Oral, Vaginal, and Placental Microbiota Profiles in Japanese Pregnancies with Preterm Birth and Chronic Abruption-Oligohydramnios Sequence (CAOS): A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Participants
2.3. Procedures
2.4. 16S rRNA Gene Sequencing and Microbiota Profiling
2.5. Statistical Analysis
3. Results
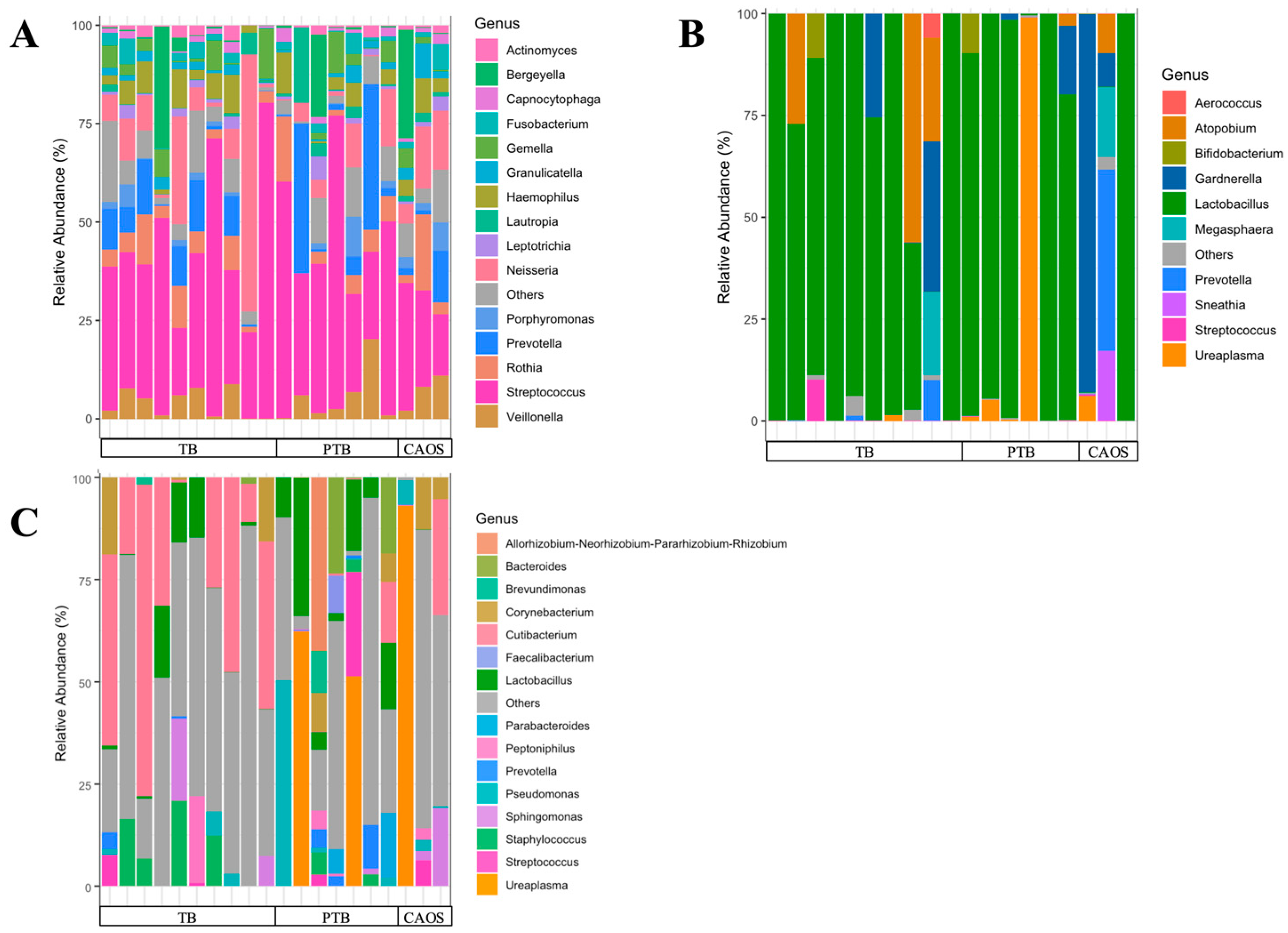
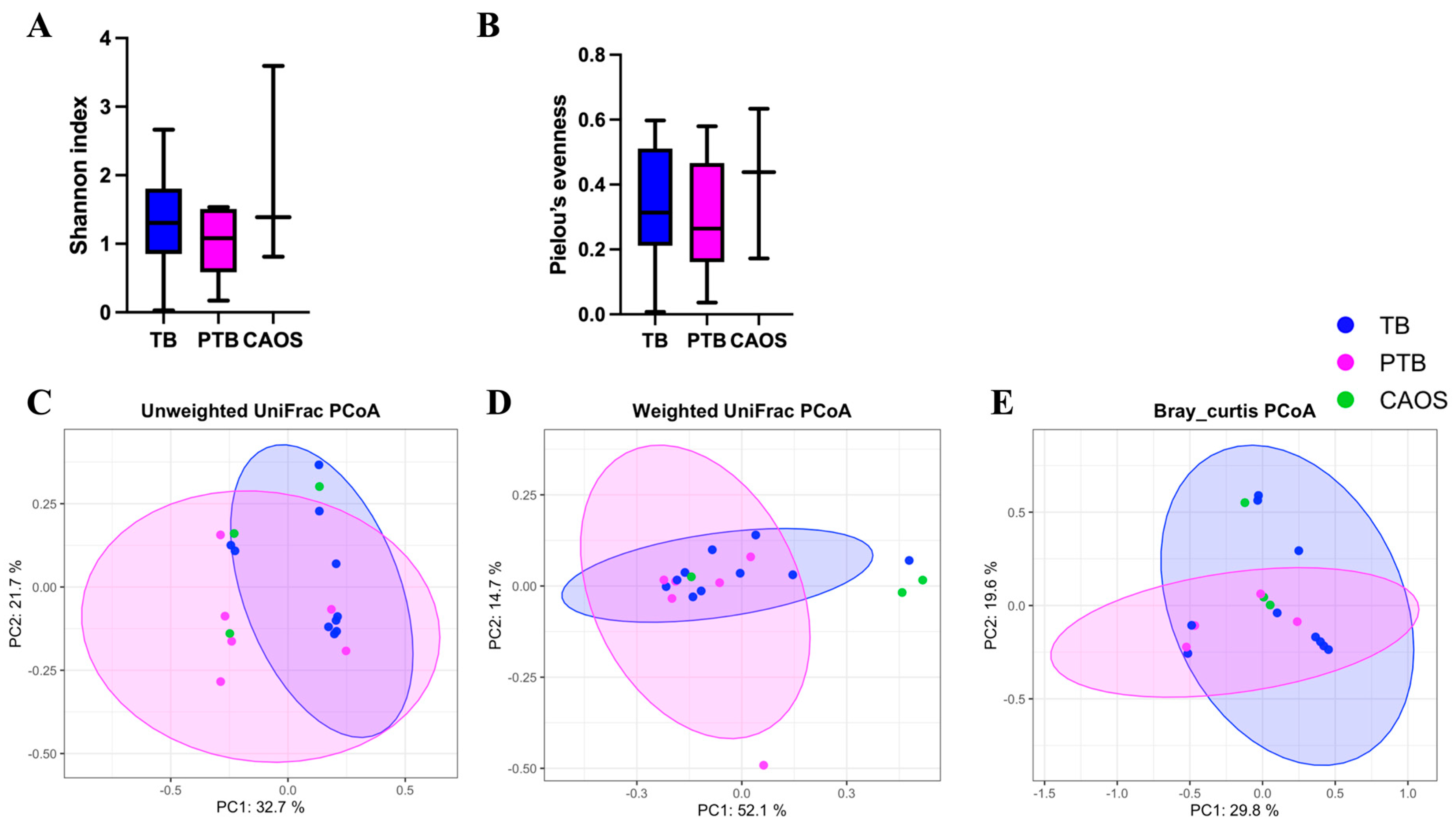
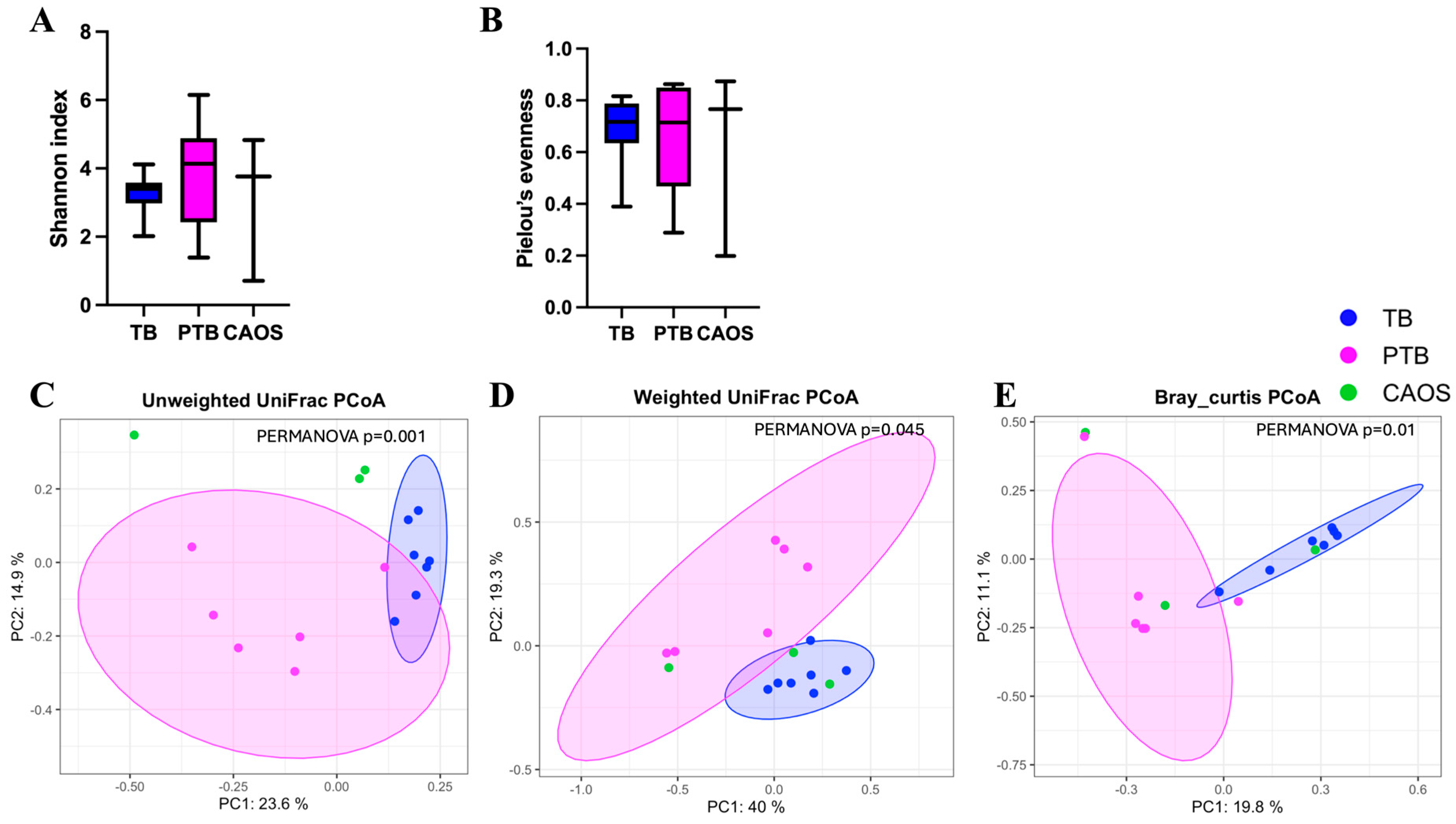
3.1. Oral Microbiota
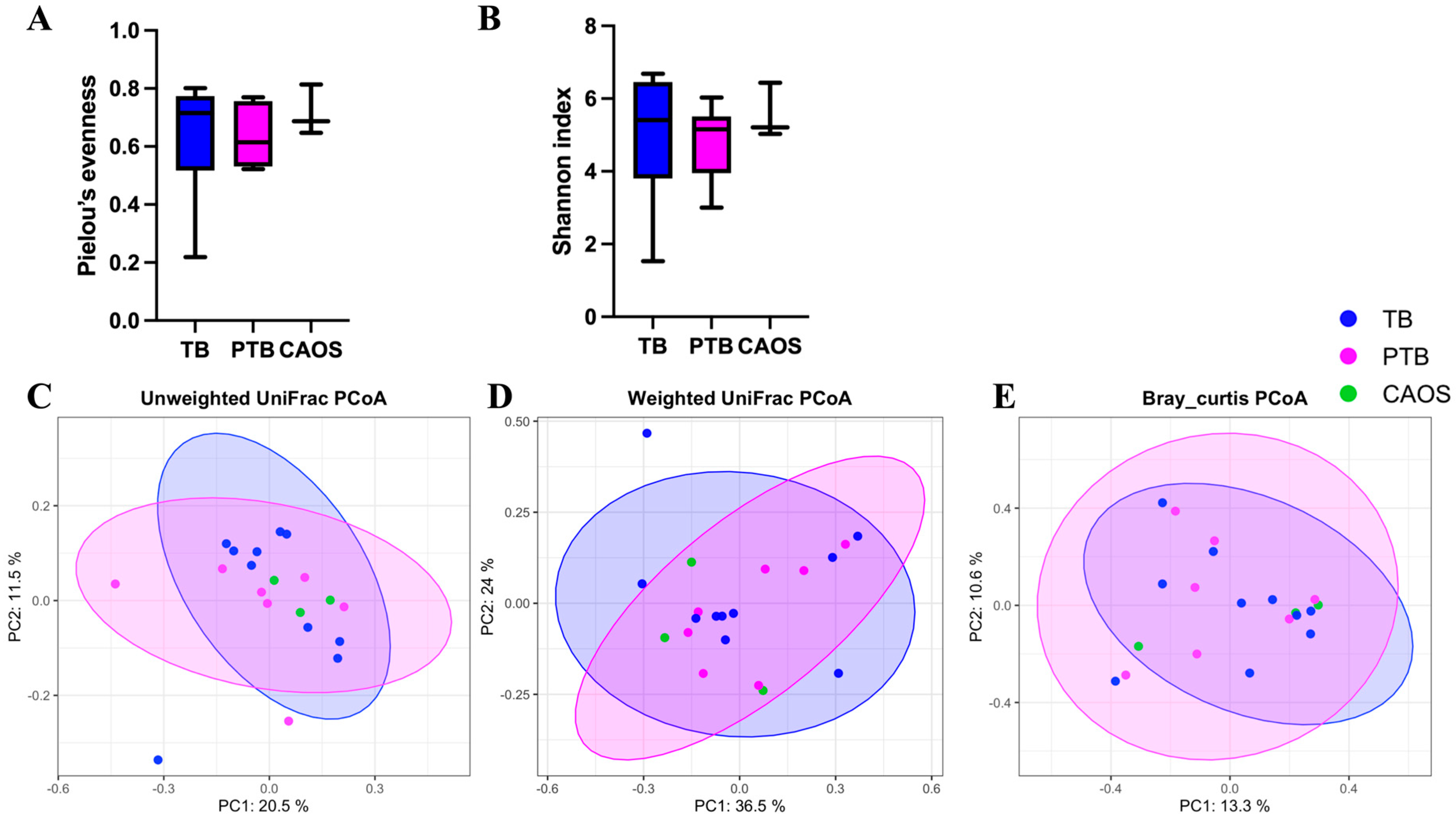
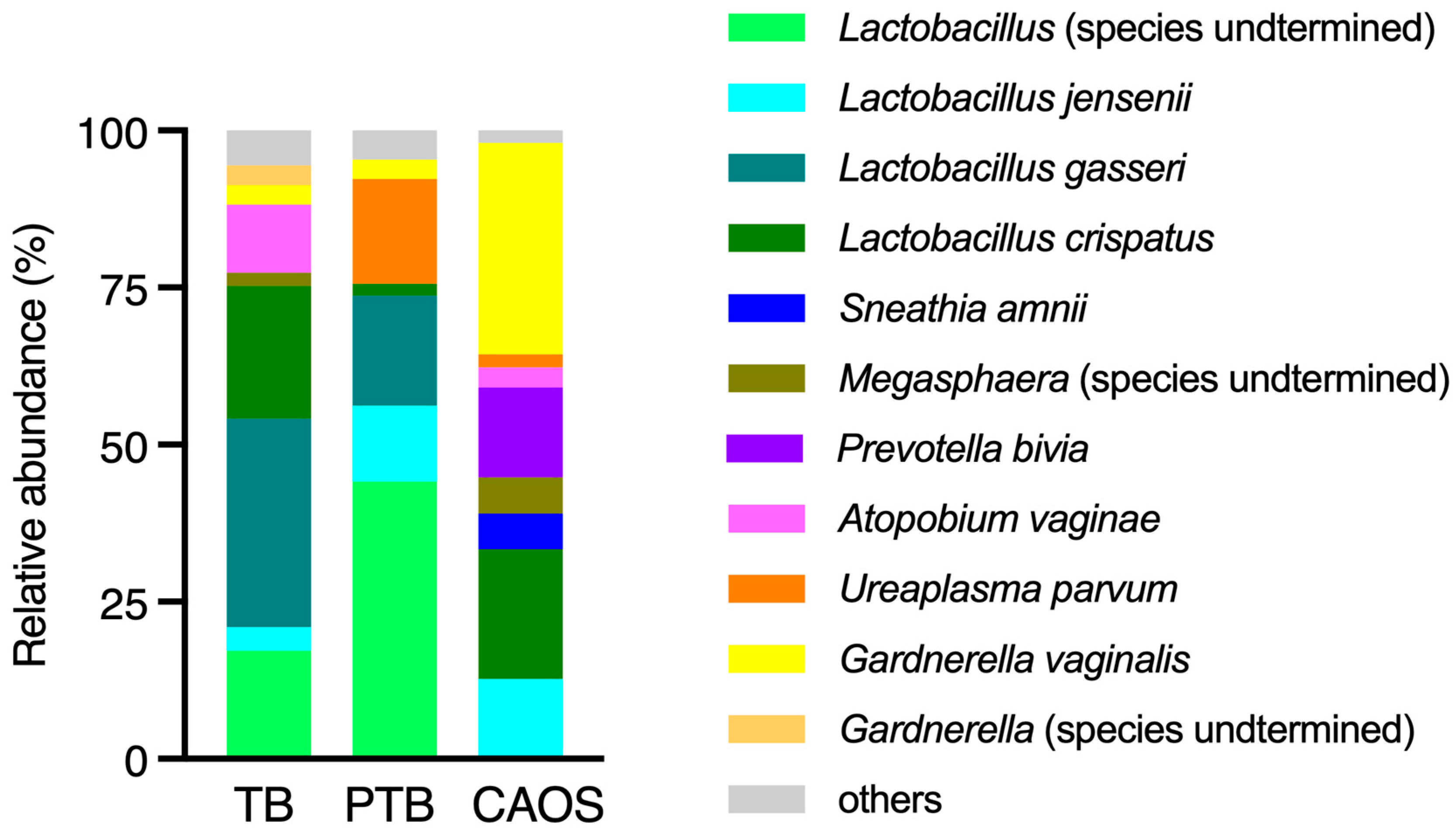
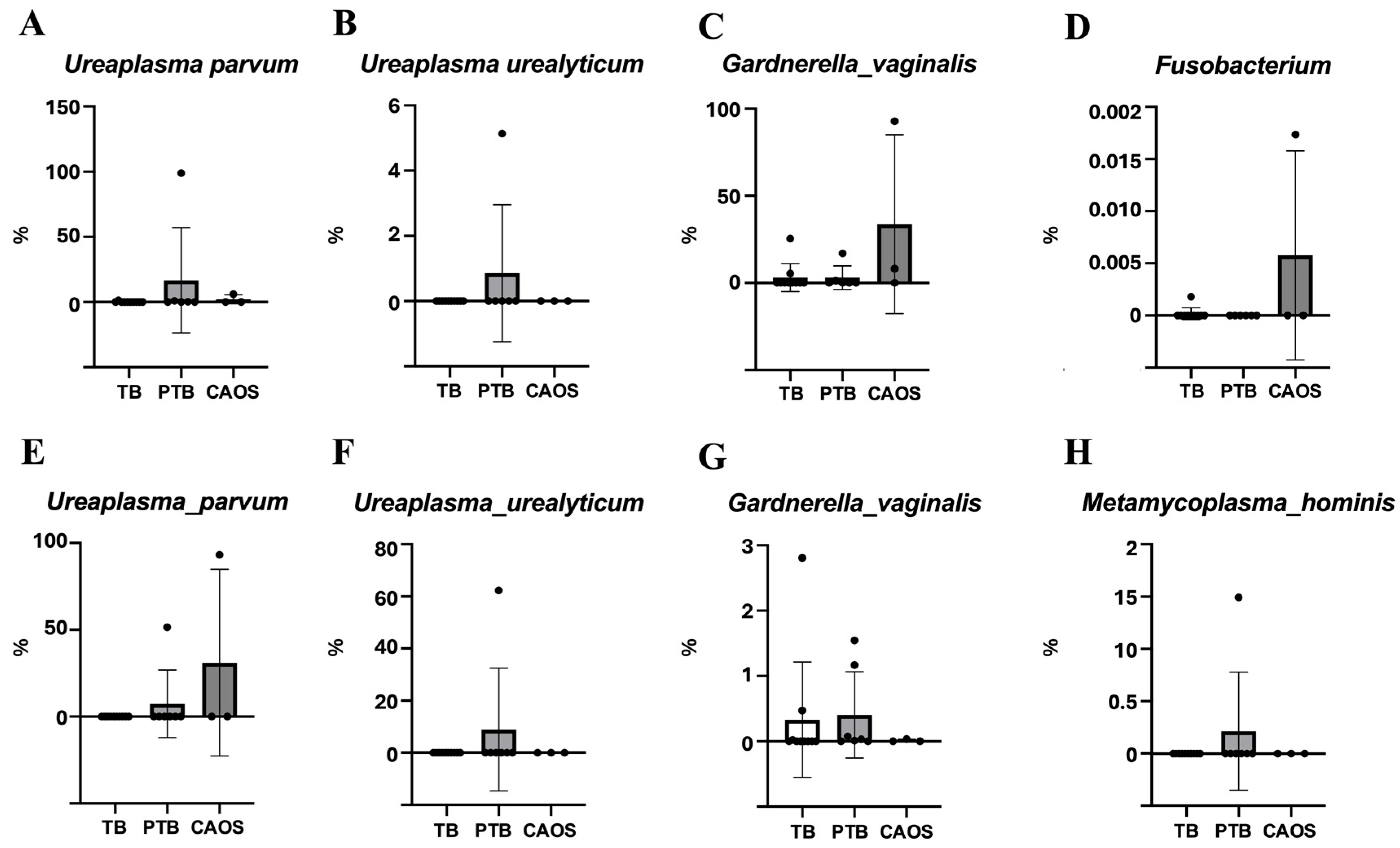
3.2. Vaginal Microbiota
3.3. Placental Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PTB | Preterm Birth |
| TB | Term Birth |
| CAOS | Chronic Abruption-Oligohydramnios Sequence |
| CAM | Chorioamnionitis |
| PCR | Polymerase Chain Reaction |
| ASVs | Alignment of obtained amplicon Sequence Variants |
Appendix A
Appendix A.1. Genomic DNA Extraction, and 16S rDNA Metagenomic Sequencing Library Preparation
Appendix A.2. Illumina Sequencing
Appendix A.3. Data Analysis
References
- Ohuma, E.O.; Moller, A.B.; Bradley, E.; Chakwera, S.; Hussain-Alkhateeb, L.; Lewin, A.; Okwaraji, Y.B.; Mahanani, W.R.; Johansson, E.W.; Lavin, T.; et al. National, regional, and global estimates of preterm birth in 2020, with trends from 2010: A systematic analysis. Lancet 2023, 402, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Chawanpaiboon, S.; Vogel, J.P.; Moller, A.B.; Lumbiganon, P.; Petzold, M.; Hogan, D.; Landoulsi, S.; Jampathong, N.; Kongwattanakul, K.; Laopaiboon, M.; et al. Global, regional, and national estimates of levels of preterm birth in 2014: A systematic review and modelling analysis. Lancet Glob. Health 2019, 7, e37–e46. [Google Scholar] [CrossRef]
