Lumbar Interlaminar Ventral Epidural Injection Without Catheter at L5–S1 for Lumbosacral Radicular Pain: A Pilot Feasibility Study
Abstract
1. Introduction
2. Materials and Methods
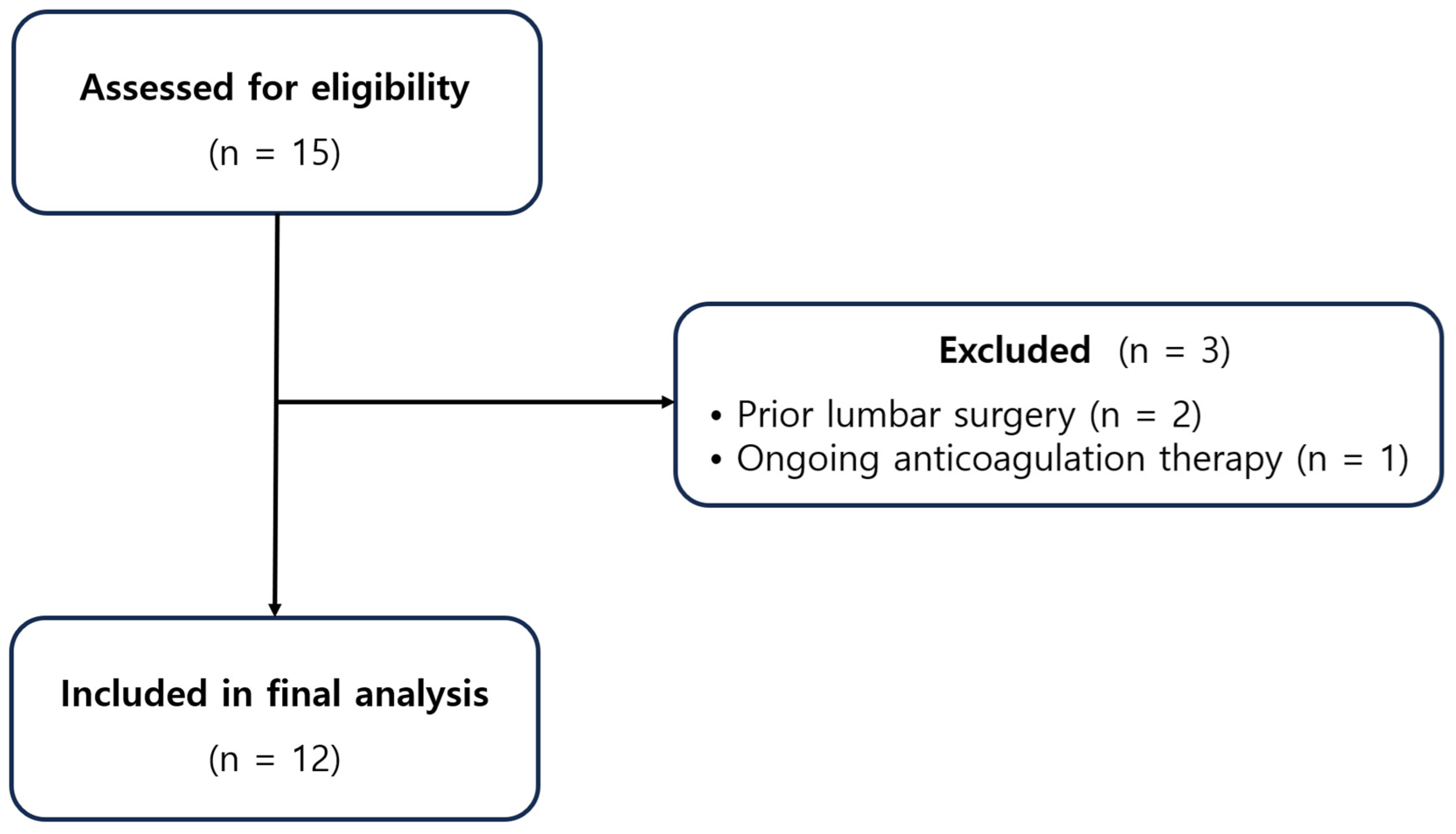
2.1. Study Design and Participants
