An Integrative Machine Learning Model for Predicting Early Safety Outcomes in Patients Undergoing Transcatheter Aortic Valve Implantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Data Collection
2.3. Model Selection
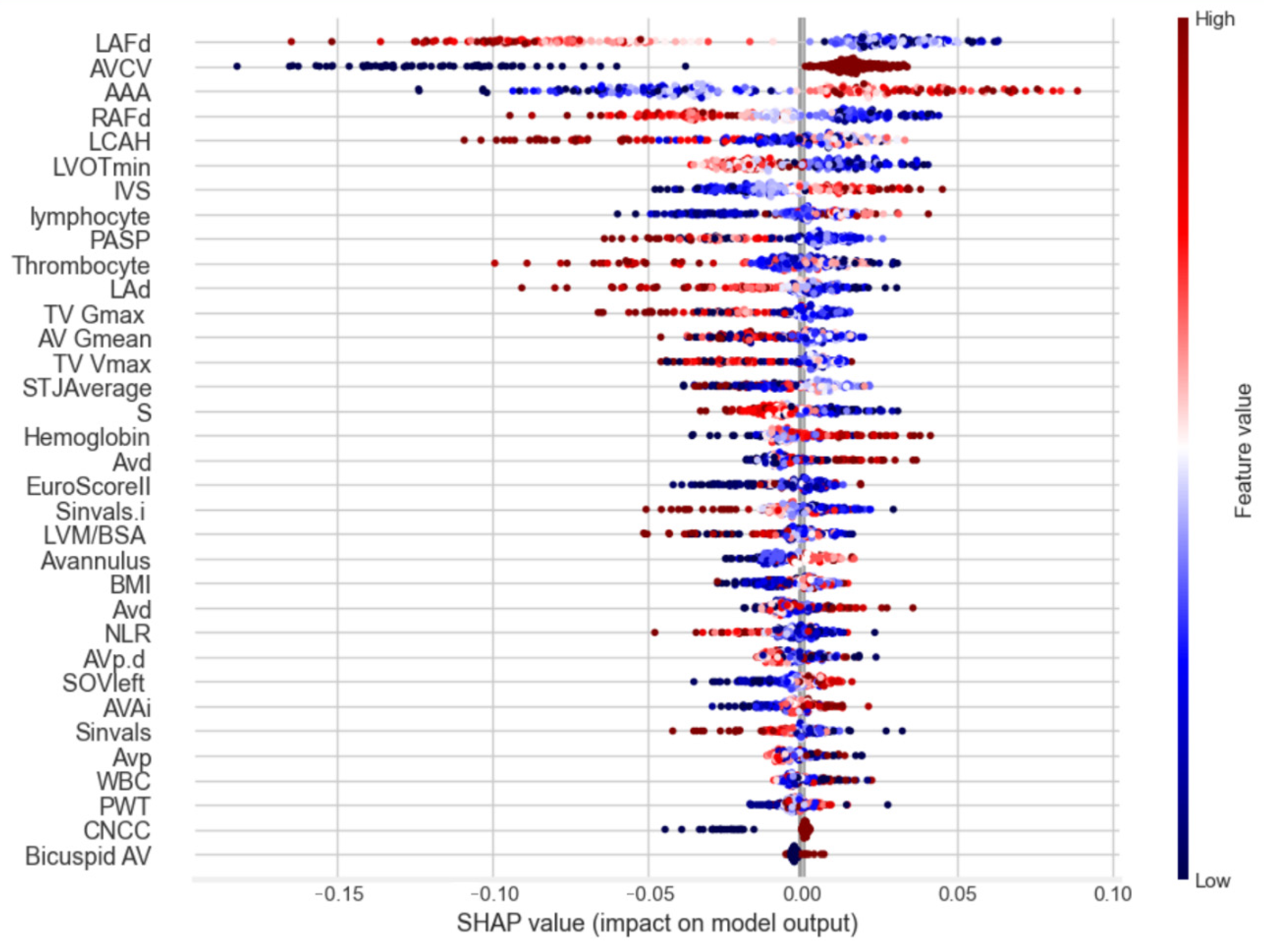
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AS | Aortic stenosis |
| AV | Aortic valve |
| BSA | Body surface area |
| BEVs | Balloon-expandable valves |
| CABG | Coronary artery bypass graft |
| CT | Computed tomography |
| CAD | Coronary artery disease |
| EuroSCORE II | European System for Cardiac Operative Risk Evaluation |
| LVEF | Left ventricular ejection fraction |
| MI | Myocardial infarction |
| MR | Mitral valve regurgitation |
| NYHA | New York Heart Association |
| PCI | Percutaneous coronary intervention |
| SAVR | Surgical aortic valve replacement |
| SEV | Self-expandable valve |
| SPSS | Statistical analysis was performed using SPSS |
| TAVI | Transcatheter aortic valve implantation |
| VARC-2 | Valve Academic Research Consortium II |
References
- Krishnaswami, A.; Forman, D.E.; Maurer, M.S.; Lee, S.J. A Decision-Making Framework for Objective Risk Assessment in Older Adults With Severe Symptomatic Aortic Stenosis. Curr. Geriatr. Rep. 2015, 4, 338–346. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary. J. Am. Coll. Cardiol. 2020, 73, e139–e228. [Google Scholar] [CrossRef] [PubMed]
- Leon, M.B.; Mack, M.J.; Hahn, R.T.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Alu, M.C.; Russo, M.J.; Babaliaros, V.; Douglas, P.S.; et al. Outcomes of Balloon-Expandable Transcatheter Aortic Valve Replacement Among Patients Younger Than 65 Years in the Low–Surgical Risk Era. JAMA Cardiol. 2021, 6, 139–149. [Google Scholar] [CrossRef]
- Adams, D.H.; Popma, J.J.; Reardon, M.J.; Yakubov, S.J.; Coselli, J.S.; Deeb, G.M.; Gleason, T.G.; Buchbinder, M.; Hermiller, J.B.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Prosthesis. N. Engl. J. Med. 2014, 370, 1790–1798. [Google Scholar] [CrossRef]
- Smith, C.R.; Leon, M.B.; Mack, M.J.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter versus Surgical Aortic-Valve Replacement in High-Risk Patients. N. Engl. J. Med. 2011, 364, 2187–2198. [Google Scholar] [CrossRef]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef]
