Immunological Landscape of Non-Melanoma Skin Neoplasms: Role of CTLA4+IFN-γ+ Lymphocytes in Tumor Microenvironment Suppression
Abstract
1. Introduction
2. Materials and Methods
2.1. Immunohistological Protocol and Microscopy
2.2. Multichannel Immunofluorescence (IF) Protocol and Microscopy
2.3. Statistical Analysis
3. Results
3.1. Epidemiological Characteristics of Cohorts and Pathohistological Characteristics of Keratoacanthoma (KA)
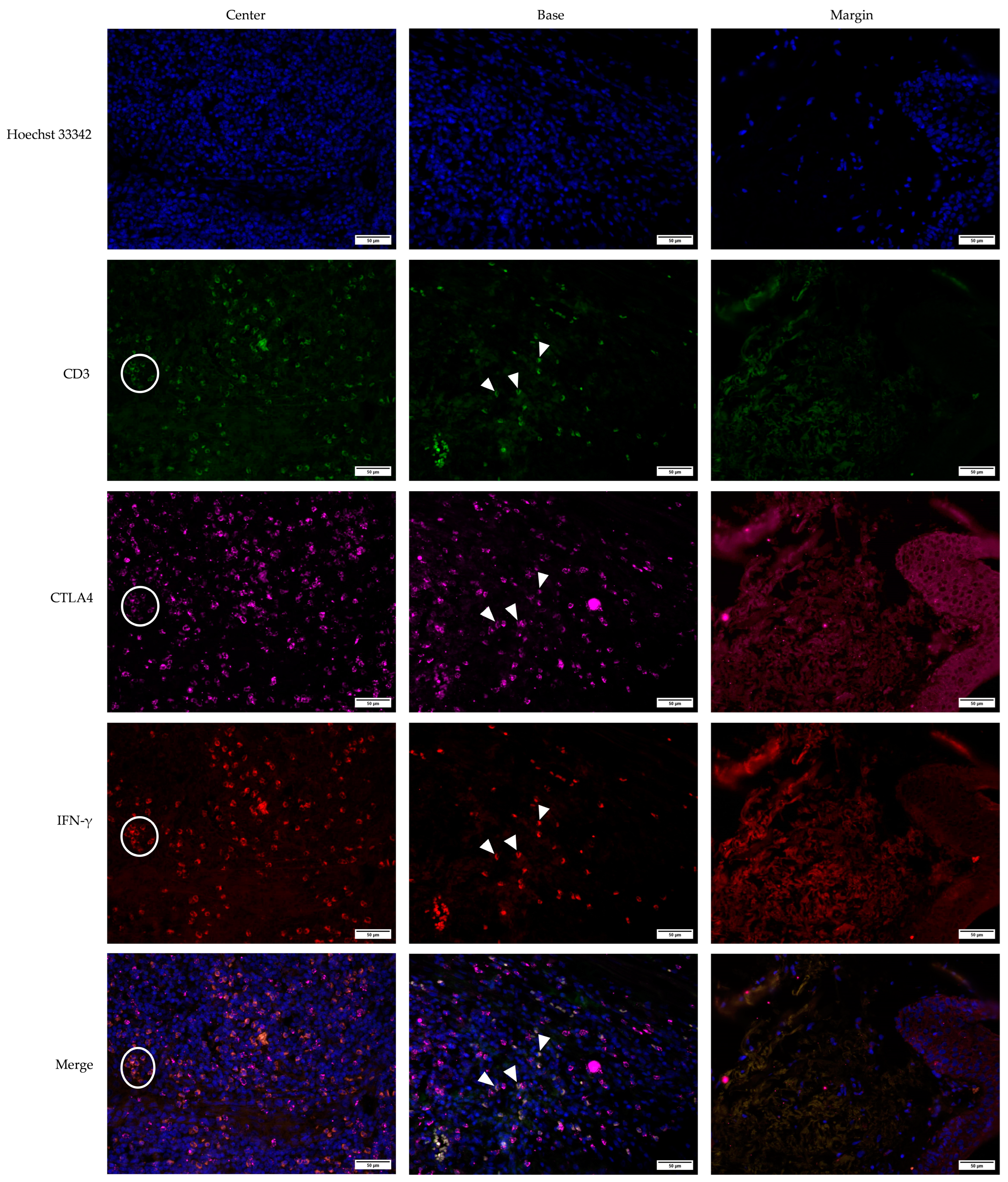
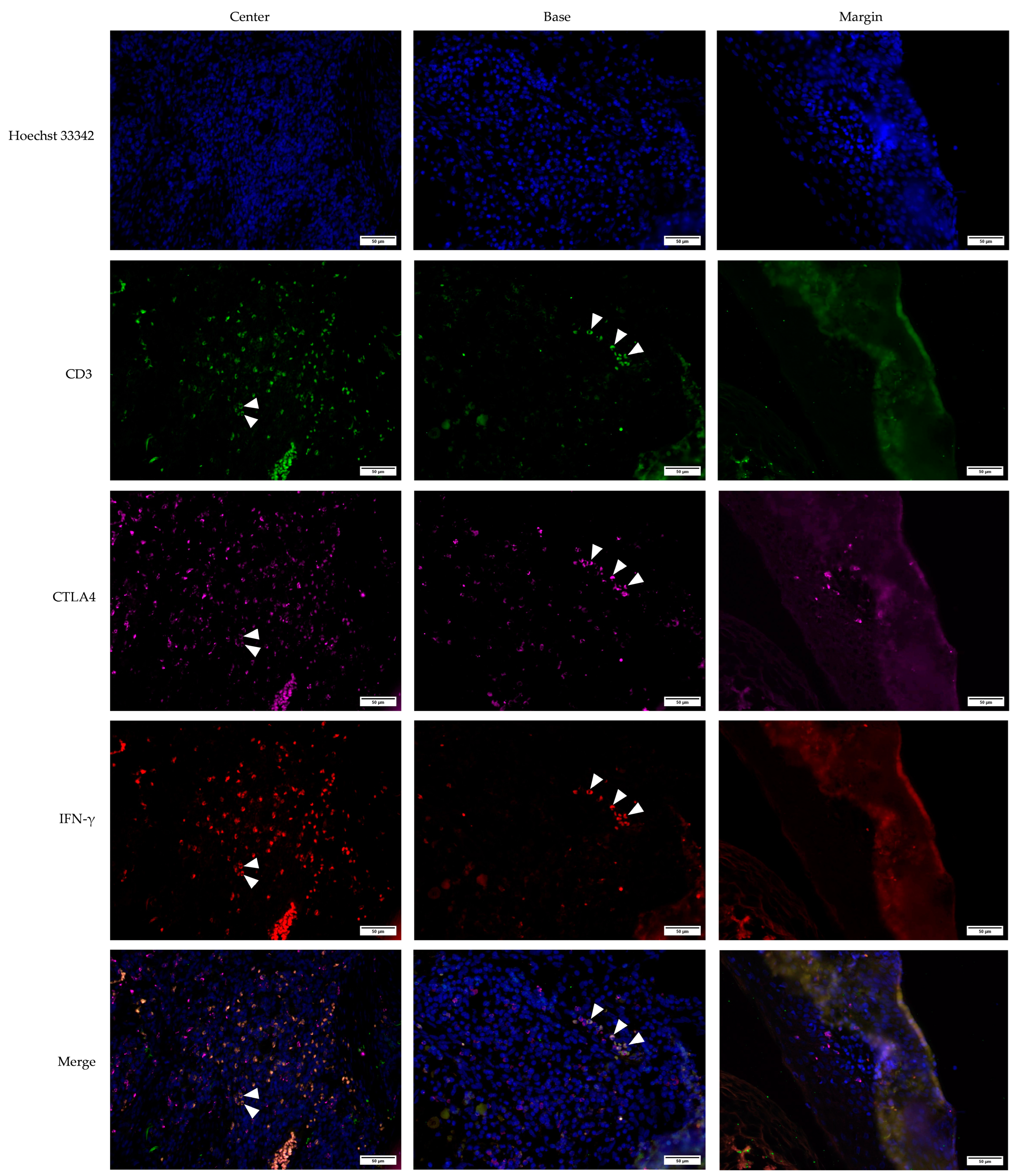
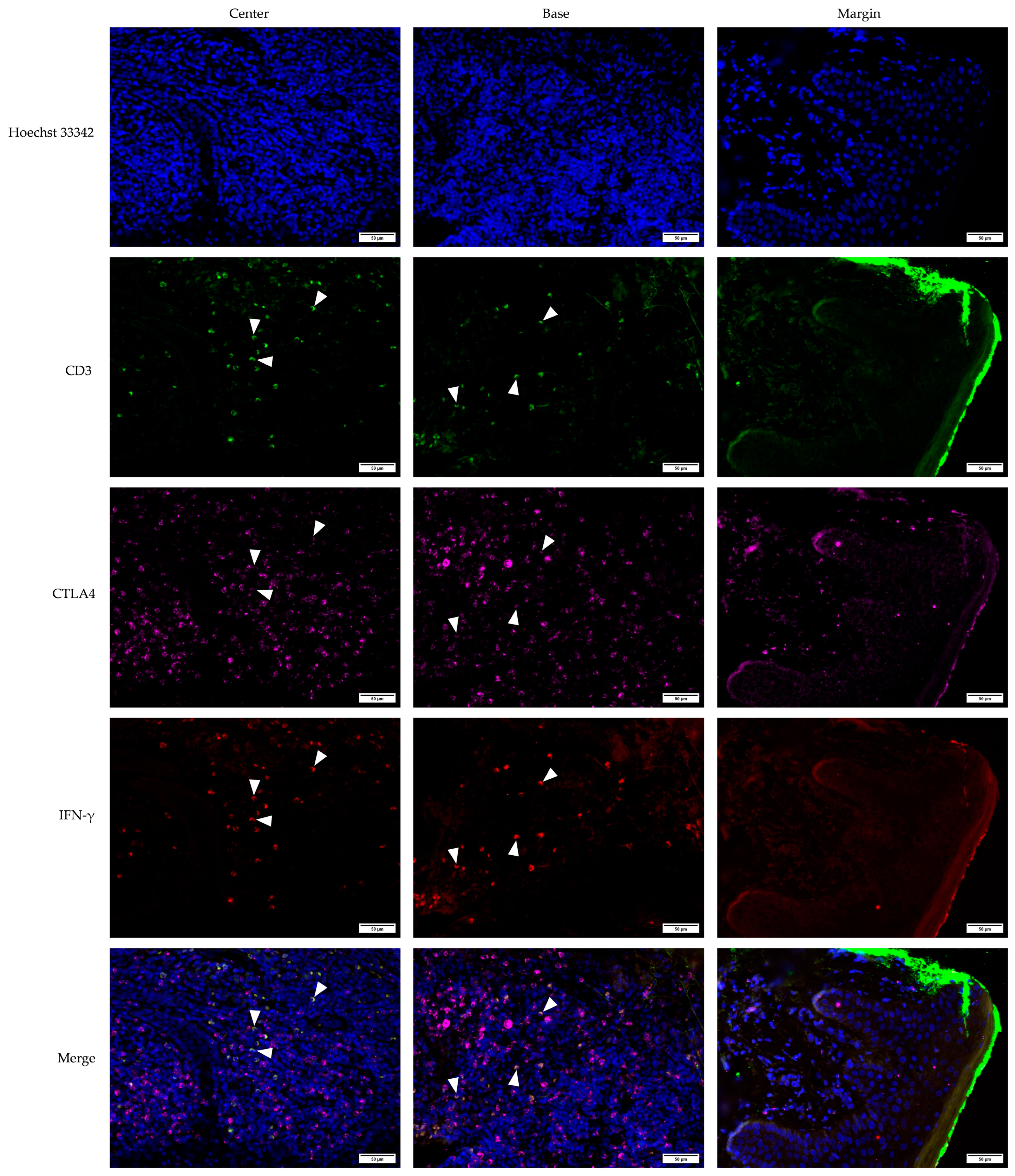
3.2. Cross-Sectional CTLA4 and IFN-γ Immunofluorescent Staining of KA, SCC, and VV
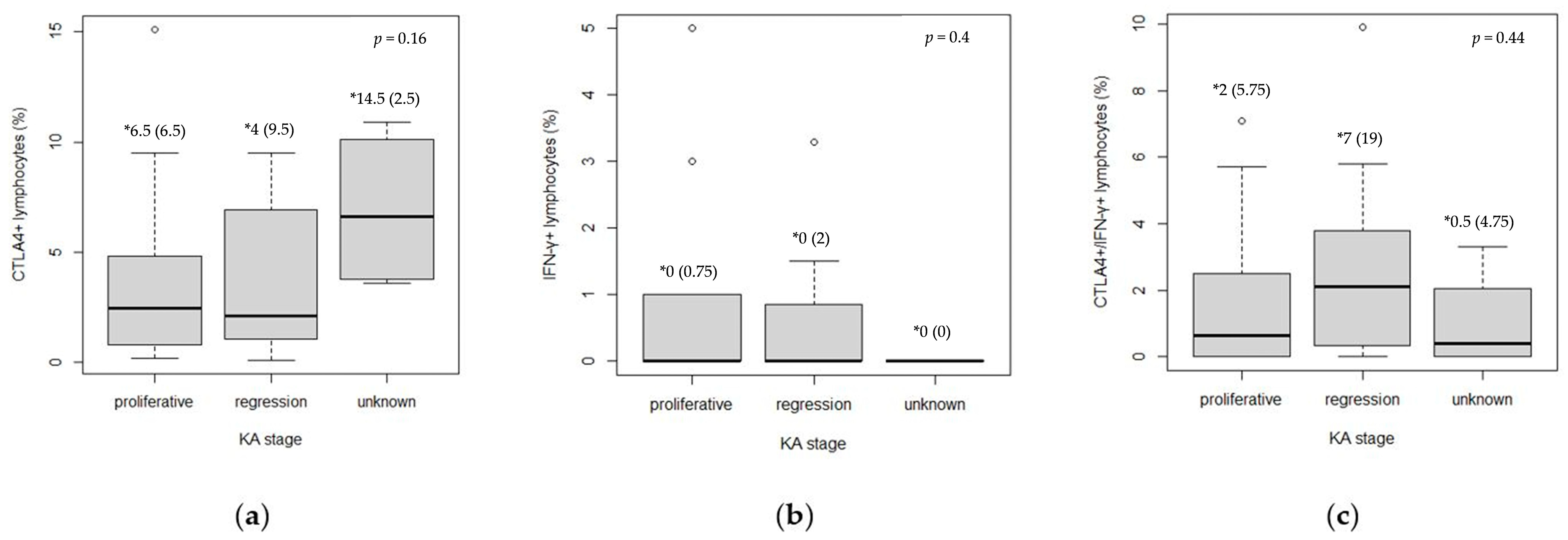
3.3. Analysis of CTLA4 and IFN-γ Expression in Keratoacanthoma
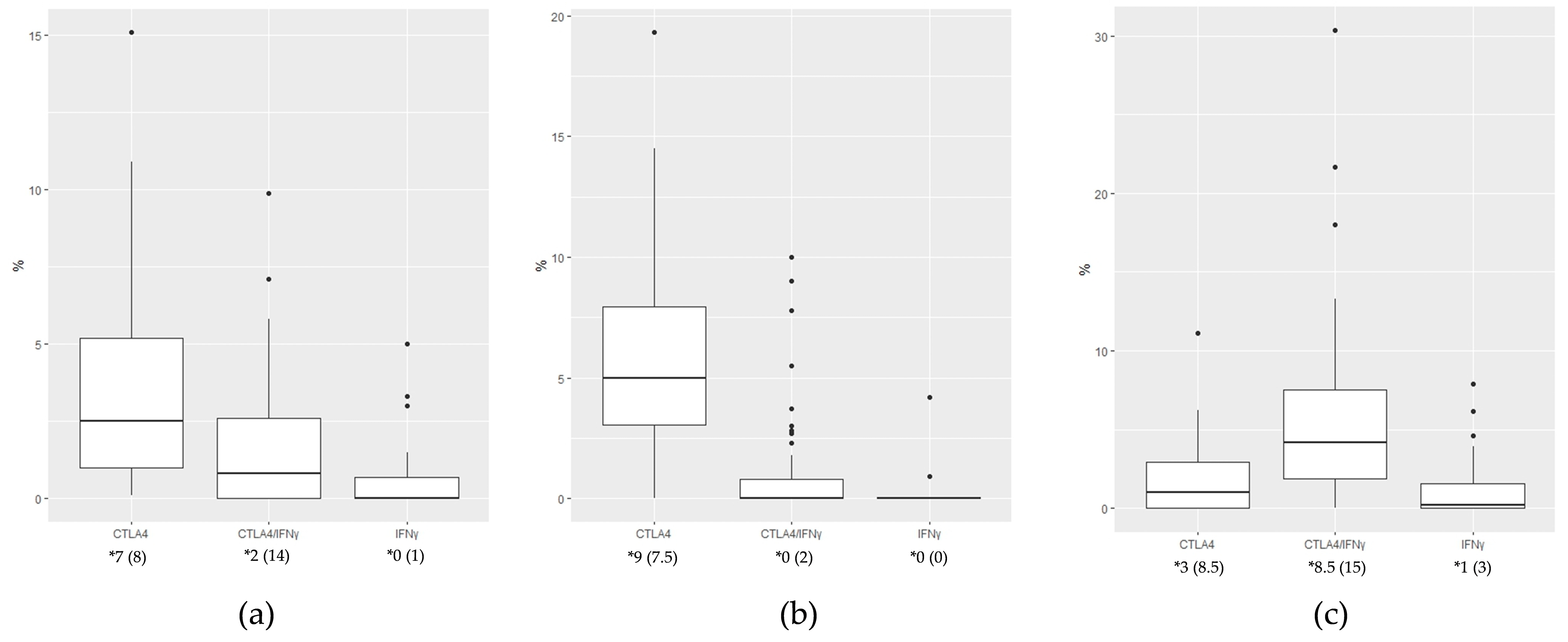
3.4. Expression of CTLA4+, IFN-γ+, and CTLA4+IFN-γ+ Cells Between KA, SCC, and VV Tumor Microenvironments
3.5. Defining CTLA4+, IFN-γ+, and CTLA4+IFN-γ+ Cells Using the CD3 Marker for T Lymphocytes in KA, SCC, and, VV Tumor Microenvironments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Small, J.; Barton, V.; Peterson, B.; Alberg, A.J. Keratinocyte Carcinoma as a Marker of a High Cancer-Risk Phenotype. Adv. Cancer Res. 2016, 130, 257–291. [Google Scholar] [CrossRef] [PubMed]
- Brantsch, K.D.; Meisner, C.; Schönfisch, B.; Trilling, B.; Wehner-Caroli, J.; Röcken, M.; Breuninger, H. Analysis of Risk Factors Determining Prognosis of Cutaneous Squamous-Cell Carcinoma: A Prospective Study. Lancet Oncol. 2008, 9, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Eisemann, N.; Waldmann, A.; Geller, A.C.; Weinstock, M.A.; Volkmer, B.; Greinert, R.; Breitbart, E.W.; Katalinic, A. Non-Melanoma Skin Cancer Incidence and Impact of Skin Cancer Screening on Incidence. J. Investig. Dermatol. 2014, 134, 43–50. [Google Scholar] [CrossRef]
- Schmitz, L.; Kanitakis, J. Histological Classification of Cutaneous Squamous Cell Carcinomas with Different Severity. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 11–15. [Google Scholar] [CrossRef]