2.2. Patient Selection and Clinical Evaluation
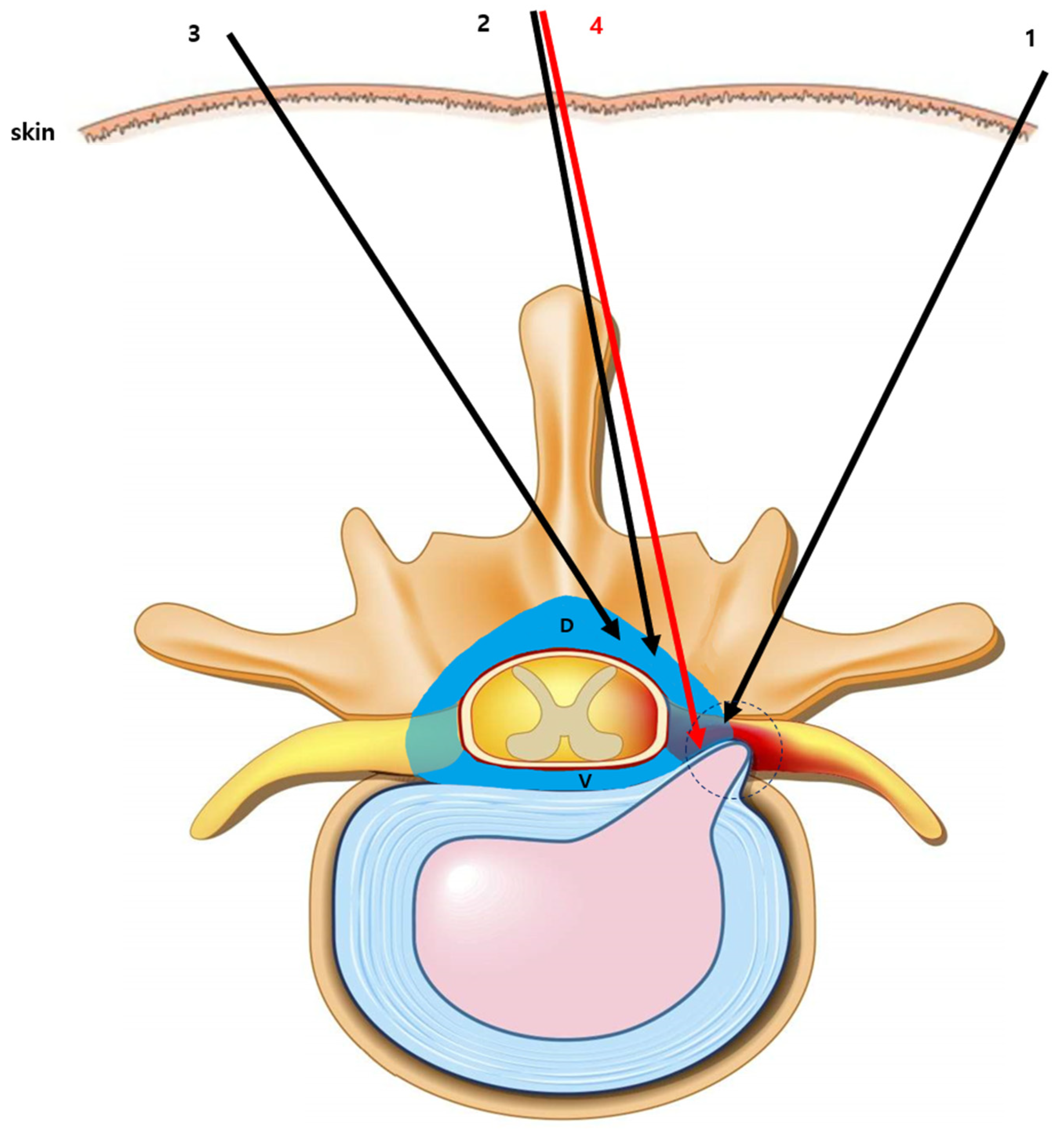
2.3. Procedural Technique for Catheter-Free LIVEI at L5–S1
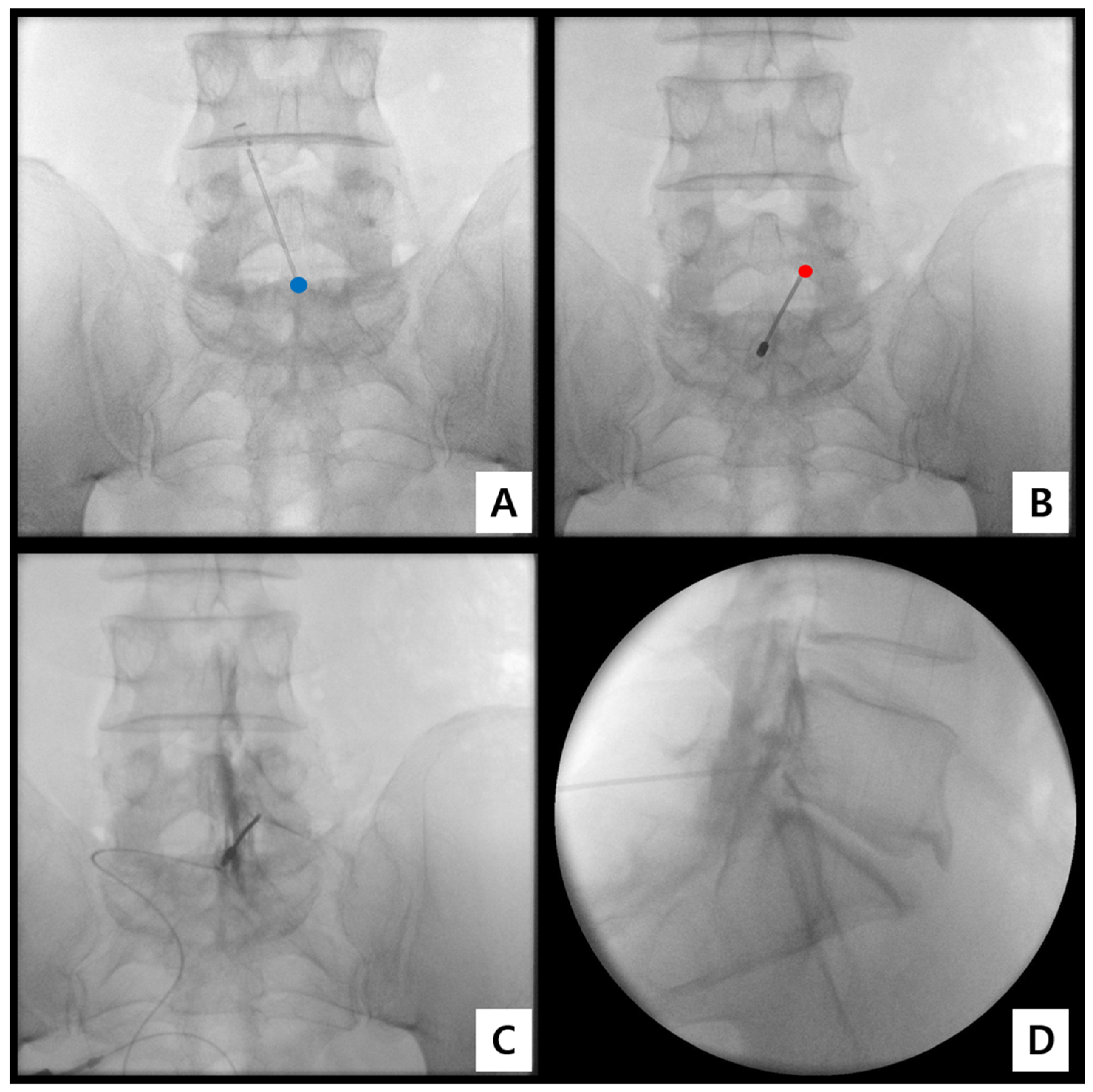
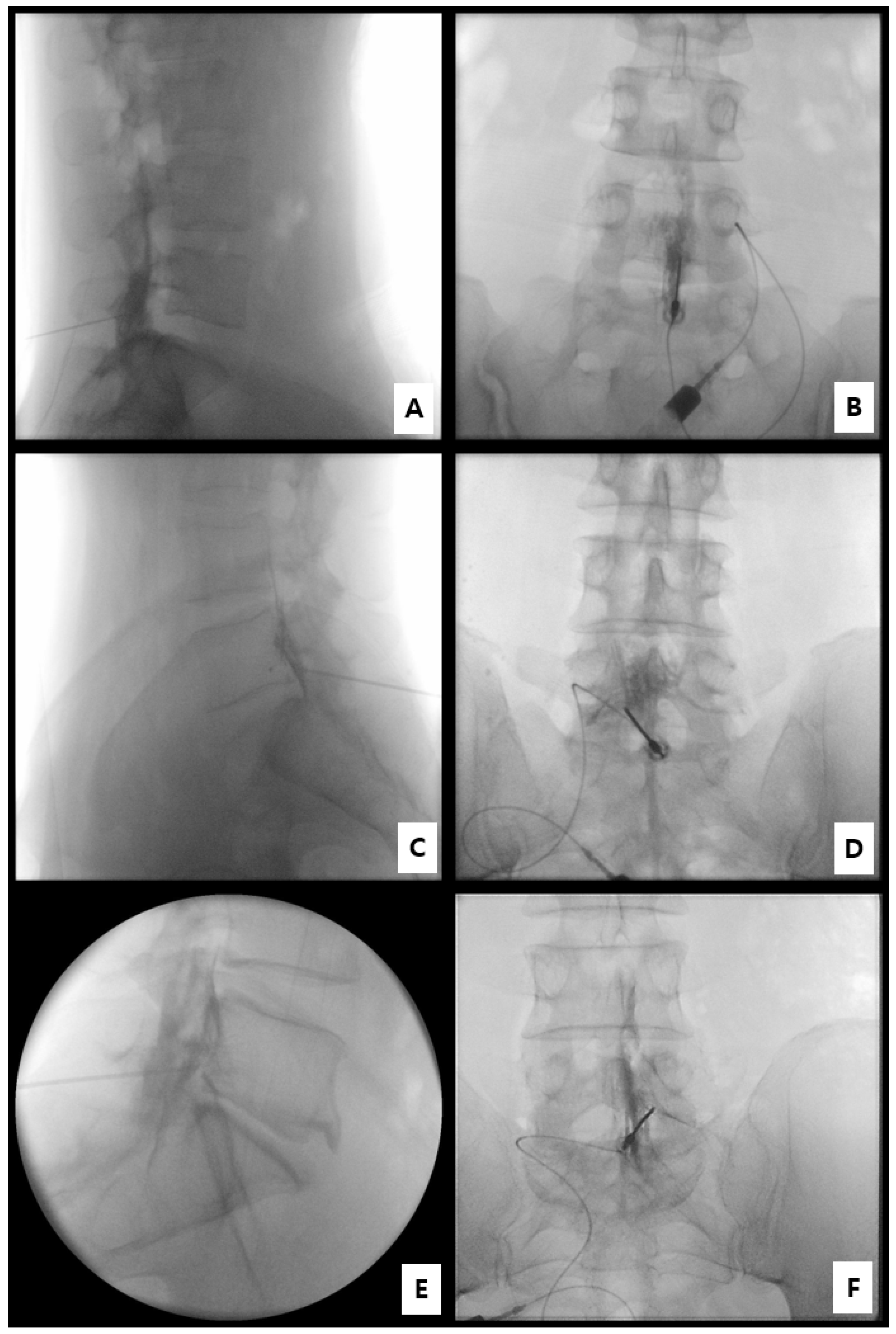
- Step 1. Fluoroscopic target identification (AP)
- Step 2. Midline entry and bone docking
