An Assessment of the Effectiveness of Preoperative İmaging Modalities (MRI, CT, and 18F-FDG PET/CT) in Determining the Extent of Disease Spread in Epithelial Ovarian–Tubal–Peritoneal Cancer (EOC)
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection and Data Collection
2.2. Surgical Criteria
2.3. Histopathologic Evaluation
2.4. Imaging Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
4.1. Limitations
- The small sample size may have limited the generalizability of the findings.
- The single-center design may have limited the generalizability of our findings.
4.2. Strenghts
- This study involved a homogenous study population consisting exclusively of EOC patients.
- This study had a prospective study design.
- Histopathological confirmation was used as the gold standard.
- For each imaging modality (CT, MRI, and PET/CT), standard institutional protocols were adhered to.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUC | Area under the curve |
| CA-125 | Cancer Antigen 125 |
| CI | Confidence interval |
| CT | Computed tomography |
| EOC | Epithelial ovarian–tubal–peritoneal cancer |
| FDG | Fluorodeoxyglucose |
| FIGO | International Federation of Gynecology and Obstetrics |
| MRI | Magnetic resonance imaging |
| NACT | Neoadjuvant chemotherapy |
| NPV | Negative predictive value |
| PET/CT | Positron emission tomography/computed tomography |
| PPV | Positive predictive value |
| sROC | Summary receiver operating characteristic |
| WB-DWI | Whole-Body Diffusion-Weighted Imaging |
References
- Selvaggi, S.M. Tumors of the ovary, maldeveloped gonads, fallopian tube, and broad ligament. Arch. Pathol. Lab. Med. 2000, 124, 477. [Google Scholar] [CrossRef] [PubMed]
- Mohaghegh, P.; Rockall, A.G. Imaging strategy for early ovarian cancer: Characterization of adnexal masses with conventional and advanced imaging techniques. Radiographics 2012, 32, 1751–1773. [Google Scholar] [CrossRef] [PubMed]
- Kurman, R.J.; Shih Ie, M. The origin and pathogenesis of epithelial ovarian cancer: A proposed unifying theory. Am. J. Surg. Pathol. 2010, 34, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Berek, J.S.; Renz, M.; Kehoe, S.; Kumar, L.; Friedlander, M. Cancer of the ovary, fallopian tube, and peritoneum: 2021 update. Int. J. Gynecol. Obstet. 2021, 155, 61–85. [Google Scholar] [CrossRef]