- Berger, R.; Abele, H.; Bahlmann, F.; Doubek, K.; Felderhoff-Müser, U.; Fluhr, H.; Garnier, Y.; Grylka-Baeschlin, S.; Hayward, A.; Helmer, H.; et al. Prevention and Therapy of Preterm Birth. Guideline of the DGGG, OEGGG and SGGG (S2k Level, AWMF Registry Number 015/025, September 2022)—Part 2 with Recommendations on the Tertiary Prevention of Preterm Birth and on the Management of Preterm Premature Rupture of Membranes. Geburtshilfe Frauenheilkd 2023, 83, 569–601. [Google Scholar] [CrossRef] [PubMed]
- Glover, A.V.; Manuck, T.A. Screening for spontaneous preterm birth and resultant therapies to reduce neonatal morbidity and mortality: A review. Semin. Fetal Neonatal Med. 2018, 23, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Koire, A.; Chu, D.M.; Aagaard, K. Family history is a predictor of current preterm birth. Am. J. Obstet. Gynecol. MFM 2021, 3, 100277. [Google Scholar] [CrossRef]
- Cornish, R.P.; Magnus, M.C.; Urhoj, S.K.; Santorelli, G.; Smithers, L.G.; Odd, D.; Fraser, A.; Haberg, S.E.; Nybo Andersen, A.M.; Birnie, K.; et al. Maternal pre-pregnancy body mass index and risk of preterm birth: A collaboration using large routine health datasets. BMC Med. 2024, 22, 10. [Google Scholar] [CrossRef]
- Dekker, G.A.; Lee, S.Y.; North, R.A.; McCowan, L.M.; Simpson, N.A.; Roberts, C.T. Risk factors for preterm birth in an international prospective cohort of nulliparous women. PLoS ONE 2012, 7, e39154. [Google Scholar] [CrossRef]
- Kasuga, Y.; Miyakoshi, K.; Nishio, H.; Akiba, Y.; Otani, T.; Fukutake, M.; Ikenoue, S.; Ochiai, D.; Matsumoto, T.; Tanaka, K.; et al. Mid-trimester residual cervical length and the risk of preterm birth in pregnancies after abdominal radical trachelectomy: A retrospective analysis. BJOG 2017, 124, 1729–1735. [Google Scholar] [CrossRef]
