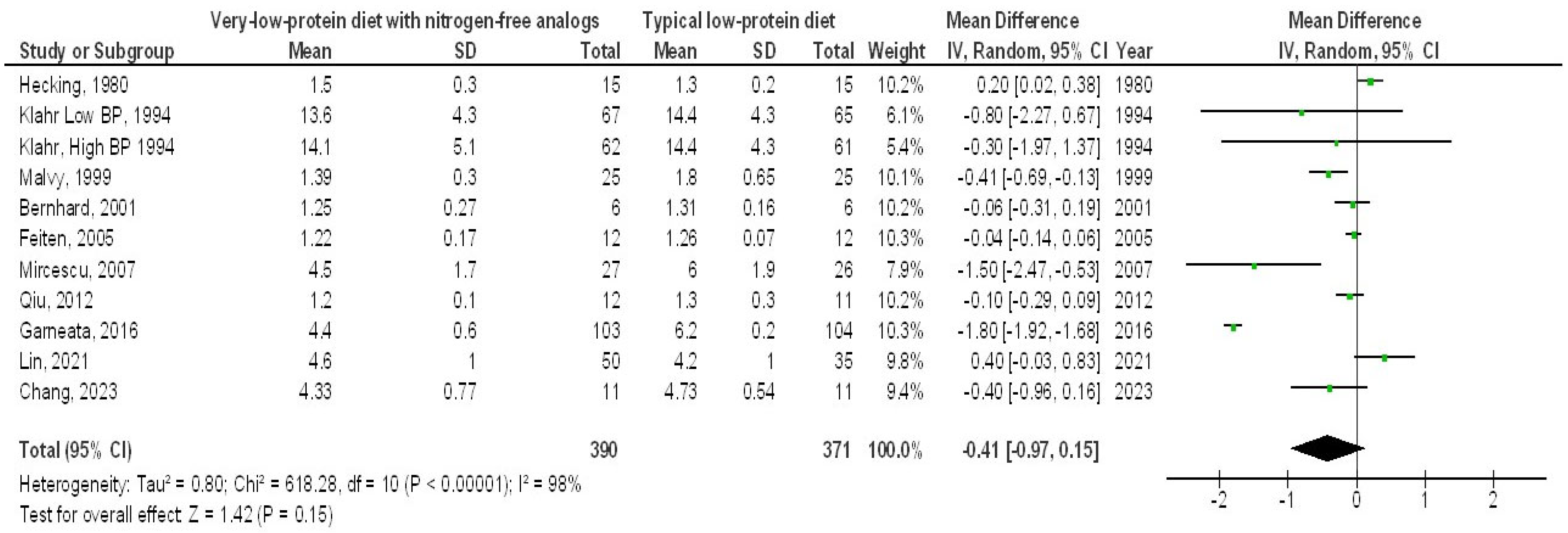
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mitch, W.E. Dietary protein restriction in chronic renal failure: Nutritional efficacy, compliance, and progression of renal insufficiency. J. Am. Soc. Nephrol. 1991, 2, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.; Porter, J. Dietary mobile apps and their effect on nutritional indicators in chronic renal disease: A systematic review. Nephrology 2015, 20, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Bellizzi, V.; Calella, P.; Carrero, J.J.; Fouque, D. Very low-protein diet to postpone renal failure: Pathophysiology and clinical applications in chronic kidney disease. Chronic Dis. Transl. Med. 2018, 4, 45–50. [Google Scholar] [CrossRef]
- Khan, I.A.; Nasiruddin, M.; Haque, S.F.; Khan, R.A. Comparative evaluation of efficacy and safety profile of rhubarb and α-keto analogs of essential amino acids supplementation in patients with diabetic nephropathy. Saudi J. Kidney Dis. Transplant. 2016, 27, 710. [Google Scholar] [CrossRef] [PubMed]
- Garneata, L.; Stancu, A.; Dragomir, D.; Stefan, G.; Mircescu, G. Ketoanalogue-supplemented vegetarian very low–protein diet and CKD progression. J. Am. Soc. Nephrol. 2016, 27, 2164–2176. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhang, X.; Yang, L.; Li, Z.; Qin, W. Effect of restricted protein diet supplemented with keto analogues in chronic kidney disease: A systematic review and meta-analysis. Int. Urol. Nephrol. 2016, 48, 409–418. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Gupta, A.; Das, A.; Majumder, K.; Arora, N.; Mayo, H.G.; Singh, P.P.; Beg, M.S.; Singh, S. Obesity is Independently Associated With Increased Risk of Hepatocellular Cancer–related Mortality. Am. J. Clin. Oncol. 2018, 41, 874–881. [Google Scholar] [CrossRef]
- Hecking, E.; Andrzejewski, L.; Prellwitz, W.; Opferkuch, W.; Müller, D. Double-blind cross-over study with oral α-ketoacids in patients with chronic renal failure. Am. J. Clin. Nutr. 1980, 33, 1678–1681. [Google Scholar] [CrossRef] [PubMed]
- Klahr, S.; Levey, A.S.; Beck, G.J.; Caggiula, A.W.; Hunsicker, L.; Kusek, J.W.; Striker, G. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. N. Engl. J. Med. 1994, 330, 877–884. [Google Scholar] [CrossRef]
- Malvy, D.; Maingourd, C.; Pengloan, J.; Bagros, P.; Nivet, H. Effects of severe protein restriction with ketoanalogues in advanced renal failure. J. Am. Coll. Nutr. 1999, 18, 481–486. [Google Scholar] [CrossRef]
- Bernhard, J.; Beaufre, B.; Laville, M.; Fouque, D. Adaptive response to a low-protein diet in predialysis chronic renal failure patients. J. Am. Soc. Nephrol. 2001, 12, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S.; Pande, D.P.; Sharma, S.; Sharma, D.; Bal, C.S.; Kulkarni, H. Randomized, double-blind, placebo-controlled trial to evaluate efficacy of ketodiet in predialytic chronic renal failure. J. Ren. Nutr. 2004, 14, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Feiten, S.; Draibe, S.; Watanabe, R.; Duenhas, M.; Baxmann, A.; Nerbass, F.; Cuppari, L. Short-term effects of a very-low-protein diet supplemented with ketoacids in