- Lengyel, E. Ovarian cancer development and metastasis. Am. J. Pathol. 2010, 177, 1053–1064. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA A Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef]
- Griffiths, C.T.; Fuller, A.F. Intensive surgical and chemotherapeutic management of advanced ovarian cancer. Surg. Clin. N. Am. 1978, 58, 131–142. [Google Scholar] [CrossRef]
- Hoskins, W.J.; McGuire, W.P.; Brady, M.F.; Homesley, H.D.; Creasman, W.T.; Berman, M.; Ball, H.; Berek, J.S. The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. Am. J. Obstet. Gynecol. 1994, 170, 974–980. [Google Scholar] [CrossRef]
- Du Bois, A.; Reuss, A.; Pujade-Lauraine, E.; Harter, P.; Ray-Coquard, I.; Pfisterer, J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: A combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: By the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer 2009, 115, 1234–1244. [Google Scholar]
- Forstner, R.; Sala, E.; Kinkel, K.; Spencer, J.A. ESUR guidelines: Ovarian cancer staging and follow-up. Eur. Radiol. 2010, 20, 2773–2780. [Google Scholar] [CrossRef]
- Coleridge, S.L.; Bryant, A.; Lyons, T.J.; Goodall, R.J.; Kehoe, S.; Morrison, J. Chemotherapy versus surgery for initial treatment in advanced ovarian epithelial cancer. Cochrane Database Syst. Rev. 2019, 2019, CD005343. [Google Scholar] [CrossRef] [PubMed]
- Engbersen, M.P.; Van Driel, W.; Lambregts, D.; Lahaye, M. The role of CT, PET-CT, and MRI in ovarian cancer. Br. J. Radiol. 2021, 94, 20210117. [Google Scholar] [CrossRef]
- Prat, J.; FIGO Committee on Gynecologic Oncology. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int. J. Gynecol. Obstet. 2014, 124, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Young, R. WHO classification of tumours of female reproductive organs. In Monodermal Teratomas and Somatic-Type Tumours Arising from a Dermoid Cyst; International Agency for Research on Cancer: Lyon, France, 2014; pp. 63–66. [Google Scholar]
- De Iaco, P.; Musto, A.; Orazi, L.; Zamagni, C.; Rosati, M.; Allegri, V.; Cacciari, N.; Al-Nahhas, A.; Rubello, D.; Venturoli, S.; et al. FDG-PET/CT in advanced ovarian cancer staging: Value and pitfalls in detecting lesions in different abdominal and pelvic quadrants compared with laparoscopy. Eur. J. Radiol. 2011, 80, e98–e103. [Google Scholar] [CrossRef]