- Kyrgiou, M.; Athanasiou, A.; Paraskevaidi, M.; Mitra, A.; Kalliala, I.; Martin-Hirsch, P.; Arbyn, M.; Bennett, P.; Paraskevaidis, E. Adverse obstetric outcomes after local treatment for cervical preinvasive and early invasive disease according to cone depth: Systematic review and meta-analysis. BMJ 2016, 354, i3633. [Google Scholar] [CrossRef]
- Liu, B.; Xu, G.; Sun, Y.; Qiu, X.; Ryckman, K.K.; Yu, Y.; Snetselaar, L.G.; Bao, W. Maternal cigarette smoking before and during pregnancy and the risk of preterm birth: A dose-response analysis of 25 million mother-infant pairs. PLoS Med. 2020, 17, e1003158. [Google Scholar] [CrossRef]
- Iams, J.D.; Goldenberg, R.L.; Meis, P.J.; Mercer, B.M.; Moawad, A.; Das, A.; Thom, E.; McNellis, D.; Copper, R.L.; Johnson, F.; et al. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N. Engl. J. Med. 1996, 334, 567–572. [Google Scholar] [CrossRef]
- Gao, T.; Zhou, J.; Yang, L.; Wang, T. Association of multiple pregnancies with risk of preterm birth in the United States: A retrospective cohort study. Transl. Pediatr. 2025, 14, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, S.; Azami, M.; Badfar, G.; Parizad, N.; Sayehmiri, K. The relationship between maternal anemia during pregnancy with preterm birth: A systematic review and meta-analysis. J. Matern. Fetal Neonatal Med. 2020, 33, 2679–2689. [Google Scholar] [CrossRef] [PubMed]
- Hossain, R.; Harris, T.; Lohsoonthorn, V.; Williams, M.A. Risk of preterm delivery in relation to vaginal bleeding in early pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2007, 135, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Peelen, M.J.; Kazemier, B.M.; Ravelli, A.C.; De Groot, C.J.; Van Der Post, J.A.; Mol, B.W.; Hajenius, P.J.; Kok, M. Impact of fetal gender on the risk of preterm birth, a national cohort study. Acta Obstet. Gynecol. Scand. 2016, 95, 1034–1041. [Google Scholar] [CrossRef]
- Mohanty, T.; Doke, P.P.; Khuroo, S.R. Effect of bacterial vaginosis on preterm birth: A meta-analysis. Arch. Gynecol. Obstet. 2023, 308, 1247–1255. [Google Scholar] [CrossRef]