- Goldsweig, A.M.; Lyden, E.; Aronow, H.D.; Kolte, D.; Pavlides, G.; Barton, D.; Chatzizisis, Y.; Gumina, R.J.; Abbott, J.D. Predictors of Contrast Volume in Transcatheter Aortic Valve Replacement. Cardiology 2020, 145, 608–617. [Google Scholar] [CrossRef]
- Kodali, S.K.; Williams, M.R.; Smith, C.R.; Svensson, L.G.; Webb, J.G.; Makkar, R.R.; Fontana, G.P.; Dewey, T.M.; Thourani, V.H.; Pichard, A.D.; et al. Two-Year Outcomes after Transcatheter or Surgical Aortic-Valve Replacement. N. Engl. J. Med. 2012, 366, 1686–1695. [Google Scholar] [CrossRef]
- Søndergaard, L.; De Backer, O.; Kofoed, K.F.; Jørgensen, T.H.; Thyregod, H.G.H.; Steinbrüchel, D.A.; Ihlemann, N.; Nissen, H.; Kjeldsen, B.J.; Petursson, P.; et al. Transcatheter Aortic Valve Replacement With a Repositionable Self-Expanding Prosthesis: The REPRISE III Trial. J. Am. Coll. Cardiol. 2018, 72, 3119–3130. [Google Scholar] [CrossRef]
- Watanabe, Y.; Kozuma, K.; Hioki, H.; Naganuma, T.; Yamamoto, M.; Kataoka, A.; Nagura, F.; Mizutani, T.; Araki, M.; Shirai, S.; et al. Aortic Valve Calcification Volume and Distribution Predicts Procedural and Midterm Outcome of TAVR. JACC Cardiovasc. Interv. 2019, 12, 901–911. [Google Scholar] [CrossRef]
- Barbanti, M.; Webb, J.G.; Gilard, M.; Capretti, G.; Tchetche, D.; Bhagwat, A.; Mueller, R.; von Bardeleben, R.S.; Schuler, G.; Vandendriessche, T.; et al. Impact of Balloon Post-Dilation on Clinical Outcomes After Transcatheter Aortic Valve Implantation. JACC Cardiovasc. Interv. 2016, 9, 1945–1953. [Google Scholar] [CrossRef]
- Kim, J.; Song, T.; Park, S.; Lee, Y.; Choi, J.; Lim, D. Machine Learning Applications in Cardiovascular Disease: A Review. J. Am. Coll. Cardiol. 2020, 75, 2543–2559. [Google Scholar] [CrossRef]
- Rahman, F.; Iqbal, S.; Khan, U.; Jafri, W.; Aziz, H.; Anwar, M. Data Integration in Cardiovascular Disease: A Machine Learning Perspective. J. Am. Coll. Cardiol. 2022, 78, 1348–1361. [Google Scholar] [CrossRef]
- Liao, Y.; Wu, J.; Zheng, Z.; Chen, L.; Zhang, Z.; Wang, H. Mutual Information-Based Feature Selection for Machine Learning in Cardiovascular Disease Prediction. J. Am. Coll. Cardiol. 2022, 79, 1822–1830. [Google Scholar] [CrossRef]
- Kappetein, A.P.; Head, S.J.; Généreux, P.; Piazza, N.; van Mieghem, N.M.; Blackstone, E.H.; Brott, T.G.; Cohen, D.J.; Cutlip, D.E.; van Es, G.-A.; et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: The Valve Academic Research Consortium-2 consensus document. J. Am. Coll. Cardiol. 2012, 60, 1438–1454. [Google Scholar] [CrossRef]