- Step 3. Interlaminar epidural access confirmation
- Step 4. Controlled ventral advancement (“saline cushion” technique)
- Step 5. Fluoroscopic confirmation of ventral epidural positioning
- Step 6. Final injectate delivery
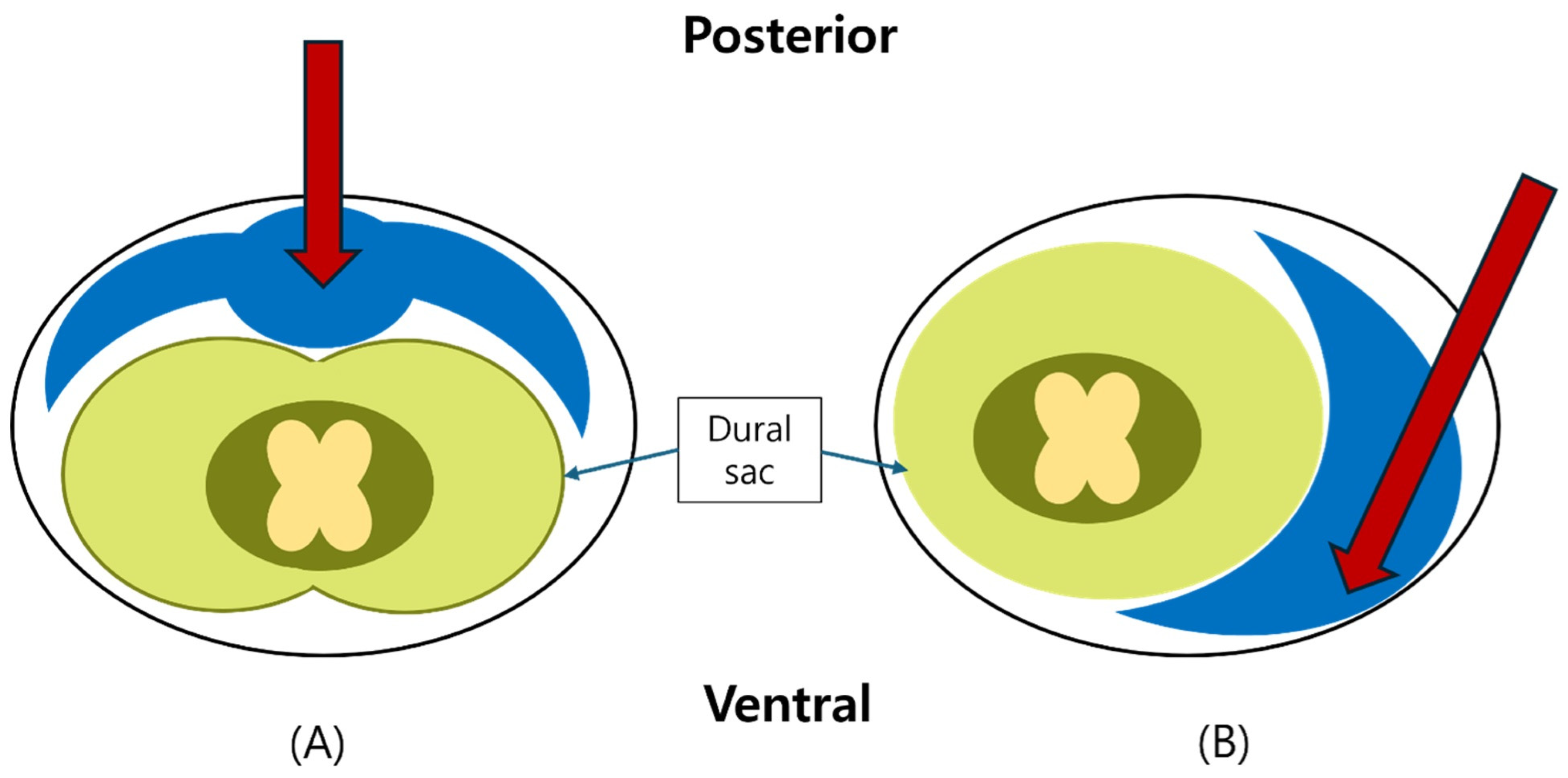
2.4. Fluoroscopic Assessment and Grading of Spread
- Grade 3: Anterior (ventral) epidural spread with foraminal extension at two or more levels;
- Grade 2: Anterior spread with limited foraminal or cranio-caudal extent;
- Grade 1: Posterior-only or failed anterior spread.