- Romero, R.; Oyarzun, E.; Mazor, M.; Sirtori, M.; Hobbins, J.C.; Bracken, M. Meta-analysis of the relationship between asymptomatic bacteriuria and preterm delivery/low birth weight. Obstet. Gynecol. 1989, 73, 576–582. [Google Scholar]
- Khader, Y.S.; Ta’ani, Q. Periodontal diseases and the risk of preterm birth and low birth weight: A meta-analysis. J. Periodontol. 2005, 76, 161–165. [Google Scholar] [CrossRef]
- Elliott, J.P.; Gilpin, B.; Strong, T.H., Jr.; Finberg, H.J. Chronic abruption-oligohydramnios sequence. J. Reprod. Med. 1998, 43, 418–422. [Google Scholar]
- Kobayashi, A.; Minami, S.; Tanizaki, Y.; Shiro, M.; Yamamoto, M.; Yagi, S.; Okutani, T.; Kumagai, T.; Higuchi, R.; Ino, K. Adverse perinatal and neonatal outcomes in patients with chronic abruption-oligohydramnios sequence. J. Obstet. Gynaecol. Res. 2014, 40, 1618–1624. [Google Scholar] [CrossRef]
- Han, C.S.; Schatz, F.; Lockwood, C.J. Abruption-associated prematurity. Clin. Perinatol. 2011, 38, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Kasuga, Y.; Fukuma, Y.; Kajikawa, K.; Akita, K.; Tamai, J.; Tanaka, Y.; Otani, T.; Fukutake, M.; Ikenoue, S.; Tanaka, M. Perinatal Outcomes of Chronic Abruption Oligohydramnios Sequence: A Multicenter Retrospective Observational Study. J. Clin. Med. 2025, 14, 5523. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, M.; Itani, Y.; Yamanaka, M.; Goto, A.; Kato, K.; Ijiri, R.; Tanaka, Y. Maternal, neonatal, and placental features associated with diffuse chorioamniotic hemosiderosis, with special reference to neonatal morbidity and mortality. Pediatrics 2004, 113, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Dey, S.K.; Fisher, S.J. Preterm labor: One syndrome, many causes. Science 2014, 345, 760–765. [Google Scholar] [CrossRef]