- Que, S.K.T.; Zwald, F.O.; Schmults, C.D. Cutaneous Squamous Cell Carcinoma. J. Am. Acad. Dermatol. 2018, 78, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Yus, E.S.; Simón, P.; Requena, L.; Ambrojo, P.; de Eusebio, E. Solitary Keratoacanthoma. Am. J. Dermatopathol. 2000, 22, 305–310. [Google Scholar] [CrossRef]
- Ogita, A.; Ansai, S. What Is a Solitary Keratoacanthoma? A Benign Follicular Neoplasm, Frequently Associated with Squamous Cell Carcinoma. Diagnostics 2021, 11, 1848. [Google Scholar] [CrossRef]
- Patil, S. Tumor Immunotherapy—A Lot to Learn from Keratoacanthoma. Med. Hypotheses 2020, 141, 109719. [Google Scholar] [CrossRef]
- Elder, D.E.; Massi, D.; Scolyer, R.A.; Willemze, R. WHO Classification of Skin Tumours, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2018. [Google Scholar]
- Kwiek, B.; Schwartz, R.A. Keratoacanthoma (KA): An Update and Review. J. Am. Acad. Dermatol. 2016, 74, 1220–1233. [Google Scholar] [CrossRef]
- Forslund, O.; DeAngelis, P.M.; Beigi, M.; Schjølberg, A.R.; Clausen, O.P.F. Identification of Human Papillomavirus in Keratoacanthomas. J. Cutan. Pathol. 2003, 30, 423–429. [Google Scholar] [CrossRef]
- Ekström, J.; Mühr, L.S.A.; Bzhalava, D.; Söderlund-Strand, A.; Hultin, E.; Nordin, P.; Stenquist, B.; Paoli, J.; Forslund, O.; Dillner, J. Diversity of Human Papillomaviruses in Skin Lesions. Virology 2013, 447, 300–311. [Google Scholar] [CrossRef]
- Ekström, J.; Bzhalava, D.; Svenback, D.; Forslund, O.; Dillner, J. High Throughput Sequencing Reveals Diversity of Human Papillomaviruses in Cutaneous Lesions. Int. J. Cancer 2011, 129, 2643–2650. [Google Scholar] [CrossRef]
- Arroyo Mühr, L.S.; Hultin, E.; Bzhalava, D.; Eklund, C.; Lagheden, C.; Ekström, J.; Johansson, H.; Forslund, O.; Dillner, J. Human Papillomavirus Type 197 Is Commonly Present in Skin Tumors. Int. J. Cancer 2015, 136, 2546–2555. [Google Scholar] [CrossRef]
- Karagas, M.R.; Waterboer, T.; Li, Z.; Nelson, H.H.; Michael, K.M.; Bavinck, J.N.B.; Perry, A.E.; Spencer, S.K.; Daling, J.; Green, A.C.; et al. Genus Human Papillomaviruses and Incidence of Basal Cell and Squamous Cell Carcinomas of Skin: Population Based Case-Control Study. BMJ 2010, 341, c2986. [Google Scholar] [CrossRef]