nondialyzed chronic kidney disease patients. Eur. J. Clin. Nutr. 2005, 59, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Greene, T.; Sarnak, M.J.; Wang, X.; Beck, G.J.; Kusek, J.W.; Collins, A.J.; Kopple, J.D. Effect of dietary protein restriction on the progression of kidney disease: Long-term follow-up of the Modification of Diet in Renal Disease (MDRD) Study. Am. J. Kidney Dis. 2006, 48, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Bellizzi, V.; Di Iorio, B.; De Nicola, L.; Minutolo, R.; Zamboli, P.; Trucillo, P.; Catapano, F.; Cristofano, C.; Scalfi, L.; Conte, G. Very low protein diet supplemented with ketoanalogs improves blood pressure control in chronic kidney disease. Kidney Int. 2007, 71, 245–251. [Google Scholar] [CrossRef]
- Mircescu, G.; Gârneaţă, L.; Stancu, S.H.; Căpuşă, C. Effects of a supplemented hypoproteic diet in chronic kidney disease. J. Ren. Nutr. 2007, 17, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Liu, F.; Zhao, L.; Huang, S.; Zuo, C.; Zhong, H.; Chen, F. Comparison of the effects of alpha-keto/amino acid supplemented low protein diet and diabetes diet in patients with diabetic nephropathy. Sichuan Da Xue Xue Bao. Yi Xue Ban = J. Sichuan Univ. Med. Sci. Ed. 2012, 43, 425–428. [Google Scholar]
- Milovanova, L.; Fomin, V.; Moiseev, S.; Taranova, M.; Milovanov, Y.; Lysenko, L.; Kozlov, V.; Kozevnikova, E.; Milovanova, S.; Lebedeva, M. Effect of essential amino acid ketoanalogues and protein restriction diet on morphogenetic proteins (FGF-23 and Klotho) in 3b–4 stages chronic kidney disease patients: A randomized pilot study. Clin. Exp. Nephrol. 2018, 22, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Khadka, S.; Amgain, K.; Acharya, S.; Panta, P.P. Rationale of α-Ketoanalogue supplemented with low protein diet for the treatment of chronic kidney disease. J. Karnali Acad. Health Sci. 2020, 3, 1012. [Google Scholar]
- Lin, Y.-L.; Hou, J.-S.; Wang, C.-H.; Su, C.-Y.; Liou, H.-H.; Hsu, B.-G. Effects of ketoanalogues on skeletal muscle mass in patients with advanced chronic kidney disease: Real-world evidence. Nutrition 2021, 91, 111384. [Google Scholar] [CrossRef]
- Chang, G.; Shih, H.-M.; Pan, C.-F.; Wu, C.-J.; Lin, C.-J. Effect of Low Protein Diet Supplemented with Ketoanalogs on Endothelial Function and Protein-Bound Uremic Toxins in Patients with Chronic Kidney Disease. Biomedicines 2023, 11, 1312. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, G.; Solini, A.; Bonora, E.; Orsi, E.; Zerbini, G.; Giorgino, F.; Cavalot, F.; Pontiroli, A.E.; Baroni, M.G.; Morano, S. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation provides a better definition of cardiovascular burden associated with CKD than the Modification of Diet in Renal Disease (MDRD) Study formula in subjects with type 2 diabetes. Atherosclerosis 2011, 218, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Whitman, I.R.; Feldman, H.I.; Deo, R. CKD and sudden cardiac death: Epidemiology, mechanisms, and therapeutic approaches. J. Am. Soc. Nephrol. 2012, 23, 1929–1939. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int. Suppl. 2009, 76, S1–S130. [Google Scholar]