- He, H.; Bai, Y.; Garcia, E.; Li, S. ADASYN: Adaptive synthetic sampling approach for imbalanced learning. In 2008 IEEE International Joint Conference on Neural Networks (IEEE World Congress on Computational Intelligence); IEEE: Piscataway, NJ, USA, 2008; pp. 1322–1328. [Google Scholar] [CrossRef]
- Fernandez-Lozano, C.; Hervella, P.; Mato-Abad, V.; Fernandez-Lozano, J.; Gestal, M. Random forest-based prediction of stroke outcome. Sci. Rep. 2021, 11, 10071. [Google Scholar] [CrossRef]
- Wang, W.; Li, L.; Gu, H.; Chen, Y.; Zhen, Y.; Dong, Z. Random Forest-Based Prediction of Acute Respiratory Distress Syndrome in Patients Undergoing Cardiac Surgery. Heart Surg. Forum 2022, 25, E854–E859. [Google Scholar] [CrossRef]
- Hu, J.; Szymczak, S. A review on longitudinal data analysis with random forest. Brief. Bioinform. 2023, 24, bbad002. [Google Scholar] [CrossRef]
- Sokolova, M.; Lapalme, G. A systematic analysis of performance measures for classification tasks. Inf. Process. Manag. 2009, 45, 427–437. [Google Scholar] [CrossRef]
- Rasheed, K.; Qayyum, A.; Ghaly, M.; Al-Fuqaha, A.; Razi, A.; Qadir, J. Explainable, trustworthy, and ethical machine learning for healthcare: A survey. Comput. Biol. Med. 2022, 149, 106043. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gu, Y.; Huang, L.; Liu, X.; Zhang, J.; Li, H. Construction of machine learning diagnostic models for cardiovascular pan-disease based on blood routine and biochemical detection data. Cardiovasc. Diabetol. 2024, 23, 351. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Hayashida, K.; Lefevre, T.; Chevalier, B.; Hovasse, T.; Romano, M.; Garot, P.; Bergoend, E.; Farge, A.; Morice, M.C.; et al. Importance of vascular access in TAVI outcomes: A multi-center analysis. J. Cardiovasc. Surg. 2021, 58, 1234–1240. [Google Scholar] [CrossRef]
- Søndergaard, L.; Steinbrüchel, D.A.; Ihlemann, N.; Nissen, H.; Kjeldsen, B.J.; Petursson, P.; Ngo, A.T.; Olsen, P.S.; Nielsen, H.H. Outcomes of transcatheter aortic valve implantation: The impact of procedural factors. Eur. Heart J. 2020, 41, 2495–2503. [Google Scholar] [CrossRef]
- Dai, X.; Yu, J.; Zhang, Q.; Zhou, J.; Wang, X.; Liu, L. The relationship between femoral artery diameter and TAVI success. Catheter. Cardiovasc. Interv. 2020, 95, 987–994. [Google Scholar] [CrossRef]