- Viarisio, D.; Mueller-Decker, K.; Kloz, U.; Aengeneyndt, B.; Kopp-Schneider, A.; Gröne, H.-J.; Gheit, T.; Flechtenmacher, C.; Gissmann, L.; Tommasino, M. E6 and E7 from Beta Hpv38 Cooperate with Ultraviolet Light in the Development of Actinic Keratosis-Like Lesions and Squamous Cell Carcinoma in Mice. PLoS Pathog. 2011, 7, e1002125. [Google Scholar] [CrossRef]
- Viarisio, D.; Müller-Decker, K.; Accardi, R.; Robitaille, A.; Dürst, M.; Beer, K.; Jansen, L.; Flechtenmacher, C.; Bozza, M.; Harbottle, R.; et al. Beta HPV38 Oncoproteins Act with a Hit-and-Run Mechanism in Ultraviolet Radiation-Induced Skin Carcinogenesis in Mice. PLoS Pathog. 2018, 14, e1006783. [Google Scholar] [CrossRef]
- Plasencia, J.M. Cutaneous warts: Diagnosis and treatment. Prim. Care Clin. Off. Pract. 2000, 27, 423–434. [Google Scholar] [CrossRef]
- Bacelieri, R.; Johnson, S.M. Cutaneous Warts: An Evidence-Based Approach to Therapy. Am. Fam. Physician 2005, 72, 647–652. [Google Scholar]
- Gogia, P.; Thami, G.P.; Poonia, K.; Bhalla, M.; Garg, G. Efficacy of Tuberculin Immunotherapy in Verruca Vulgaris: Our Experience from Single Center from North-West India and Review of Literature. Dermatol. Ther. 2021, 34, e14843. [Google Scholar] [CrossRef]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef]
- Fakhry, C.; Westra, W.H.; Li, S.; Cmelak, A.; Ridge, J.A.; Pinto, H.; Forastiere, A.; Gillison, M.L. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J. Natl. Cancer Ins. 2008, 100, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Albers, A.E.; Qian, X.; Kaufmann, A.M.; Coordes, A. Meta analysis: HPV and p16 pattern determine survival in patients with HNSCC and identifies potential new biologic subtype. Sci. Rep. 2017, 7, 16715. [Google Scholar] [CrossRef]
- Lechien, J.R.; Descamps, G.; Seminerio, I.; Furgiuele, S.; Dequanter, D.; Mouawad, F.; Badoual, C.; Journe, F.; Saussez, S. HPV Involvement in the Tumor Microenvironment and Immune Treatment in Head and Neck Squamous Cell Carcinomas. Cancers 2020, 12, 1060. [Google Scholar] [CrossRef]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 Pathways. Am. J. Clin. Oncol. 2016, 39, 98–106. [Google Scholar] [CrossRef]