- Fagotti, A.; Fanfani, F.; Rossitto, C.; Lorusso, D.; De Gaetano, A.M.; Giordano, A.; Vizzielli, G.; Scambia, G. A treatment selection protocol for recurrent ovarian cancer patients: The role of FDG-PET/CT and staging laparoscopy. Oncology 2008, 75, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Gouy, S.; Belghiti, J.; Uzan, C.; Canlorbe, G.; Gauthier, T.; Morice, P. Accuracy and reproducibility of the peritoneal cancer index in advanced ovarian cancer during laparoscopy and laparotomy. Int. J. Gynecol. Cancer 2013, 23, 1699–1703. [Google Scholar] [CrossRef]
- Michielsen, K.; Vergote, I.; Op de Beeck, K.; Amant, F.; Leunen, K.; Moerman, P.; Deroose, C.; Souverijns, G.; Dymarkowski, S.; De Keyzer, F.; et al. Whole-body MRI with diffusion-weighted sequence for staging of patients with suspected ovarian cancer: A clinical feasibility study in comparison to CT and FDG-PET/CT. Eur. Radiol. 2014, 24, 889–901. [Google Scholar] [CrossRef]
- Low, R.N.; Barone, R.M. Imaging for Peritoneal Metastases. Surg. Oncol. Clin. N. Am. 2018, 27, 425–442. [Google Scholar] [CrossRef]
- Bailly, C.; Bailly-Glatre, A.; Alfidja, A.; Vincent, C.; Dauplat, J.; Pomel, C. Peritoneal carcinosis in ovarian cancer: Conventional imaging (CT-scan and MRI). Bull. Du Cancer 2009, 96, 1155–1162. [Google Scholar] [CrossRef]
- Coakley, F.V.; Choi, P.H.; Gougoutas, C.A.; Pothuri, B.; Venkatraman, E.; Chi, D.; Bergman, A.; Hricak, H. Peritoneal metastases: Detection with spiral CT in patients with ovarian cancer. Radiology 2002, 223, 495–499. [Google Scholar] [CrossRef]
- Marin, D.; Catalano, C.; Baski, M.; Di Martino, M.; Geiger, D.; Di Giorgio, A.; Sibio, S.; Passariello, R. 64-Section multi-detector row CT in the preoperative diagnosis of peritoneal carcinomatosis: Correlation with histopathological findings. Abdom. Imaging 2010, 35, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, P.; Jelinek, J.S.; Steves, M.A.; Sugarbaker, P.H. Evaluation of computed tomography in patients with peritoneal carcinomatosis. Cancer 1993, 72, 1631–1636. [Google Scholar] [CrossRef] [PubMed]