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Fluoroscopic Findings
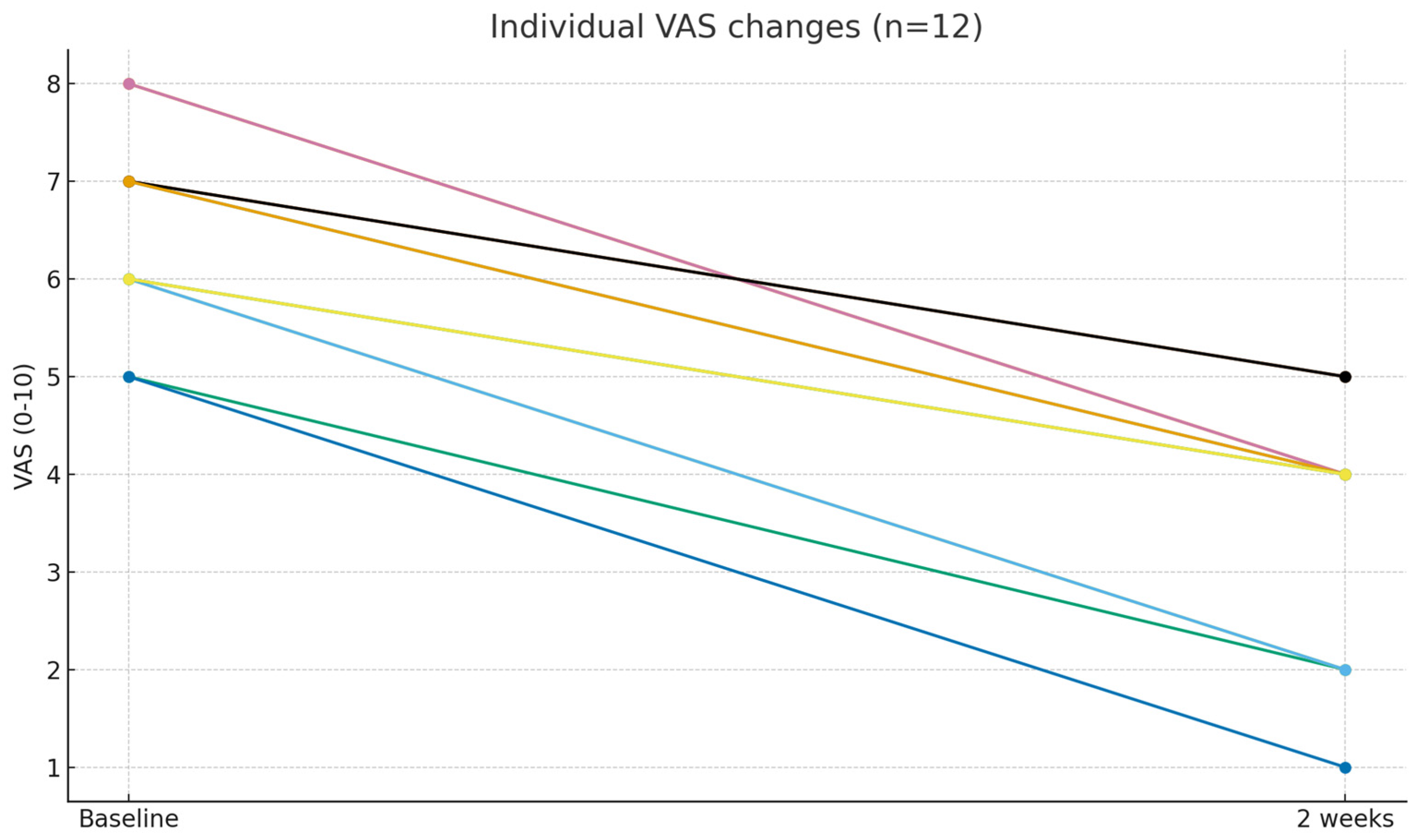
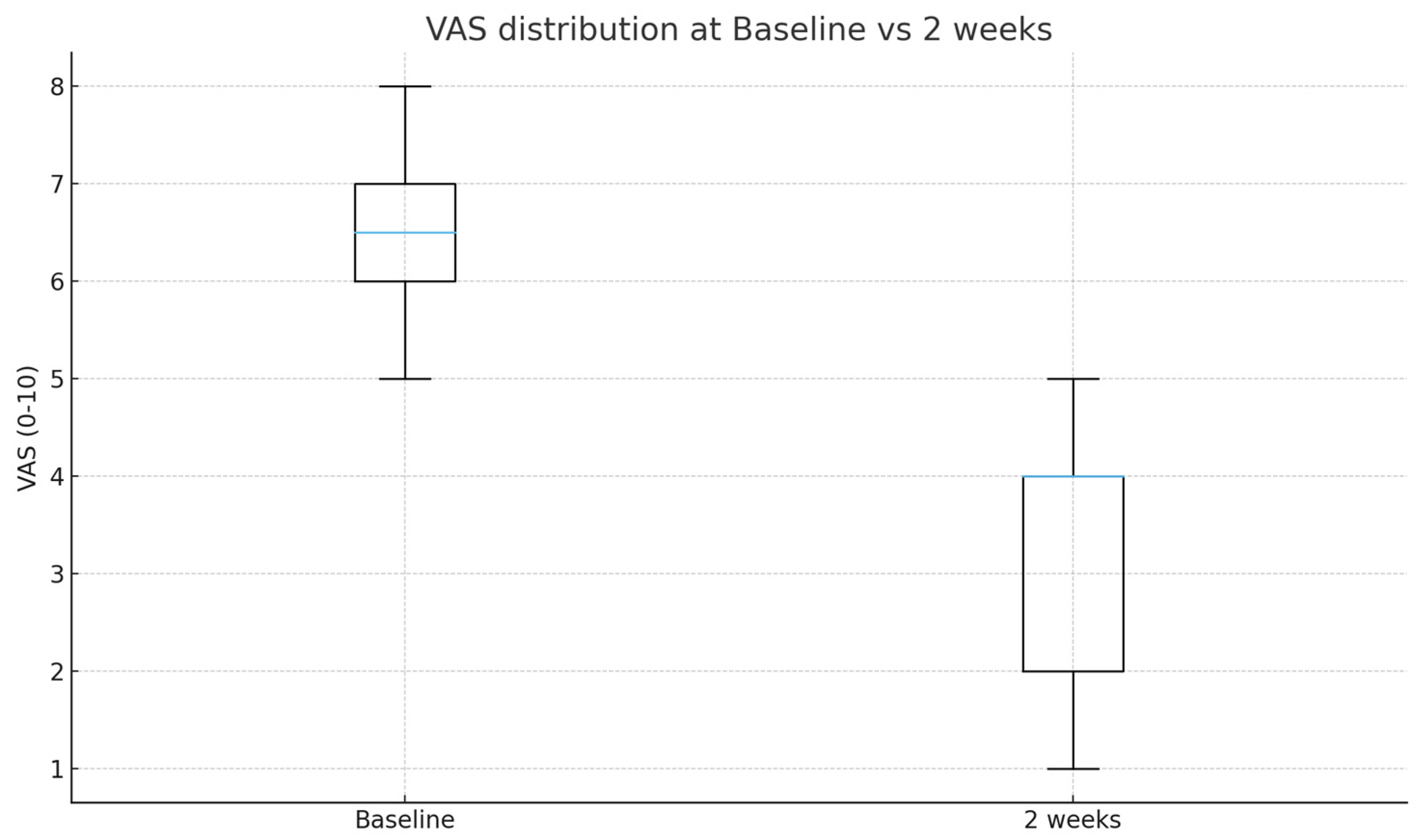
3.3. Pain Reduction
3.4. Safety Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LIVEI | Lumbar interlaminar ventral epidural injection |
| TFEI | Transforaminal epidural injection |
| VAS | Visual Analog Scale |
| IRB | Institutional Review Board |
References
- Abdi, S.; Datta, S.; Trescot, A.M.; Schultz, D.M.; Adlaka, R.; Atluri, S.L.; Smith, H.S.; Manchikanti, L. Epidural steroids in the management of chronic spinal pain: A systematic review. Pain Physician 2007, 10, 185–212. [Google Scholar] [CrossRef] [PubMed]
- Airaksinen, O.; Brox, J.I.; Cedraschi, C.; Hildebrandt, J.; Klaber-Moffett, J.; Kovacs, F.; Mannion, A.F.; Reis, S.; Staal, J.B.; Ursin, H.; et al. Chapter 4. European guidelines for the management of chronic nonspecific low back pain. Eur. Spine J. 2006, 15 (Suppl. S2), S192–S300. [Google Scholar] [CrossRef] [PubMed]
- Benyamin, R.M.; Wang, V.C.; Vallejo, R.; Singh, V.; Helm, S., II. A systematic evaluation of thoracic interlaminar epidural injections. Pain Physician 2012, 15, E497–E514. [Google Scholar] [CrossRef]
- Pinto, R.Z.; Maher, C.G.; Ferreira, M.L.; Hancock, M.; Oliveira, V.C.; McLachlan, A.J.; Koes, B.; Ferreira, P.H. Epidural corticosteroid injections in the management of sciatica: A systematic review and meta-analysis. Ann. Intern. Med. 2012, 157, 865–877. [Google Scholar] [CrossRef]
- Manchikanti, L.; Buenaventura, R.M.; Manchikanti, K.N.; Ruan, X.; Gupta, S.; Smith, H.S.; Christo, P.J.; Ward, S.P. Effectiveness of therapeutic lumbar transforaminal epidural steroid injections in managing lumbar spinal pain. Pain Physician 2012, 15, E199–E245. [Google Scholar] [CrossRef] [PubMed]
- Atluri, S.; Glaser, S.E.; Shah, R.V.; Sudarshan, G. Needle position analysis in cases of paralysis from transforaminal epidurals: Consider alternative approaches to traditional technique. Pain Physician 2013, 16, 321–334. [Google Scholar] [CrossRef]