- Kindinger, L.M.; Bennett, P.R.; Lee, Y.S.; Marchesi, J.R.; Smith, A.; Cacciatore, S.; Holmes, E.; Nicholson, J.K.; Teoh, T.G.; MacIntyre, D.A. The interaction between vaginal microbiota, cervical length, and vaginal progesterone treatment for preterm birth risk. Microbiome 2017, 5, 6. [Google Scholar] [CrossRef]
- Freitas, A.C.; Chaban, B.; Bocking, A.; Rocco, M.; Yang, S.; Hill, J.E.; Money, D.M.; Group, V.R. The vaginal microbiome of pregnant women is less rich and diverse, with lower prevalence of Mollicutes, compared to non-pregnant women. Sci. Rep. 2017, 7, 9212. [Google Scholar] [CrossRef]
- Fettweis, J.M.; Serrano, M.G.; Brooks, J.P.; Edwards, D.J.; Girerd, P.H.; Parikh, H.I.; Huang, B.; Arodz, T.J.; Edupuganti, L.; Glascock, A.L.; et al. The vaginal microbiome and preterm birth. Nat. Med. 2019, 25, 1012–1021. [Google Scholar] [CrossRef]
- Gudnadottir, U.; Debelius, J.W.; Du, J.; Hugerth, L.W.; Danielsson, H.; Schuppe-Koistinen, I.; Fransson, E.; Brusselaers, N. The vaginal microbiome and the risk of preterm birth: A systematic review and network meta-analysis. Sci. Rep. 2022, 12, 7926. [Google Scholar] [CrossRef]
- Vidmar Simic, M.; Maver, A.; Zimani, A.N.; Hocevar, K.; Peterlin, B.; Kovanda, A.; Premru-Srsen, T. Oral microbiome and preterm birth. Front. Med. 2023, 10, 1177990. [Google Scholar] [CrossRef]
- Hong, Y.M.; Lee, J.; Cho, D.H.; Jeon, J.H.; Kang, J.; Kim, M.G.; Lee, S.; Kim, J.K. Predicting preterm birth using machine learning techniques in oral microbiome. Sci. Rep. 2023, 13, 21105. [Google Scholar] [CrossRef]
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The placenta harbors a unique microbiome. Sci. Transl. Med. 2014, 6, 237ra65. [Google Scholar] [CrossRef] [PubMed]