- Strickley, J.D.; Messerschmidt, J.L.; Awad, M.E.; Li, T.; Hasegawa, T.; Ha, D.T.; Nabeta, H.W.; Bevins, P.A.; Ngo, K.H.; Asgari, M.M.; et al. Immunity to commensal papillomaviruses protects against skin cancer. Nature 2019, 575, 519–522. [Google Scholar] [CrossRef]
- Anderson, N.M.; Simon, M.C. The Tumor Microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Chiang, E.; Stafford, H.; Buell, J.; Ramesh, U.; Amit, M.; Nagarajan, P.; Migden, M.; Yaniv, D. Review of the Tumor Microenvironment in Basal and Squamous Cell Carcinoma. Cancers 2023, 15, 2453. [Google Scholar] [CrossRef]
- Schwarz, A.; Beissert, S.; Grosse-Heitmeyer, K.; Gunzer, M.; Bluestone, J.A.; Grabbe, S.; Schwarz, T. Evidence for Functional Relevance of CTLA-4 in Ultraviolet-Radiation-Induced Tolerance. J. Immunol. 2000, 165, 1824–1831. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, J.; Song, Q.; Sun, X.; Xue, M.; Yang, Z.; Shang, J. Comprehensive Analysis of CTLA-4 in the Tumor Immune Microenvironment of 33 Cancer Types. Int. Immunopharmacol. 2020, 85, 106633. [Google Scholar] [CrossRef]
- Hosseini, A.; Gharibi, T.; Marofi, F.; Babaloo, Z.; Baradaran, B. CTLA-4: From Mechanism to Autoimmune Therapy. Int. Immunopharmacol. 2020, 80, 106221. [Google Scholar] [CrossRef]
- Van Coillie, S.; Wiernicki, B.; Xu, J. Molecular and Cellular Functions of CTLA-4. Adv. Exp. Med. Biol. 2020, 1248, 7–32. [Google Scholar] [CrossRef] [PubMed]
- Mojic, M.; Takeda, K.; Hayakawa, Y. The Dark Side of IFN-γ: Its Role in Promoting Cancer Immunoevasion. Int. J. Mol. Sci. 2017, 19, 89. [Google Scholar] [CrossRef] [PubMed]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-γ in Tumor Progression and Regression: A Review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef]
- Bayer-Garner, I.B.; Ivan, D.; Schwartz, M.R.; Tschen, J.A. The Immunopathology of Regression in Benign Lichenoid Keratosis, Keratoacanthoma and Halo Nevus. Clin. Med. Res. 2004, 2, 89–97. [Google Scholar] [CrossRef][Green Version]
- Savage, J.A.; Maize, J.C. Keratoacanthoma Clinical Behavior. Am. J. Dermatopathol. 2014, 36, 422–429. [Google Scholar] [CrossRef]
- Walunas, T.L.; Lenschow, D.J.; Bakker, C.Y.; Linsley, P.S.; Freeman, G.J.; Green, J.M.; Thompson, C.B.; Bluestone, J.A. CTLA-4 Can Function as a Negative Regulator of T Cell Activation. Immunity 1994, 1, 405–413. [Google Scholar] [CrossRef]