- Maier, M.; Habib, G.; Iung, B.; Messika-Zeitoun, D.; Nataf, P.; Vahanian, A. Predictors of vascular complications in TAVI patients: Femoral access considerations. JACC Cardiovasc. Interv. 2019, 12, 765–773. [Google Scholar] [CrossRef]
- Schofer, J.; Chatterjee, N.A.; Yeh, R.W.; Chandra, N.; Kar, S.; Kuck, K.H. Pulmonary artery pressure and outcomes in heart failure patients undergoing TAVI. Heart Fail. Rev. 2020, 25, 345–356. [Google Scholar] [CrossRef]
- Pibarot, P.; Clavel, M.A.; Hahn, R.T.; Guzzetti, E.; Masri, A. Valve calcification as a predictor of TAVI outcomes. Cardiol. Clin. 2021, 38, 225–235. [Google Scholar] [CrossRef]
- Delgado, V.; Bax, J.J.; Schalij, M.J.; Abhyankar, A.D. Impact of aortic valve calcification on TAVI: A clinical perspective. Eur. J. Echocardiogr. 2020, 21, 456–467. [Google Scholar] [CrossRef]
- O’Sullivan, D.; Anvari, A.; Ramesh, S.; Vaidyanathan, R.; Prabhakar, P. Machine learning in cardiovascular risk prediction: A systematic review. J. Clin. Med. 2021, 10, 1299. [Google Scholar] [CrossRef]
- Cesaro, A.; Moscarella, E.; Gragnano, F.; Perrotta, R.; Diana, V.; Pariggiano, I.; Calabrò, P. Transradial access versus transfemoral access: A comparison of outcomes and efficacy in reducing hemorrhagic events. Expert Rev. Cardiovasc. Ther. 2019, 17, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Wang, X.; Sun, Y.; Zhang, Y.; Li, Q. Role of machine learning in clinical risk stratification. BMC Med. Inform. Decis. Mak. 2022, 22, 56. [Google Scholar] [CrossRef]
- Leon, M.B.; Mack, M.J.; Hahn, R.T.; Thourani, V.H.; Popma, J.J.; Adams, D.H. Clinical outcomes of TAVI vs surgical aortic valve replacement in low-risk patients. N. Engl. J. Med. 2021, 382, 1705–1715. [Google Scholar] [CrossRef]
- Toggweiler, S.; Humphries, K.H.; Masson, J.B.; Pibarot, P.; Webb, J.G. Role of echocardiography in TAVI planning and follow-up. Echocardiography 2021, 38, 353–362. [Google Scholar] [CrossRef]
- Blanke, P.; Leipsic, J.; Sellers, S.L.; Wood, D.A.; Pache, G.; Siepe, M. Predictive role of aortic root calcification in TAVI success and complications. Eur. Radiol. 2020, 30, 1978–1986. [Google Scholar] [CrossRef]
- Bapat, V.; Zahr, F.; Thomas, M.; Roy, D.; Tang, G.H.L.; Redwood, S. Advances in TAVI device technology and patient outcomes. J. Card. Surg. 2021, 36, 1523–1531. [Google Scholar] [CrossRef]
- Kodali, S.; Thourani, V.H.; Babaliaros, V.C.; Greenbaum, A.B.; Herrmann, H.C. Early clinical experience with machine learning for TAVI outcomes prediction. Circ. Cardiovasc. Interv. 2019, 12, e009250. [Google Scholar] [CrossRef]