- Candido, K.D.; Katz, J.A.; Chinthagada, M.; McCarthy, R.A.; Knezevic, N.N. Incidence of intradiscal injection during lumbar fluoroscopically guided transforaminal and interlaminar epidural steroid injections. Anesth. Analg. 2010, 110, 1464–1467. [Google Scholar] [CrossRef]
- Kennedy, D.J.; Dreyfuss, P.; Aprill, C.N.; Bogduk, N. Paraplegia following image-guided transforaminal lumbar spine epidural steroid injection: Two case reports. Pain Med. 2009, 10, 1389–1394. [Google Scholar] [CrossRef]
- Mandell, J.C.; Czuczman, G.J.; Gaviola, G.C.; Ghazikhanian, V.; Cho, C.H. The lumbar neural foramen and transforaminal epidural steroid injections: An anatomic review with key safety considerations in planning the percutaneous approach. AJR Am. J. Roentgenol. 2017, 209, W26–W35. [Google Scholar] [CrossRef]
- Wadhwa, H.; Rohde, M.; Koltsov, J.C.B.; Cabell, A.; Smuck, M.; Hu, S.S.; Kleimeyer, J.P. Incidence and risk factors for complications following cervical epidural steroid injections. Spine J. 2025, 25, 886–902. [Google Scholar] [CrossRef]
- Rozin, L.; Rozin, R.; Koehler, S.A.; Shakir, A.; Ladham, S.; Barmada, M.; Dominick, J.; Wecht, C.H. Death during transforaminal epidural steroid nerve root block (C7) due to perforation of the left vertebral artery. Am. J. Forensic Med. Pathol. 2003, 24, 351–355. [Google Scholar] [CrossRef]
- Ghai, B.; Vadaje, K.S.; Wig, J.; Dhillon, M.S. Lateral parasagittal versus midline interlaminar lumbar epidural steroid injection for management of low back pain with lumbosacral radicular pain: A double-blind, randomized study. Anesth. Analg. 2013, 117, 219–227. [Google Scholar] [CrossRef]
- Hashemi, M.; Mofrad, M.K.; Mohajerani, S.A.; Kazemi, S.M.; Radpey, B.; Zali, A. Anatomical flow pattern of contrast in lumbar epidural space: A human study with a midline vs. parasagittal interlaminar approach under fluoroscopy. Pain Physician 2015, 18, 317–324. [Google Scholar] [CrossRef]
- Kim, E.D.; Roh, M.S.; Park, J.J.; Jo, D. Comparison of the ventral epidural spreading in modified interlaminar approach and transforaminal approach: A randomized, double-blind study. Pain Med. 2016, 17, 1620–1627. [Google Scholar] [CrossRef]