- Tonelli, M.; Wiebe, N.; Culleton, B.; House, A.; Rabbat, C.; Fok, M.; McAlister, F.; Garg, A.X. Chronic kidney disease and mortality risk: A systematic review. J. Am. Soc. Nephrol. 2006, 17, 2034–2047. [Google Scholar] [CrossRef] [PubMed]
- McClellan, W.; Jurkovitz, C.; Abramson, J. The epidemiology and control of anaemia among pre-ESRD patients with chronic kidney disease. Eur. J. Clin. Investig. 2005, 35, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, C.; Borrelli, S.; De Stefano, T.; Provenzano, M.; Andreucci, M.; Cabiddu, G.; La Milia, V.; Vizzardi, V.; Sandrini, M.; Cancarini, G. Incremental dialysis in ESRD: Systematic review and meta-analysis. J. Nephrol. 2019, 32, 823–836. [Google Scholar] [CrossRef]
- Noce, A.; Vidiri, M.; Marrone, G.; Moriconi, E.; Bocedi, A.; Capria, A.; Rovella, V.; Ricci, G.; De Lorenzo, A.; Di Daniele, N. Is low-protein diet a possible risk factor of malnutrition in chronic kidney disease patients? Cell Death Discov. 2016, 2, 1–6. [Google Scholar] [CrossRef]
- Fouque, D.; Aparicio, M. Eleven reasons to control the protein intake of patients with chronic kidney disease. Nat. Clin. Pract. Nephrol. 2007, 3, 383–392. [Google Scholar] [CrossRef]
- Garneata, L.; Stancu, A.; Luca, P.; Stefan, G.; Mircescu, G. Vegetarian very low protein diet supplemented with ketoanalogues may reduce nephrotic-range proteinuria in predialysis CKD patients. In Nephrology Dialysis Transplantation; Oxford Univ Press: Oxford, UK, 2016; p. 202. [Google Scholar]
- Driver, T.H.; Shlipak, M.G.; Katz, R.; Goldenstein, L.; Sarnak, M.J.; Hoofnagle, A.N.; Siscovick, D.S.; Kestenbaum, B.; de Boer, I.H.; Ix, J.H. Low serum bicarbonate and kidney function decline: The Multi-Ethnic Study of Atherosclerosis (MESA). Am. J. Kidney Dis. 2014, 64, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Raphael, K.L.; Zhang, Y.; Ying, J.; Greene, T. Prevalence of and risk factors for reduced serum bicarbonate in chronic kidney disease. Nephrology 2014, 19, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Pupim, L.B.; Flakoll, P.J.; Majchrzak, K.M.; Guy, D.L.A.; Stenvinkel, P.; Ikizler, T.A. Increased muscle protein breakdown in chronic hemodialysis patients with type 2 diabetes mellitus. Kidney Int. 2005, 68, 1857–1865. [Google Scholar] [CrossRef]
- Amdur, R.L.; Feldman, H.I.; Gupta, J.; Yang, W.; Kanetsky, P.; Shlipak, M.; Rahman, M.; Lash, J.P.; Townsend, R.R.; Ojo, A. Inflammation and progression of CKD: The CRIC study. Clin. J. Am. Soc. Nephrol. 2016, 11, 1546–1556. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.; Kopple, J.D.; Wang, X.; Beck, G.J.; Collins, A.J.; Kusek, J.W.; Greene, T.; Levey, A.S.; Sarnak, M.J. Effect of a very low-protein diet on outcomes: Long-term follow-up of the Modification of Diet in Renal Disease (MDRD) Study. Am. J. Kidney Dis. 2009, 53, 208–217. [Google Scholar] [CrossRef]
- Ikizler, T.A. The use and misuse of serum albumin as a nutritional marker in kidney disease. Clin. J. Am. Soc. Nephrol. 2012, 7, 1375–1377. [Google Scholar] [CrossRef]