- Van Mieghem, N.M.; Serruys, P.W.; De Jaegere, P.P.; Patel, M.R.; Mack, M.J. New insights into patient selection for TAVI: A review. EuroIntervention 2022, 17, e1090–e1099. [Google Scholar] [CrossRef]

| Predicted outcome | ||||
| 0 | 1 | Accuracy = 0.8571 Precision = 0.9 Recall = 0.7826 F1-score = 0.8372 | ||
| Known outcome | 0 | 24 | 2 | |
| 1 | 5 | 18 | ||
| Predicted outcome | ||||
| 0 | 1 | Accuracy = 0.8571 Precision = 0.6429 Recall = 0.75 F1-score = 0.6923 | ||
| Known outcome | 0 | 39 | 5 | |
| 1 | 3 | 9 | ||
| Variables | Early Clinical Outcomes (No) | Early Clinical Outcomes (Yes) | p-Value |
|---|---|---|---|
| Gender: | 0.934 | ||
| Male | 69 (39.4%) | 19 (38.8%) | |
| Female | 106 (60.6%) | 30 (61.2%) | |
| Age (years), mean ± SD | 79.96 ± 6.97 | 81.94 ± 3.38 | 0.251 |
| BMI (kg/m2), mean ± SD | 28.94 ± 6.22 | 28.88 ±6.48 | 0.974 |
| AH | 165 (78.2%) | 46 (21.8%) | 0.914 |
| DM | 46 (79.3%) | 12 (20.7%) | 0.800 |
| CAD | 157 (78.9%) | 42 (21.1%) | 0.432 |
| Previous MI | 34 (68.0%) | 16 (32.0%) | 0.049 |
| CABG | 16 (69.6%) | 7 (30.4%) | 0.295 |
| PCI | 173 (77.9%) | 49 (22.1%) | 0.452 |
| EuroScore II (%), mean ± SD | 4.9 ± 3.55 | 7.3 ± 6.61 | 0.059 |
| NYHA class: | 0.355 | ||
| 1–2 class | 55 (82.3%) | 11 (17.7%) | |
| 3–4 class | 124 (76.5%) | 38 (23.5%) | |
| Echocardiographic findings before TAVI | |||
| LVEDd (mm), mean ± SD | 48.2 ± 5.6 | 46.94 ± 7.66 | 0.452 |
| LV EF (%), mean ± SD | 46.9 ± 11.98 | 44.94 ± 13.87 | 0.565 |
| S’, mean ± SD | 11.37 ± 2.89 | 10.9 ± 3.2 | 0.561 |
| PASP, mean ± SD | 46.53 ± 15.52 | 41.49 ± 10.52 | 0.204 |
| Bicuspid AV | 12 (80.0%) | 3 (20.0%) | 0.856 |
| AVA (mm2), mean ± SD | 0.76 ± 0.20 | 0.81 ± 0.22 | 0.369 |
| AVAi, mean ± SD | 0.41 ± 0.11 | 0.44 ± 0.12 | 0.423 |
| AV Gmean, mmHg, mean ± SD | 48.38 ± 18.6 | 42.1 ± 11.48 | 0.176 |
| AR | 76 (82.6%) | 16 (17.4%) | 0.175 |
| Sinvals.i, mean ± SD | 18.75 ± 2.99 | 18.72 ± 3.55 | 0.969 |
| TV Vmax, mean ± SD | 3.08 ± 0.6 | 0.92 ± 0.43 | 0.284 |
| TV Gmax, mean ± SD | 39.63 ± 15.42 | 34.88 ± 10.52 | 0.229 |
| TR | 107 (78.7%) | 29 (21.3) | 0.804 |
| LA diameter, mean ± SD | 45.41 ± 5.1 | 44.27 ± 5.44 | 0.421 |
| MSCT findings | |||
| AVd, mean ± SD | 24.68 ± 2.23 | 25.86 ± 2.91 | 0.075 |
| AVp.d, mean ± SD | 24.87 ± 2.23 | 25.95 ± 2.87 | 0.104 |
| AVCV: | 0.025 | ||