- Manchikanti, L.; Knezevic, N.N.; Navani, A.; Christo, P.J.; Limerick, G.; Calodney, A.K.; Grider, J.; Harned, M.E.; Cintron, L.; Gharibo, C.G.; et al. Epidural interventions in the management of chronic spinal pain: American Society of Interventional Pain Physicians (ASIPP) comprehensive evidence-based guidelines. Pain Physician 2021, 24, S27–S208. [Google Scholar] [CrossRef]
- Gebrekristos, B.; Turcu, R.; Kotler, D.; Gureck, A.E.; Meleger, A.L. An update on technical and safety practice patterns of interlaminar epidural steroid injections. Interv. Pain Med. 2023, 2, 100371. [Google Scholar] [CrossRef]
- Choi, E.; Nahm, F.S.; Lee, P.B. Comparison of contrast flow and clinical effectiveness between a modified paramedian interlaminar approach and transforaminal approach in cervical epidural steroid injection. Br. J. Anaesth. 2015, 115, 768–774. [Google Scholar] [CrossRef]
- Choi, Y.K.; Barbella, J.D. Evaluation of epidurographic contrast patterns with fluoroscopically guided lumbar interlaminar ventral epidural injection. Pain Pract. 2009, 9, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, S.H.; Lee, S.; Park, S.B. Fluoroscopic analysis of ventral epidural spread in parasagittal interlaminar approach compared with transforaminal epidural injection. Pain Physician 2018, 21, 269–278. [Google Scholar] [PubMed]
- Manchikanti, L.; Boswell, M.V.; Singh, V.; Benyamin, R.M.; Fellows, B.; Falco, F.J. Safety and complications of epidural steroids: Evaluation of intravascular injection and epidurograms. Pain Physician 2009, 12, 864–871. [Google Scholar]
- Choi, E.; Park, K.D.; Lee, J.; Kim, S.; Lee, M.; Kim, H.; Yang, Y.; Lee, D.; Kwon, T.; Jeong, C.; et al. Modified interlaminar epidural approach versus transforaminal injection in cervical radicular pain: Contrast flow comparison under fluoroscopy. Korean J Pain. 2022, 25, E1379–E1388. [Google Scholar]
- Seidel, R.; Tietke, M.; Heese, O.; Walter, U. Serious complications after epidural catheter placement: Two case reports. Local Reg. Anesth. 2021, 14, 117–124. [Google Scholar] [CrossRef]
- van Kassel, M.N.; Hermanides, J.; Lirk, P.; Hollmann, M.W. Prevention of epidural catheter migration and inflammation by tunneling: A systematic review and meta-analysis. J. Clin. Med. 2025, 14, 5788. [Google Scholar] [CrossRef] [PubMed]