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef]
- Sobhani, N.; Tardiel-Cyril, D.R.; Davtyan, A.; Generali, D.; Roudi, R.; Li, Y. CTLA-4 in Regulatory T Cells for Cancer Immunotherapy. Cancers 2021, 13, 1440. [Google Scholar] [CrossRef]
- Fuertes Marraco, S.A.; Neubert, N.J.; Verdeil, G.; Speiser, D.E. Inhibitory Receptors Beyond T Cell Exhaustion. Front. Immunol. 2015, 6, 310. [Google Scholar] [CrossRef]
- Oyewole-Said, D.; Konduri, V.; Vazquez-Perez, J.; Weldon, S.A.; Levitt, J.M.; Decker, W.K. Beyond T-Cells: Functional Characterization of CTLA-4 Expression in Immune and Non-Immune Cell Types. Front. Immunol. 2020, 11, 608024. [Google Scholar] [CrossRef]
- Boulch, M.; Cazaux, M.; Cuffel, A.; Guerin, M.V.; Garcia, Z.; Alonso, R.; Lemaître, F.; Beer, A.; Corre, B.; Menger, L.; et al. Tumor-Intrinsic Sensitivity to the pro-Apoptotic Effects of IFN-γ Is a Major Determinant of CD4+ CAR T-Cell Antitumor Activity. Nat. Cancer 2023, 4, 968–983. [Google Scholar] [CrossRef]
- Moon, J.W.; Kong, S.-K.; Kim, B.S.; Kim, H.J.; Lim, H.; Noh, K.; Kim, Y.; Choi, J.-W.; Lee, J.-H.; Kim, Y.-S. IFNγ Induces PD-L1 Overexpression by JAK2/STAT1/IRF-1 Signaling in EBV-Positive Gastric Carcinoma. Sci. Rep. 2017, 7, 17810. [Google Scholar] [CrossRef] [PubMed]
- Gocher, A.M.; Workman, C.J.; Vignali, D.A.A. Interferon-γ: Teammate or Opponent in the Tumour Microenvironment? Nat. Rev. Immunol. 2022, 22, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Rothenberger, N.; Somasundaram, A.; Stabile, L. The Role of the Estrogen Pathway in the Tumor Microenvironment. Int. J. Mol. Sci. 2018, 19, 611. [Google Scholar] [CrossRef] [PubMed]
- Lang, T.J. Estrogen as an Immunomodulator. Clin. Immunol. 2004, 113, 224–230. [Google Scholar] [CrossRef]
- Sandmand, M.; Bruunsgaard, H.; Kemp, K.; Andersen-Ranberg, K.; Pedersen, A.N.; Skinhøj, P. Is Ageing Associated with a Shift in the Balance between Type 1 and Type 2 Cytokines in Humans? Clin. Exp. Immunol. 2002, 127, 107–114. [Google Scholar] [CrossRef]