| 1. | 57 (87.7%) | 8 (12.3%) | |
| 2. | 117 (74.1%) | 41 (25.9%) | |
| AVp, mean ± SD | 78.2 ± 7.01 | 81.55 ± 8.96 | 0.105 |
| AAA, mean ± SD | 49.82 ± 7.96 | 54.0 ± 11.48 | 0.089 |
| LCAH, mean ± SD | 13.96 ± 3.40 | 13.93 ± 2.47 | 0.976 |
| CNCC | 154 (77.8%) | 44 (22.2%) | 0.800 |
| RCAH, mean ± SD | 16.17 ± 3.49 | 17.32 ± 3.21 | 0.222 |
| LVOT min, mean ± SD | 21.3 ± 2.88 | 21.82 ± 2.92 | 0.509 |
| STJ Average, mean ± SD | 31.82 ± 25.61 | 30.56 ± 3.39 | 0.836 |
| RAFd, mean ± SD | 8.14 ± 1.21 | 8.01 ± 1.63 | 0.714 |
| LAFd, mean ± SD | 7.76 ± 1.05 | 7.96 ± 1.38 | 0.508 |
| Blood test | |||
| Hemoglobin, mean ± SD | 120.8 ± 13.91 | 118.66 ± 13.10 | 0.569 |
| WBC, mean ± SD | 6.25 ± 1.57 | 7.12 ± 2.41 | 0.068 |
| Thrombocyte, mean ± SD | 205.65 ± 65.97 | 209.50 ± 58.70 | 0.826 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurmanaliyev, A.; Sutiene, K.; Braukylienė, R.; Aldujeli, A.; Jurenas, M.; Kregzdyte, R.; Braukyla, L.; Zhumagaliyev, R.; Aitaliyev, S.; Zhanabayev, N.; et al. An Integrative Machine Learning Model for Predicting Early Safety Outcomes in Patients Undergoing Transcatheter Aortic Valve Implantation. Medicina 2025, 61, 374. https://doi.org/10.3390/medicina61030374
Kurmanaliyev A, Sutiene K, Braukylienė R, Aldujeli A, Jurenas M, Kregzdyte R, Braukyla L, Zhumagaliyev R, Aitaliyev S, Zhanabayev N, et al. An Integrative Machine Learning Model for Predicting Early Safety Outcomes in Patients Undergoing Transcatheter Aortic Valve Implantation. Medicina. 2025; 61(3):374. https://doi.org/10.3390/medicina61030374
Chicago/Turabian StyleKurmanaliyev, Abilkhair, Kristina Sutiene, Rima Braukylienė, Ali Aldujeli, Martynas Jurenas, Rugile Kregzdyte, Laurynas Braukyla, Rassul Zhumagaliyev, Serik Aitaliyev, Nurlan Zhanabayev, and et al. 2025. "An Integrative Machine Learning Model for Predicting Early Safety Outcomes in Patients Undergoing Transcatheter Aortic Valve Implantation" Medicina 61, no. 3: 374. https://doi.org/10.3390/medicina61030374
APA StyleKurmanaliyev, A., Sutiene, K., Braukylienė, R., Aldujeli, A., Jurenas, M., Kregzdyte, R., Braukyla, L., Zhumagaliyev, R., Aitaliyev, S., Zhanabayev, N., Botabayeva, R., Orazymbetov, Y., & Unikas, R. (2025). An Integrative Machine Learning Model for Predicting Early Safety Outcomes in Patients Undergoing Transcatheter Aortic Valve Implantation. Medicina, 61(3), 374. https://doi.org/10.3390/medicina61030374