- de Goffau, M.C.; Lager, S.; Sovio, U.; Gaccioli, F.; Cook, E.; Peacock, S.J.; Parkhill, J.; Charnock-Jones, D.S.; Smith, G.C.S. Human placenta has no microbiome but can contain potential pathogens. Nature 2019, 572, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Itakura, A.; Shoji, S.; Shigeru, A.; Kotaro, F.; Junichi, H.; Hironobu, H.; Kamei, Y.; Eiji, K.; Shintaro, M.; Ryu, M.; et al. Guidelines for obstetrical practice in Japan: Japan Society of Obstetrics and Gynecology and Japan Association of Obstetricians and Gynecologists 2020 edition. J. Obstet. Gynaecol. Res. 2023, 49, 5–53. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Saadaoui, M.; Djekidel, M.N.; Murugesan, S.; Kumar, M.; Elhag, D.; Singh, P.; Kabeer, B.S.A.; Marr, A.K.; Kino, T.; Brummaier, T.; et al. Exploring the composition of placental microbiome and its potential origin in preterm birth. Front. Cell. Infect. Microbiol. 2024, 14, 1486409. [Google Scholar] [CrossRef]
- Huang, C.; Gin, C.; Fettweis, J.; Foxman, B.; Gelaye, B.; MacIntyre, D.A.; Subramaniam, A.; Fraser, W.; Tabatabaei, N.; Callahan, B. Meta-analysis reveals the vaginal microbiome is a better predictor of earlier than later preterm birth. BMC Biol. 2023, 21, 199. [Google Scholar] [CrossRef]
- Ferrante, M.; Oliveri Conti, G.; Pulvirenti, E.; Favara, C.; Fiore, M.; Cristaldi, A. The vaginal microbiota and preterm birth: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2025, 311, 114007. [Google Scholar] [CrossRef]
- Serrano, M.G.; Parikh, H.I.; Brooks, J.P.; Edwards, D.J.; Arodz, T.J.; Edupuganti, L.; Huang, B.; Girerd, P.H.; Bokhari, Y.A.; Bradley, S.P.; et al. Racioethnic diversity in the dynamics of the vaginal microbiome during pregnancy. Nat. Med. 2019, 25, 1001–1011. [Google Scholar] [CrossRef]
- Baud, A.; Hillion, K.H.; Plainvert, C.; Tessier, V.; Tazi, A.; Mandelbrot, L.; Poyart, C.; Kennedy, S.P. Microbial diversity in the vaginal microbiota and its link to pregnancy outcomes. Sci. Rep. 2023, 13, 9061. [Google Scholar] [CrossRef]