- Rambod, M.; Bross, R.; Zitterkoph, J.; Benner, D.; Pithia, J.; Colman, S.; Kovesdy, C.P.; Kopple, J.D.; Kalantar-Zadeh, K. Association of Malnutrition-Inflammation Score with quality of life and mortality in hemodialysis patients: A 5-year prospective cohort study. Am. J. Kidney Dis. 2009, 53, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Gama-Axelsson, T.; Heimbürger, O.; Stenvinkel, P.; Bárány, P.; Lindholm, B.; Qureshi, A.R. Serum albumin as predictor of nutritional status in patients with ESRD. Clin. J. Am. Soc. Nephrol. 2012, 7, 1446–1453. [Google Scholar] [CrossRef] [PubMed]
- Moe, S.M.; Drüeke, T.; Lameire, N.; Eknoyan, G. Chronic kidney disease–mineral-bone disorder: A new paradigm. Adv. Chronic Kidney Dis. 2007, 14, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Stevens, L.A.; Djurdjev, O.; Cardew, S.; Cameron, E.; Levin, A. Calcium, phosphate, and parathyroid hormone levels in combination and as a function of dialysis duration predict mortality: Evidence for the complexity of the association between mineral metabolism and outcomes. J. Am. Soc. Nephrol. 2004, 15, 770–779. [Google Scholar] [CrossRef]
- Block, G.A.; Klassen, P.S.; Lazarus, J.M.; Ofsthun, N.; Lowrie, E.G.; Chertow, G.M. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J. Am. Soc. Nephrol. 2004, 15, 2208–2218. [Google Scholar] [CrossRef] [PubMed]
- Young, E.W.; Albert, J.M.; Satayathum, S.; Goodkin, D.A.; Pisoni, R.L.; Akiba, T.; Akizawa, T.; Kurokawa, K.; Bommer, J.; Piera, L. Predictors and consequences of altered mineral metabolism: The Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2005, 67, 1179–1187. [Google Scholar] [CrossRef]
- Li, A.; Lee, H.-Y.; Lin, Y.-C. The effect of ketoanalogues on chronic kidney disease deterioration: A meta-analysis. Nutrients 2019, 11, 957. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Su, X.; Xu, B.; Qiao, X.; Wang, L. Effect of diet protein restriction on progression of chronic kidney disease: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0206134. [Google Scholar] [CrossRef] [PubMed]
- Sim, I. Two Ways of Knowing: Big Data and Evidence-Based Medicine; American College of Physicians: Philadelphia, PA, USA, 2016. [Google Scholar]





| Study | Country | Subjects | Age (Years) | Study Design | Treatment Period | Main Results |
|---|---|---|---|---|---|---|
| Hecking, 1980 [9] | Germany | 30 (15/15) | very-LPD: 43.7 ± 12.6 LPD: 43.7 ± 12.6 | RCT; 1.05 g/10 kg/day NFA vs. LPD (0.60 g/kg/d) | 6 weeks | SCL BUN SAC SC SP C |
| Klahr, 1994 [10] | USA | 255 (129/126) | NA | RCT; 0.28 g/kg/day NFA vs. LPD (0.60 g/kg/d) | 18–45 months | EGFR SCL SP |
| Malvy, 1999 [11] | France | 50 (25/25) | very-LPD: 53.6 ± 11.0 LPD: 56.0 ± 14.0 | RCT; 0.17 g/kg/day NFA vs. LPD (0.58 g/kg/d) | 3 months | PH SAC SC SP C |
| Bernhard, 2001 [12] | France | 12 (6/6) | very-LPD: 49.5 ± 7.0 LPD: 39.0 ± 5.8 | RCT; 1 pill/5 kg/day NFA vs. LPD RCT; (0.60–80 g/kg/d) | 3 months | SCL PH SAC SP C |
| Prakash, 2004 [13] | India | 34 (18/16) | very-LPD: 52.8 ± 14.1 LPD: 55.9 ± 17.6 | RCT; 1 pill/5 kg/day NFA vs. LPD (0.60 g/kg/d) | 9 months | EGFR SCL BUN SAC |
| Feiten, 2005 [14] | Brazil | 24 (12/12) | very-LPD: 49.7 ± 11.3 LPD: 43.9 ± 16.3 | RCT; 1 pill/5 kg/day NFA vs. LPD (0.60 g/kg/d) | 4 months | EGFR SCL BUN PH SAC SC SP C |