- Iafrate, F.; Ciolina, M.; Sammartino, P.; Baldassari, P.; Rengo, M.; Lucchesi, P.; Sibio, S.; Accarpio, F.; Di Giorgio, A.; Laghi, A. Peritoneal carcinomatosis: Imaging with 64-MDCT and 3T MRI with diffusion-weighted imaging. Abdom. Imaging 2012, 37, 616–627. [Google Scholar] [CrossRef]
- Koh, D.M.; Collins, D.J. Diffusion-weighted MRI in the body: Applications and challenges in oncology. Am. J. Roentgenol. 2007, 188, 1622–1635. [Google Scholar] [CrossRef]
- Kyriazi, S.; Collins, D.J.; Morgan, V.A.; Giles, S.L.; deSouza, N.M. Diffusion-weighted imaging of peritoneal disease for noninvasive staging of advanced ovarian cancer. Radiographics 2010, 30, 1269–1285. [Google Scholar] [CrossRef]
- Fujii, S.; Matsusue, E.; Kanasaki, Y.; Kanamori, Y.; Nakanishi, J.; Sugihara, S.; Kigawa, J.; Terakawa, N.; Ogawa, T. Detection of peritoneal dissemination in gynecological malignancy: Evaluation by diffusion-weighted MR imaging. Eur. Radiol. 2008, 18, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Low, R.N.; Barone, R.M.; Lacey, C.; Sigeti, J.S.; Alzate, G.D.; Sebrechts, C.P. Peritoneal tumor: MR imaging with dilute oral barium and intravenous gadolinium-containing contrast agents compared with unenhanced MR imaging and CT. Radiology 1997, 204, 513–520. [Google Scholar] [CrossRef]
- Tempany, C.M.; Zou, K.H.; Silverman, S.G.; Brown, D.L.; Kurtz, A.B.; McNeil, B.J. Staging of advanced ovarian cancer: Comparison of imaging modalities--report from the Radiological Diagnostic Oncology Group. Radiology 2000, 215, 761–767. [Google Scholar] [CrossRef]
- Subhas, N.; Patel, P.V.; Pannu, H.K.; Jacene, H.A.; Fishman, E.K.; Wahl, R.L. Imaging of pelvic malignancies with in-line FDG PET-CT: Case examples and common pitfalls of FDG PET. Radiographics 2005, 25, 1031–1043. [Google Scholar] [CrossRef]
- Soussan, M.; Des Guetz, G.; Barrau, V.; Aflalo-Hazan, V.; Pop, G.; Mehanna, Z.; Rust, E.; Aparicio, T.; Douard, R.; Benamouzig, R.; et al. Comparison of FDG-PET/CT and MR with diffusion-weighted imaging for assessing peritoneal carcinomatosis from gastrointestinal malignancy. Eur. Radiol. 2012, 22, 1479–1487. [Google Scholar] [CrossRef]
- Cook, G.J.; Maisey, M.N.; Fogelman, I. Normal variants, artefacts and interpretative pitfalls in PET imaging with 18-fluoro-2-deoxyglucose and carbon-11 methionine. Eur. J. Nucl. Med. 1999, 26, 1363–1378. [Google Scholar] [CrossRef] [PubMed]