- Jung, E.; Romero, R.; Suksai, M.; Gotsch, F.; Chaemsaithong, P.; Erez, O.; Conde-Agudelo, A.; Gomez-Lopez, N.; Berry, S.M.; Meyyazhagan, A.; et al. Clinical chorioamnionitis at term: Definition, pathogenesis, microbiology, diagnosis, and treatment. Am. J. Obstet. Gynecol. 2024, 230, S807–S840. [Google Scholar] [CrossRef] [PubMed]
- Chigusa, Y.; Mogami, H.; Minamiguchi, S.; Kido, A.; Ishida, A.; Kurata, Y.; Yasuda, E.; Kawasaki, K.; Horie, A.; Yamaguchi, K.; et al. Chronic abruption-oligohydramnios sequence (CAOS) revisited: Possible implication of premature rupture of membranes. J. Matern. Fetal Neonatal Med. 2022, 35, 6894–6900. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2--approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
| Characteristic | N | TB, N = 10 1 | PTB, N = 7 1 | CAOS, N = 3 1 | p-Value 2 |
|---|---|---|---|---|---|
| Age (year) | 20 | 37.5 (30–42) | 33.0 (29–35) | 35.0 (32–36) | <0.05 |
| Ethnicity | 20 | - | |||
| Japanese | 10 (100%) | 7 (100%) | 3 (100%) | ||
| Body mass index (kg/m2) | 20 | 21.7 (17.8–25.6) | 20.0 (17.6–24.2) | 24.6 (24.3–24.8) | 0.111 |
| Nulliparas | 20 | 7 (70%) | 5 (71%) | 2 (67%) | 1.000 |
| Mode of Delivery | 20 | ||||
| Vaginal Delivery | 2 (20%) | 5 (71%) | 0 (0%) | 0.072 | |
| Elective cesarean section | 8 (80%) | 0 (0%) | 0 (0%) | <0.05 | |
| Emergent cesarean section | 0 (0%) | 2 (29%) | 3 (100%) | <0.05 | |
| Gestational ages at vaginal sampling (week) | 20 | 31.0 (24–33) | 27.5 (24–33) | 26.0 (23–27) | 0.179 |
| Gestational ages at placental sampling (week) | 20 | 37.0 (34–37) | 32.0 (25–35) | 26.0 (23–28) | <0.05 |
| Gestational ages at oral sampling (week) | 20 | 31.0 (24–32) | 29.0 (23–33) | 26.0 (23–27) | 0.245 |
| Birth weight (g) | 20 | 2780.0 (2006–3503) | 2008.0 (724–2402) | 808.0 (374–834) | <0.05 |