- Alberti, S.; Cevenini, E.; Ostan, R.; Capri, M.; Salvioli, S.; Bucci, L.; Ginaldi, L.; De Martinis, M.; Franceschi, C.; Monti, D. Age-Dependent Modifications of Type 1 and Type 2 Cytokines within Virgin and Memory CD4+ T Cells in Humans. Mech. Ageing Dev. 2006, 127, 560–566. [Google Scholar] [CrossRef]
- Kirk, A.; Graham, S.V. The human papillomavirus late life cycle and links to keratinocyte differentiation. J. Med. Virol. 2024, 96, e29461. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, L.; Song, Y.; Ma, L.; Xiao, P.; Chen, L.; Zhen, H.; Han, R.; Chen, X.; Sun, S.; et al. Induction of co-inhibitory molecule CTLA-4 by human papillomavirus E7 protein through downregulation of histone methyltransferase JHDM1B expression. Virology 2019, 538, 111–118. [Google Scholar] [CrossRef]
- Dum, D.; Henke, T.L.; Mandelkow, T.; Yang, C.; Bady, E.; Raedler, J.B.; Simon, R.; Sauter, G.; Lennartz, M.; Büscheck, F.; et al. Semi-automated validation and quantification of CTLA-4 in 90 different tumor entities using multiple antibodies and artificial intelligence. Lab. Investig. 2022, 102, 650–657. [Google Scholar] [CrossRef]
- Pandiyan, P.; Hegel, J.K.E.; Krueger, M.; Quandt, D.; Brunner-Weinzierl, M.C. High IFN-γ Production of Individual CD8 T Lymphocytes Is Controlled by CD152 (CTLA-4). J. Immunol. 2007, 178, 2132–2140. [Google Scholar] [CrossRef] [PubMed]
- Pai, C.-C.S.; Huang, J.T.; Lu, X.; Simons, D.M.; Park, C.; Chang, A.; Tamaki, W.; Liu, E.; Roybal, K.T.; Seagal, J.; et al. Clonal Deletion of Tumor-Specific T Cells by Interferon-γ Confers Therapeutic Resistance to Combination Immune Checkpoint Blockade. Immunity 2019, 50, 477–492.e8. [Google Scholar] [CrossRef] [PubMed]
- Ivanović, T.; Božić, D.; Benzon, B.; Čapkun, V.; Vukojević, K.; Glavina Durdov, M. Histological Type, Cytotoxic T Cells and Macrophages in the Tumor Microenvironment Affect the PD-L1 Status of Gastric Cancer. Biomedicines 2023, 11, 709. [Google Scholar] [CrossRef]
- Meng, L.; Wu, H.; Wu, J.; Ding, P.; He, J.; Sang, M.; Liu, L. Mechanisms of immune checkpoint inhibitors: Insights into the regulation of circular RNAS involved in cancer hallmarks. Cell Death Dis. 2024, 15, 3. [Google Scholar] [CrossRef]