- Michielsen, K.L.; Vergote, I.; Dresen, R.; Op de Beeck, K.; Vanslembrouck, R.; Amant, F.; Leunen, K.; Moerman, P.; Fieuws, S.; De Keyzer, F.; et al. Whole-body diffusion-weighted magnetic resonance imaging in the diagnosis of recurrent ovarian cancer: A clinical feasibility study. Br. J. Radiol. 2016, 89, 20160468. [Google Scholar] [CrossRef] [PubMed]
- Satoh, Y.; Ichikawa, T.; Motosugi, U.; Kimura, K.; Sou, H.; Sano, K.; Araki, T. Diagnosis of peritoneal dissemination: Comparison of 18F-FDG PET/CT, diffusion-weighted MRI, and contrast-enhanced MDCT. Am. J. Roentgenol. 2011, 196, 447–453. [Google Scholar] [CrossRef]
- Schmidt, S.; Meuli, R.A.; Achtari, C.; Prior, J.O. Peritoneal carcinomatosis in primary ovarian cancer staging: Comparison between MDCT, MRI, and 18F-FDG PET/CT. Clin. Nucl. Med. 2015, 40, 371–377. [Google Scholar] [CrossRef]
- Gu, P.; Pan, L.-L.; Wu, S.-Q.; Sun, L.; Huang, G. CA 125, PET alone, PET–CT, CT and MRI in diagnosing recurrent ovarian carcinoma: A systematic review and meta-analysis. Eur. J. Radiol. 2009, 71, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Gu, Z.X.; Tao, X.F.; Liu, S.Y. Computer tomography, magnetic resonance imaging, and positron emission tomography or positron emission tomography/computer tomography for detection of metastatic lymph nodes in patients with ovarian cancer: A meta-analysis. Eur. J. Radiol. 2012, 81, 1002–1006. [Google Scholar] [CrossRef] [PubMed]
- Van’t Sant, I.; Engbersen, M.P.; Bhairosing, P.A.; Lambregts, D.M.J.; Beets-Tan, R.G.H.; Van Driel, W.J.; Aalbers, A.G.J.; Kok, N.F.M.; Lahaye, M.J. Diagnostic performance of imaging for the detection of peritoneal metastases: A meta-analysis. Eur. Radiol. 2020, 30, 3101–3112. [Google Scholar] [CrossRef]
- Tsili, A.C.; Alexiou, G.; Tzoumpa, M.; Siempis, T.; Argyropoulou, M.I. Imaging of Peritoneal Metastases in Ovarian Cancer Using MDCT, MRI, and FDG PET/CT: A Systematic Review and Meta-Analysis. Cancers 2024, 16, 1467. [Google Scholar] [CrossRef]
| Characteristics | n: 24 |
|---|---|
| Age (Year), mean ± SD (Min–Max) | 53.5 ± 12.3 (27–79) |
| Primary cancer, n (%) | 16 (66.7) |
| Recurrent cancer, n (%) | 8 (33.3) |
| FIGO stage | |
| Stage 1, n (%) | 2 (8.3) |
| Stage 2, n (%) | 1 (4.2) |