| Levey, 2006 [15] | USA | 585 (291/294) | NA | RCT; 1.05 g/10 kg/day NFA vs. LPD (0.65 g/kg/d) | 36 months | EGFR SAC SC |
| Bellizzi, 2007 [16] | Italy | 87 (30/57) | very-LPD: 58.0 ± 16.1 LPD: 56.3 ± 15.6 | RCT; 1 pill/5 kg/day NFA vs. LPD (0.60–80 g/kg/d) | 3–6 months | EGFR BUN PH SAC SC |
| Mircescu, 2007 [17] | Romania | 53 (27/26) | very-LPD: 55.0 ± 12.7 LPD: 53.6 ± 11.0 | RCT; 1 pill/5 kg/day NFA vs. LPD (0.60–80 g/kg/d) | 15 months | EGFR SCL BUN SP C |
| Qiu, 2012 [18] | China | 23 (12/11) | very-LPD: 63.0 ± 8.9 LPD: 61.60 ±9.67 | RCT; 1 pill/5 kg/day NFA vs. LPD (0.60–80 g/kg/d) | 52 months | EGFR SAC SP C |
| Garneata, 2016 [5] | Romania | 207 (104/103) | NA | RCT; 1 pill/5 kg/day NFA vs. LPD (0.60 g/kg/d) | 12 months | EGFR BUN SAC SC SP C |
| Milovanova, 2018 [19] | Russia | 79 (42/37) | NA | RCT; 0.1 g/kg/day NFA vs. LPD (0.60 g/kg/d) | 14 months | EGFR BUN PH SAC C |
| Sharma, 2020 [20] | Nepal | 50 (25/25) | very-LPD: 42.3 ± 13.6 LPD: 41.9 ± 11.6 | RCT; 1 pill/5 kg/day NFA vs. LPD (0.60 g/kg/d) | 24 months | SCL BUN SAC |
| Lin, 2021 [21] | Taiwan | 85 (50/35) | NA | RCT; 1 pill/5 kg/day NFA vs. LPD (0.58 g/kg/d) | 15 months | SAC SC SP C |
| Chang, 2023 [22] | Taiwan | 22 (11/11) | very-LPD: 45.6 ± 11.0 LPD: 44.8 ± 14.5 | RCT; 1 pill/5 kg/day NFA vs. LPD (0.65 g/kg/d) | 36 months | EGFR SCL BUN SAC SC SP C |
| Total | 1596 (797/799) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imam, M.S.; Alrasheedi, L.S.T.; Alyami, S.A.H.; Aljamaan, M.M.A.; Alnaim, K.S.K.; Alenzi, H.M.A.; Alnufeai, N.N.; Almalki, D.A.S.; Alanazi, A.S.; Alotaibi, S.S.F.; et al. A Meta-Analysis Examining the Impact of Consuming Nitrogen-Free Analogs of Essential Amino Acids on the Progression of Chronic Renal Disease. Medicina 2025, 61, 423. https://doi.org/10.3390/medicina61030423
Imam MS, Alrasheedi LST, Alyami SAH, Aljamaan MMA, Alnaim KSK, Alenzi HMA, Alnufeai NN, Almalki DAS, Alanazi AS, Alotaibi SSF, et al. A Meta-Analysis Examining the Impact of Consuming Nitrogen-Free Analogs of Essential Amino Acids on the Progression of Chronic Renal Disease. Medicina. 2025; 61(3):423. https://doi.org/10.3390/medicina61030423
Chicago/Turabian StyleImam, Mohamed S., Lama Saud Turki Alrasheedi, Saleh Ali Hassan Alyami, Mahdi Mohammed Ahmed Aljamaan, Khaled Sami Khaled Alnaim, Hussam Mohsen Ayesh Alenzi, Nouf Nawaf Alnufeai, Daad Adnan Saad Almalki, Abdullah S. Alanazi, Saud Saad Frais Alotaibi, and et al. 2025. "A Meta-Analysis Examining the Impact of Consuming Nitrogen-Free Analogs of Essential Amino Acids on the Progression of Chronic Renal Disease" Medicina 61, no. 3: 423. https://doi.org/10.3390/medicina61030423
APA StyleImam, M. S., Alrasheedi, L. S. T., Alyami, S. A. H., Aljamaan, M. M. A., Alnaim, K. S. K., Alenzi, H. M. A., Alnufeai, N. N., Almalki, D. A. S., Alanazi, A. S., Alotaibi, S. S. F., Alshaibani, N. F. M., Abdelrahim, M. E. A., & Mohamed, B. M. E. (2025). A Meta-Analysis Examining the Impact of Consuming Nitrogen-Free Analogs of Essential Amino Acids on the Progression of Chronic Renal Disease. Medicina, 61(3), 423. https://doi.org/10.3390/medicina61030423