| Apgar score (1 min) | 20 | 7.5 (6–8) | 5.0 (4–8) | 3.0 (2–7) | 0.062 |
| Apgar score (5 min) | 20 | 8.5 (7–9) | 7.0 (6–9) | 8.0 (5–8) | 0.087 |
| Umbilical artery pH | 20 | 7.3 (7.22–7.38) | 7.3 (7.26–7.43) | 7.34 (7.19–7.38) | 0.938 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukuma, Y.; Kasuga, Y.; Akita, K.; Tamai, J.; Tanaka, Y.; Hasegawa, K.; Otani, T.; Fukutake, M.; Ikenoue, S.; Morikawa, S.; et al. Oral, Vaginal, and Placental Microbiota Profiles in Japanese Pregnancies with Preterm Birth and Chronic Abruption-Oligohydramnios Sequence (CAOS): A Cross-Sectional Study. Medicina 2025, 61, 2096. https://doi.org/10.3390/medicina61122096
Fukuma Y, Kasuga Y, Akita K, Tamai J, Tanaka Y, Hasegawa K, Otani T, Fukutake M, Ikenoue S, Morikawa S, et al. Oral, Vaginal, and Placental Microbiota Profiles in Japanese Pregnancies with Preterm Birth and Chronic Abruption-Oligohydramnios Sequence (CAOS): A Cross-Sectional Study. Medicina. 2025; 61(12):2096. https://doi.org/10.3390/medicina61122096
Chicago/Turabian StyleFukuma, Yuka, Yoshifumi Kasuga, Keisuke Akita, Junko Tamai, Yuya Tanaka, Keita Hasegawa, Toshimitsu Otani, Marie Fukutake, Satoru Ikenoue, Satoru Morikawa, and et al. 2025. "Oral, Vaginal, and Placental Microbiota Profiles in Japanese Pregnancies with Preterm Birth and Chronic Abruption-Oligohydramnios Sequence (CAOS): A Cross-Sectional Study" Medicina 61, no. 12: 2096. https://doi.org/10.3390/medicina61122096
APA StyleFukuma, Y., Kasuga, Y., Akita, K., Tamai, J., Tanaka, Y., Hasegawa, K., Otani, T., Fukutake, M., Ikenoue, S., Morikawa, S., Nakagawa, T., Ishihara, K., & Tanaka, M. (2025). Oral, Vaginal, and Placental Microbiota Profiles in Japanese Pregnancies with Preterm Birth and Chronic Abruption-Oligohydramnios Sequence (CAOS): A Cross-Sectional Study. Medicina, 61(12), 2096. https://doi.org/10.3390/medicina61122096