| Stage 3, n (%) | 21 (87.5) |
| Histology | |
| Serous, n (%) | 21 (87.5) |
| Mucinous, n (%) | 1 (4.16) |
| Clear cell, n (%) | 1 (4.16) |
| Endometriod, n (%) | 1 (4.16) |
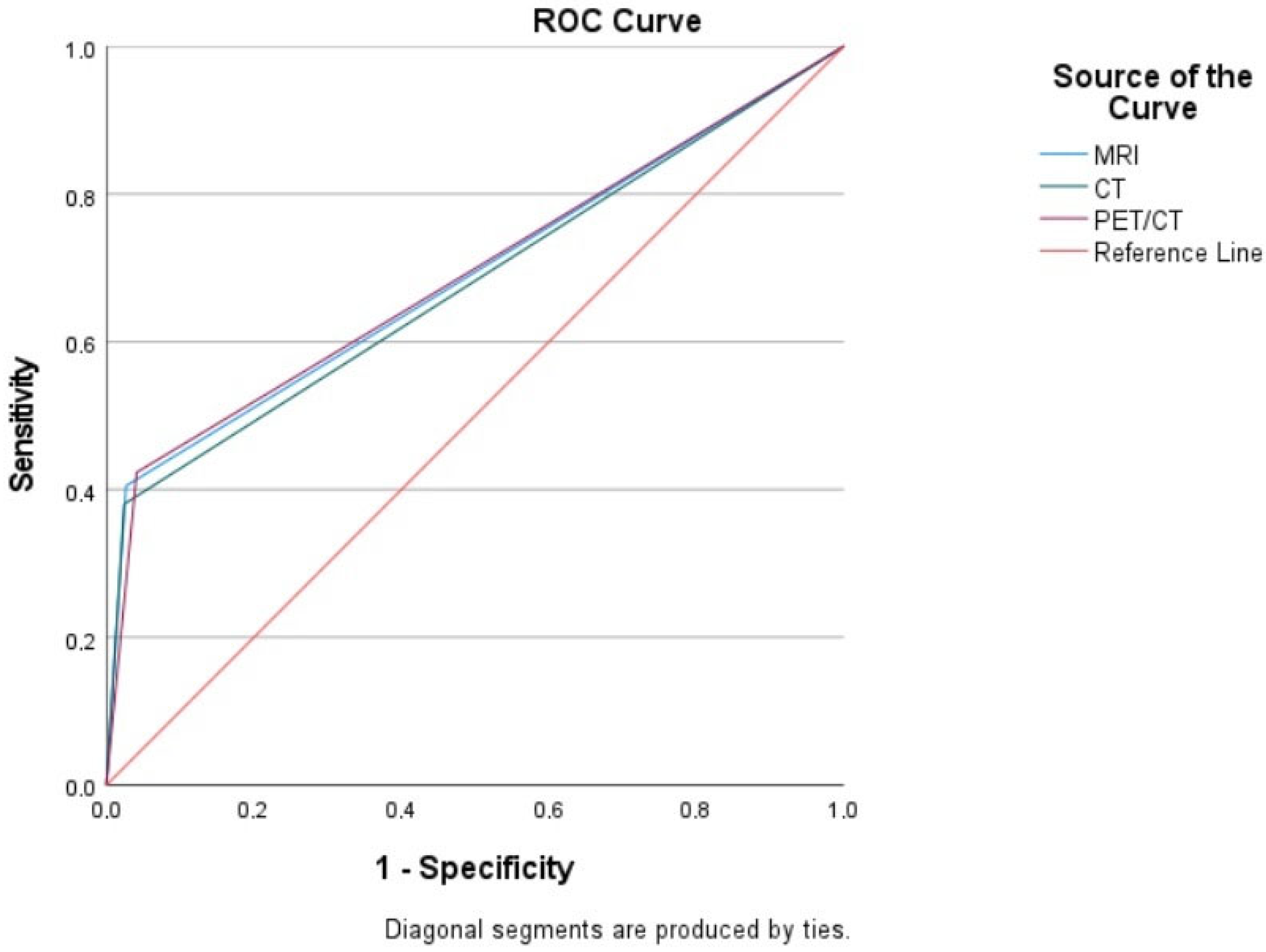
| Imaging Modalities | Sensitivity % (95% CI) | Specificity % (95% CI) | PPV % | NPV % | Accuracy % |
|---|---|---|---|---|---|
| CT Overall | 38.04 (36.32–39.74) | 97.58 (97.23–97.91) | 86.11 | 79.96 | 81.0 |
| MRI Overall | 40.49 (38.74–42.23) | 97.34 (96.97–97.69) | 85.71 | 80.56 | 81.0 |
| PET/CT Overall | 42.33 (40.58–44.08) | 95.88 (95.44–96.32) | 80.23 | 80.82 | 81.0 |
| Test Result Variable(s) | Area | Std. Error a | Asymptotic Sig. b | Asymptotic 95% Confidence Interval | |
|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||
| MRI | 0.689 | 0.027 | 0.000 | 0.636 | 0.743 |
| CT | 0.678 | 0.027 | 0.000 | 0.624 | 0.732 |
| PET/CT | 0.691 | 0.027 | 0.000 | 0.638 | 0.744 |
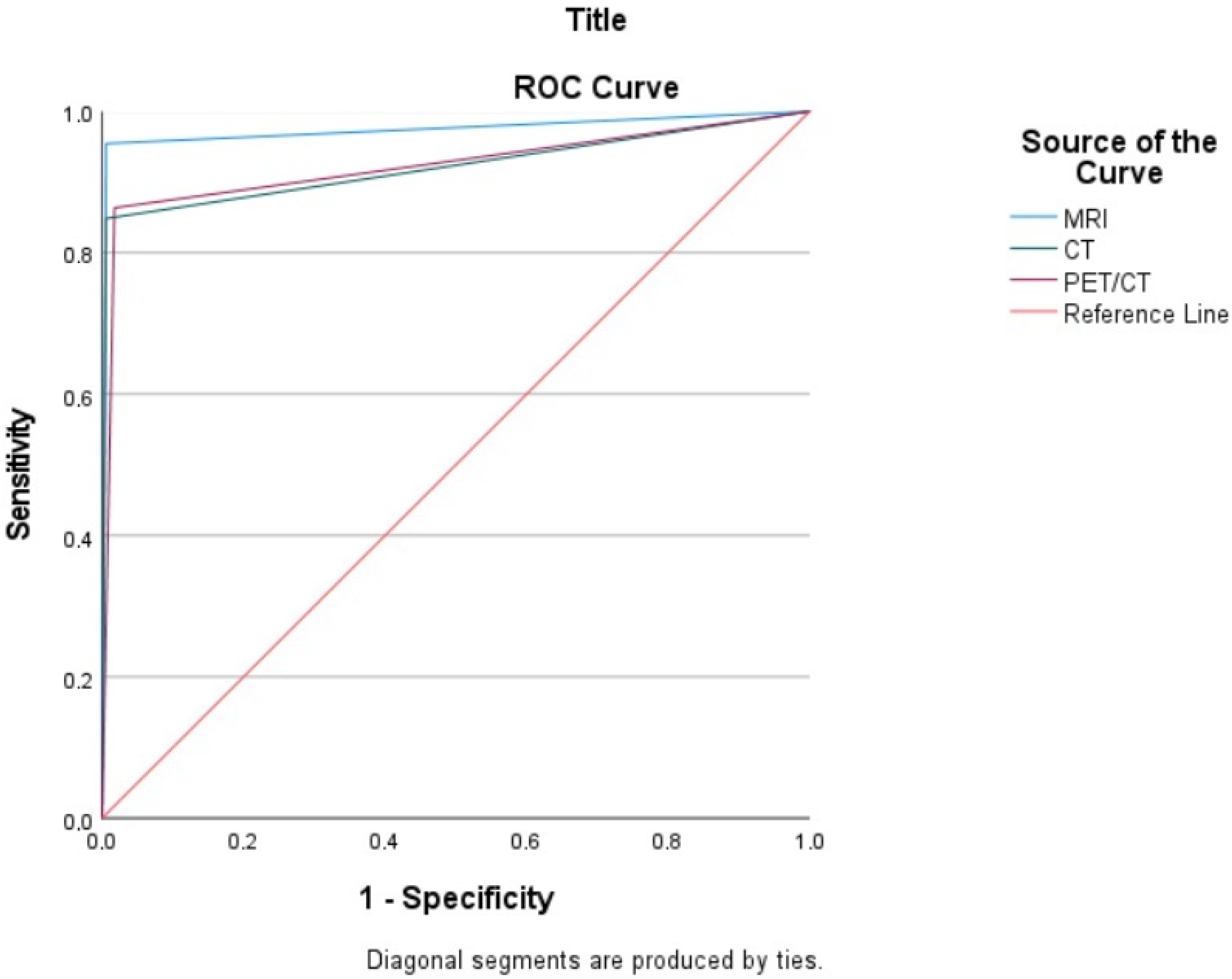
| Imaging Modalities | Sensitivity % (95% CI) | Specificity % (95% CI) | PPV % | NPV % | Accuracy % |
|---|---|---|---|---|---|
| CT Overall | 84.85 (84.51–85.18) | 99.41 (99.38–99.43) | 94.92 | 98.07 | 98.0 |
| MRI Overall | 95.45 (95.37–95.53) | 99.41 (99.40–99.42) | 95.45 | 99.41 | 99.0 |
| PET/CT Overall | 86.36 (85.92–86.80) | 98.04 (97.76–98.32) | 85.07 | 98.23 | 97.0 |
| Test Result Variable(s) | Area | Std. Error a | Asymptotic Sig. b | Asymptotic 95% Confidence Interval | |
|---|---|---|---|---|---|
| Lower Bound | Upper Boun | ||||
| MRI | 0.974 | 0.015 | 0.000 | 0.945 | 1.000 |
| CT | 0.921 | 0.026 | 0.000 | 0.870 | 0.973 |
| PET/CT | 0.923 | 0.025 | 0.000 | 0.874 | 0.972 |
| Study | Imaging Modalities | Sensitivity | Specificity | Key Findings |
|---|---|---|---|---|
| Gu, P. et al. A systematic review and meta-analysis 2009 [36] | CT MRI PET/CT | 79% 75% 91% | 84% 78% 88% | PET/CT may complement current surveillance methods, especially in patients with elevated CA 125 and negative CT or MRI |
| Yuan et al. A meta-analysis 2012 [37] | CT MRI PET/CT | 42.6% 54.7% 73.2% | 95.0% 88.3% 96.7% | PET or PET/CT is more accurate than CT and MRI in the detection of lymph node metastasis in patients with ovarian cancer. |
| Michielsen et al., 2014 [18] | CT WB-DWI/MRI PET/CT | 65% 91% 52% | 82% 91% 85% | WB-DWI/MRI shows high accuracy in characterizing primary tumors, peritoneal tumors, and distant staging. |