- Cranmer, L.D.; Hersh, E. The role of the CTLA4 blockade in the treatment of malignant melanoma. Cancer Investig. 2007, 25, 613–631. [Google Scholar] [CrossRef]
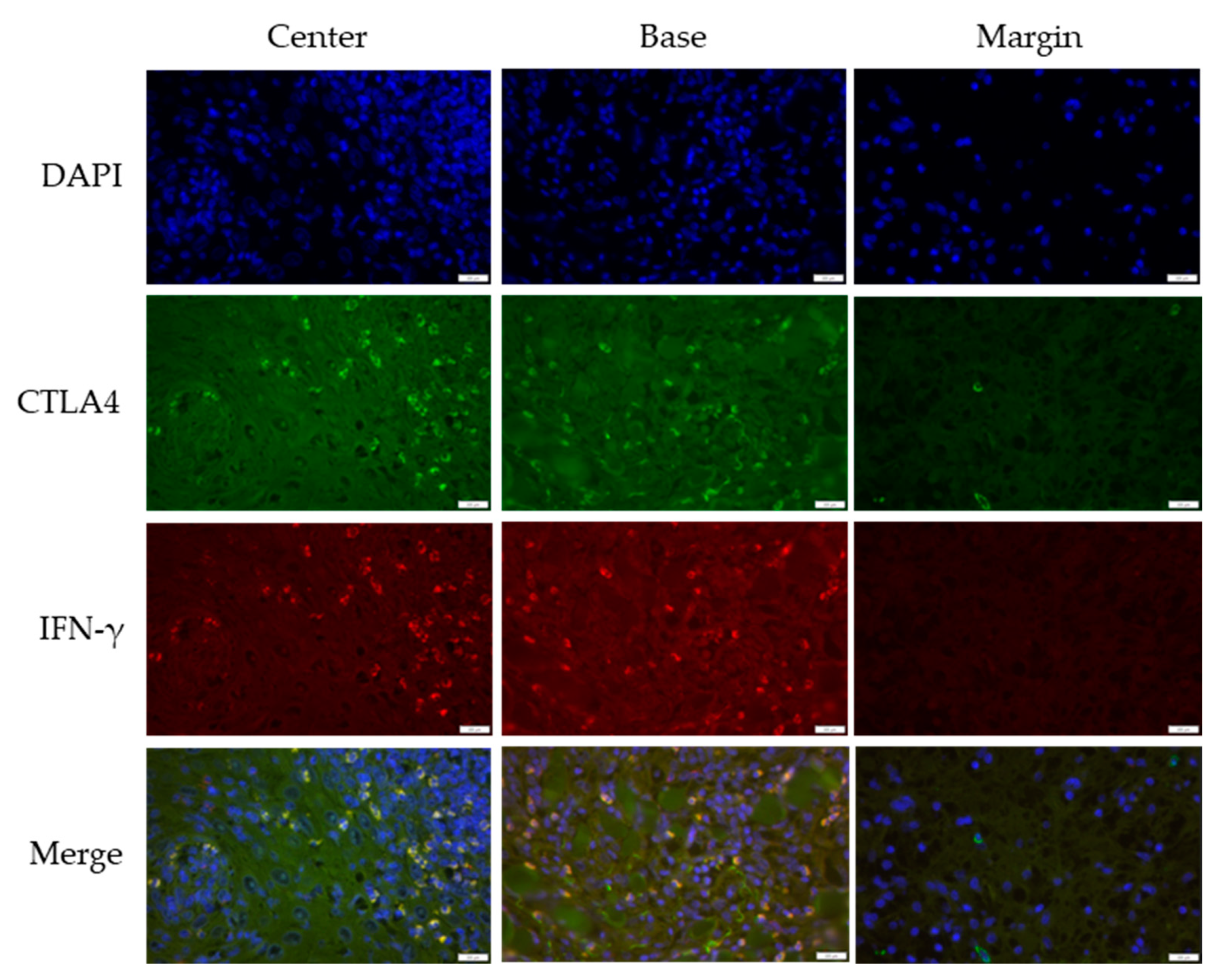
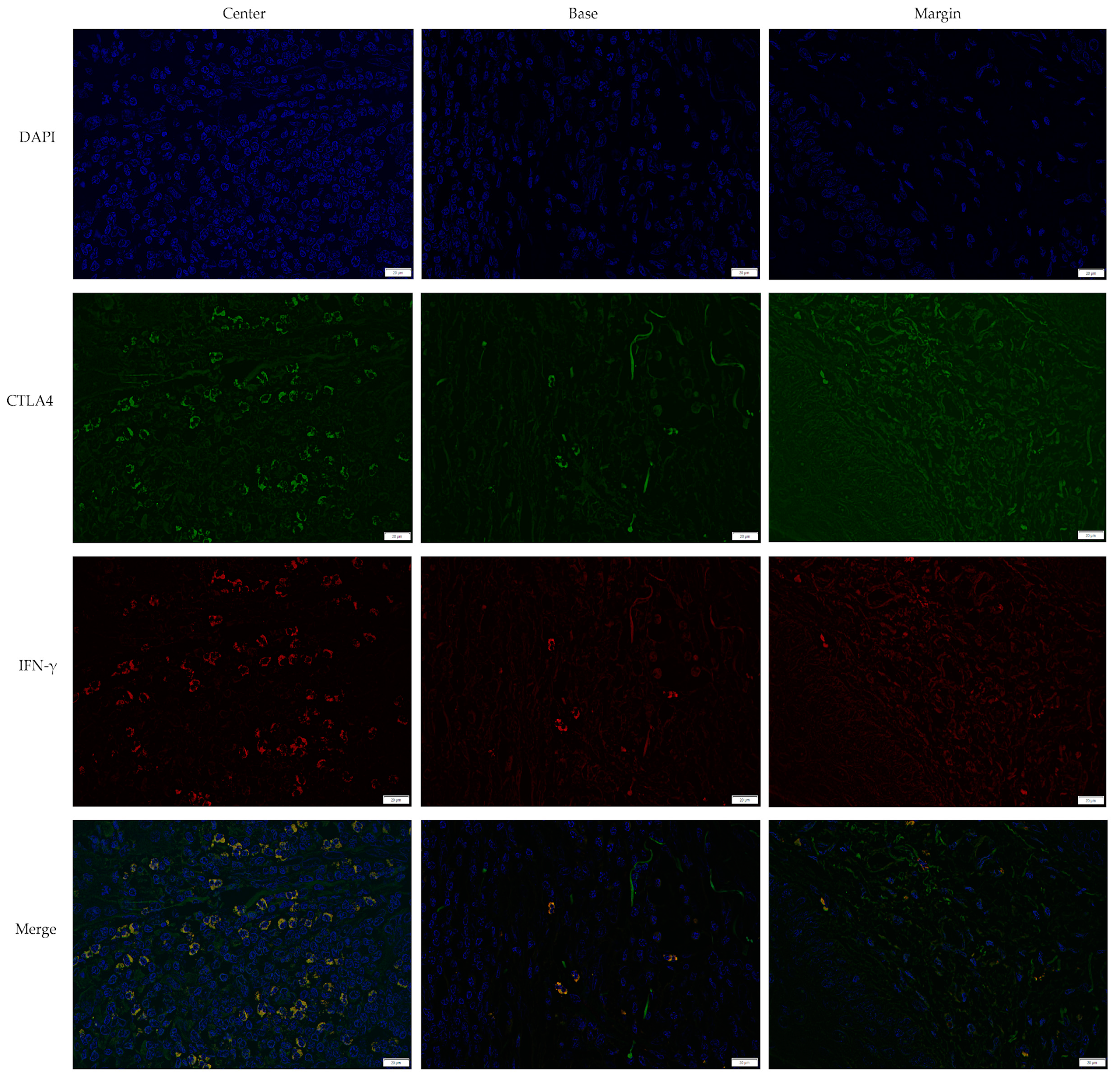
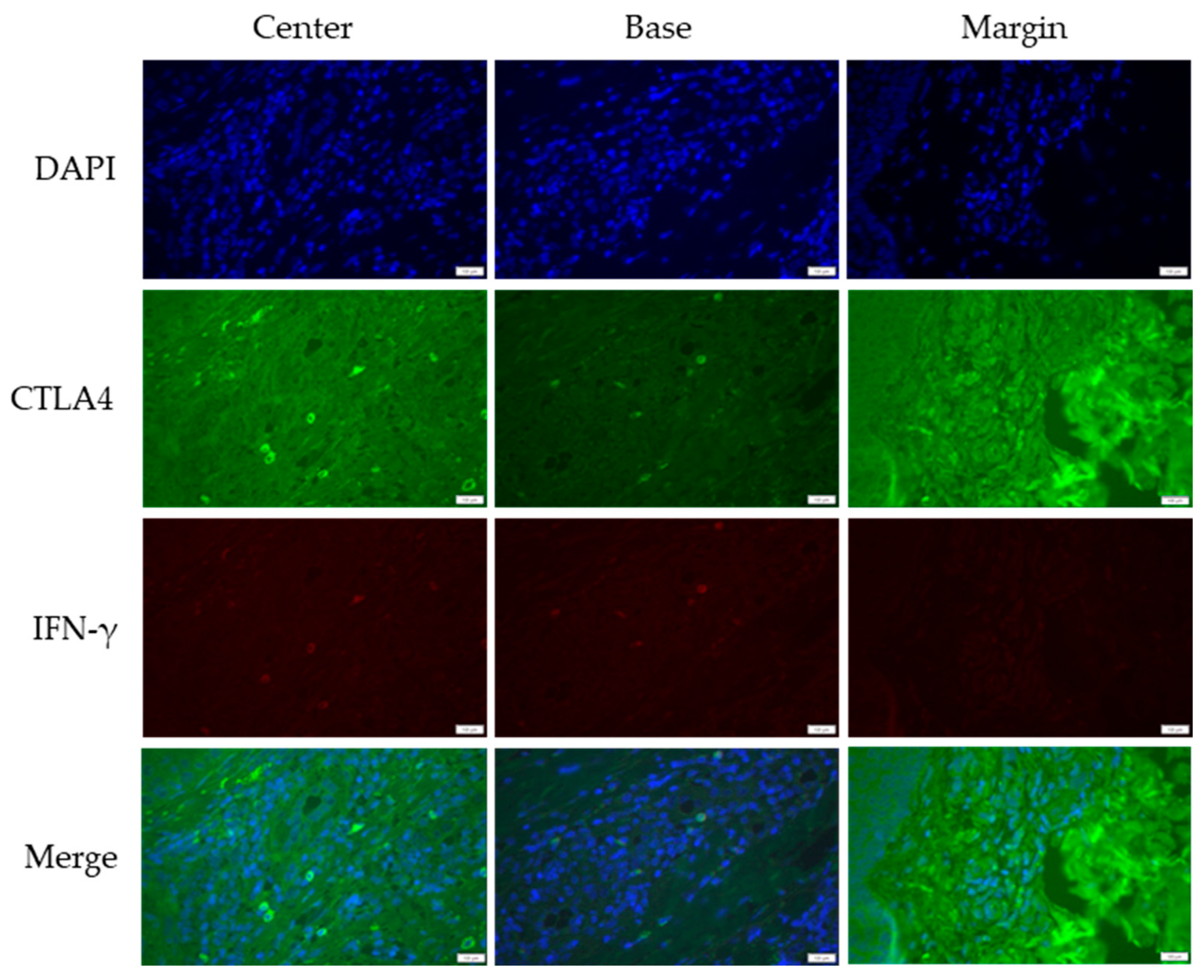
| IF Channels | Primary Antibody | Secondary Antibody | Probe | |
|---|---|---|---|---|
| Double-staining | Blue | / | / | DAPI (Sigma-Aldrich, Burlington, MA, USA) |
| Green | hCTLA4 rabbit (ab237712, Abcam, Cambridge, UK) | Alexa Fluor 488 anti-rabbit (ab150073, Abcam, Cambridge, UK), 1:400 | / | |
| Red | hIFN gamma mouse IgG2b (ab218426, Abcam, Cambridge, UK) | Rhodamine Red-X AffiniPure Donkey anti-mouse (715-295-151, Jackson ImmunoResearch, West Grove, PA, USA), 1:100 | / | |
| Triple- staining | Blue | / | / | Hoechst 33,342 (Fluka, Honeywell, Morris Plains, NJ, USA) |
| Green | hCD3 mouse IgG1 (clone F.7.2.38, Dako, Glostrup, Denmark) | Alexa Fluor 488 anti-mouse IgG1 (A21121, ThermoFisher, Waltham, MA, USA), 1:500 | / | |
| Red | hIFN gamma mouse IgG2b (ab218426, Abcam, Cambridge, UK) | CoraLite Plus 555 anti-mouse IgG2b (smsG2bCL555-1, Proteintech, San Diego, CA, USA), 1:500 | / | |
| Far red | hCTLA4 rabbit (ab237712, Abcam, Cambridge, UK) | Alexa Fluor 647 anti-rabbit (ab150083, Abcam, Cambridge, UK), 1:500 | / | |
| Characteristics | KA (n = 41) | SCC (n = 37) | VV (n = 55) | |
|---|---|---|---|---|
| Age (n) | <48 | 5 | 0 | 19 |
| 48–66 | 10 | 4 | 15 | |
| 67–79 | 11 | 11 | 12 | |
| >79 | 15 | 22 | 9 | |
| Sex (n) | Female | 20 | 12 | 22 |
| Male | 21 | 25 | 33 | |
| Characteristics | KA (n = 41) | |
|---|---|---|
| Stage (n) | proliferative | 26 |
| regression | 11 | |
| unknown | 4 | |
| Localization (n) | head and neck | 21 |
| extremities | 15 | |
| trunk | 5 | |
| Proliferative | Regression | p * | ||
|---|---|---|---|---|
| CTLA4+ † (median (IQR ‡)) | Center (%) | 0.5 (0.7) | 0.4 (1.2) | 0.70 |
| Base (%) | 1.1 (2.5) | 1.2 (1.9) | 0.78 | |
| Margin (%) | 0.2 (1.2) | 0.3 (2.9) | 0.47 | |
| IFN-γ+ § (median (IQR)) | Center (%) | 0.0 (0.1) | 0.0 (0.0) | 0.64 |
| Base (%) | 0.0 (0.0) | 0.0 (0.5) | 0.006 | |
| Margin (%) | / | / | / | |