| Schmidt et al., 2015 [35] | CT MRI PET/CT | 96% 98% 95% | 92% 84% 96% | MRI had the highest sensitivity, and FDG PET/CT had the highest specificity, with no significant differences between the three techniques. CT is the preferred choice for stand-alone examination due to its speed, cost-effectiveness, and wide availability. |
| Van’t Sant et al. A meta-analysis 2020 [38] | CT MRI PET/CT | 68% 92% 80% | 88% 85% 90% | DW-MRI and PET/CT showed comparable diagnostic performance. |
| Tisili et al. A systematic review and meta-analysis 2024 [39] | CT MRI PET/CT | 79.7% 82.7% 93.7% | 92.1% 90.3% 91.5% | Both FDG PET/CT and MRI have comparably higher per-patient diagnostic accuracy. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kandemir, H.; Sözen, H.; Kartal, M.G.; Özkan, Z.G.; Topuz, S.; Salihoğlu, M.Y. An Assessment of the Effectiveness of Preoperative İmaging Modalities (MRI, CT, and 18F-FDG PET/CT) in Determining the Extent of Disease Spread in Epithelial Ovarian–Tubal–Peritoneal Cancer (EOC). Medicina 2025, 61, 199. https://doi.org/10.3390/medicina61020199
Kandemir H, Sözen H, Kartal MG, Özkan ZG, Topuz S, Salihoğlu MY. An Assessment of the Effectiveness of Preoperative İmaging Modalities (MRI, CT, and 18F-FDG PET/CT) in Determining the Extent of Disease Spread in Epithelial Ovarian–Tubal–Peritoneal Cancer (EOC). Medicina. 2025; 61(2):199. https://doi.org/10.3390/medicina61020199
Chicago/Turabian StyleKandemir, Hülya, Hamdullah Sözen, Merve Gülbiz Kartal, Zeynep Gözde Özkan, Samet Topuz, and Mehmet Yavuz Salihoğlu. 2025. "An Assessment of the Effectiveness of Preoperative İmaging Modalities (MRI, CT, and 18F-FDG PET/CT) in Determining the Extent of Disease Spread in Epithelial Ovarian–Tubal–Peritoneal Cancer (EOC)" Medicina 61, no. 2: 199. https://doi.org/10.3390/medicina61020199
APA StyleKandemir, H., Sözen, H., Kartal, M. G., Özkan, Z. G., Topuz, S., & Salihoğlu, M. Y. (2025). An Assessment of the Effectiveness of Preoperative İmaging Modalities (MRI, CT, and 18F-FDG PET/CT) in Determining the Extent of Disease Spread in Epithelial Ovarian–Tubal–Peritoneal Cancer (EOC). Medicina, 61(2), 199. https://doi.org/10.3390/medicina61020199