| CTLA4+IFN-γ+ (median (IQR)) | Center (%) | 0.0 (1.2) | 2.1 (2.6) | 0.09 |
| Base (%) | 0.3 (0.1) | 0.0 (1.7) | 0.76 | |
| Margin (%) | / | / | / |
| KA | SCC | VV | p * | ||
|---|---|---|---|---|---|
| CTLA4+ median (IQR) | Center (%) | 0.6 (0.9) | 0.35 (1.03) | 1.9 (2.3) | <0.001 |
| Base (%) | 1.2 (2.5) | 0.0 (0.55) | 2.4 (2.4) | <0.001 | |
| Margin (%) | 0.3 (2.1) | 0.0 (0.0) | 0.0 (1.7) | 0.004 | |
| IFN-γ+ median (IQR) | Center (%) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.005 |
| Base (%) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.02 | |
| Margin (%) | / | 0.0 (1.25) | / | <0.001 | |
| CTLA4+IFN-γ+ median (IQR) | Center (%) | 0.1 (2.15) | 1.44 (2.39) | 0.0 (0.7) | <0.001 |
| Base (%) | 0.0 (0.85) | 1.45 (2.41) | 0.0 (0.0) | <0.001 | |
| Margin (%) | / | 0.0 (0.24) | / | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karabatić Knezović, S.; Knezović, D.; Ban, J.; Matana, A.; Puizina Ivić, N.; Glavina Durdov, M.; Merćep, M.; Drmić Hofman, I. Immunological Landscape of Non-Melanoma Skin Neoplasms: Role of CTLA4+IFN-γ+ Lymphocytes in Tumor Microenvironment Suppression. Medicina 2025, 61, 330. https://doi.org/10.3390/medicina61020330
Karabatić Knezović S, Knezović D, Ban J, Matana A, Puizina Ivić N, Glavina Durdov M, Merćep M, Drmić Hofman I. Immunological Landscape of Non-Melanoma Skin Neoplasms: Role of CTLA4+IFN-γ+ Lymphocytes in Tumor Microenvironment Suppression. Medicina. 2025; 61(2):330. https://doi.org/10.3390/medicina61020330
Chicago/Turabian StyleKarabatić Knezović, Silvana, Dora Knezović, Jelena Ban, Antonela Matana, Neira Puizina Ivić, Merica Glavina Durdov, Mladen Merćep, and Irena Drmić Hofman. 2025. "Immunological Landscape of Non-Melanoma Skin Neoplasms: Role of CTLA4+IFN-γ+ Lymphocytes in Tumor Microenvironment Suppression" Medicina 61, no. 2: 330. https://doi.org/10.3390/medicina61020330
APA StyleKarabatić Knezović, S., Knezović, D., Ban, J., Matana, A., Puizina Ivić, N., Glavina Durdov, M., Merćep, M., & Drmić Hofman, I. (2025). Immunological Landscape of Non-Melanoma Skin Neoplasms: Role of CTLA4+IFN-γ+ Lymphocytes in Tumor Microenvironment Suppression. Medicina, 61(2), 330. https://doi.org/10.3390/medicina61020330