- Hasoon, J.; Mahmood, S.; Mahmood, S.; Kaye, A.D.; Robinson, C.L. Safety of catheter-based cervical epidural steroid injections: A retrospective review. Orthop. Rev. 2025, 17, 137668. [Google Scholar] [CrossRef] [PubMed]
- Toomey, P.J. A technique to avoid dural puncture by the epidural catheter. Anaesthesia 1992, 47, 79. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H.; Li, A.H.; Kuo-Sheng, H.; Wu, R.S.; Tan, P.P. Prior epidural injection of 10 mL normal saline reduces the incidence of inadvertent venous puncture in epidural catheterization. Acta Anaesthesiol. Sin. 1995, 33, 27–30. [Google Scholar]
- Candido, K.D.; Raghavendra, M.S.; Chinthagada, M.; Badiee, S.; Trepashko, D.W. A prospective evaluation of iodinated contrast flow patterns with fluoroscopically guided lumbar epidural steroid injections: The lateral parasagittal interlaminar versus transforaminal approach. Anesth. Analg. 2008, 106, 638–644. [Google Scholar] [CrossRef]
| Variable | Value |
|---|---|
| Age, years | 54.17 ± 15.48 |
| Sex, n (%) | Male 9 (75.0), Female 3 (25.0) |
| Injection level, n (%) | L5–S1, 12 (100) |
| Affected side, n (%) | Left 6 (50.0), Right 6 (50.0) |
| Baseline VAS | 6.50 ± 1.00 |
| Contrast Spread Grade | Definition | N (%) |
|---|---|---|
| Grade 3 | Anterior (ventral) spread with foraminal extension involving ≥ 2 levels | 8 (66.7%) |
| Grade 2 | Anterior (ventral) spread with limited foraminal involvement | 4 (33.3%) |
| Grade 1 | Posterior-only or absence of anterior (ventral) spread | 0 (0%) |
| Total | Ventral epidural spread confirmed | 12 (100%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Lee, S.; Lee, S.; Lim, C.; Kim, Y. Lumbar Interlaminar Ventral Epidural Injection Without Catheter at L5–S1 for Lumbosacral Radicular Pain: A Pilot Feasibility Study. Medicina 2025, 61, 2069. https://doi.org/10.3390/medicina61112069
Park J, Lee S, Lee S, Lim C, Kim Y. Lumbar Interlaminar Ventral Epidural Injection Without Catheter at L5–S1 for Lumbosacral Radicular Pain: A Pilot Feasibility Study. Medicina. 2025; 61(11):2069. https://doi.org/10.3390/medicina61112069
Chicago/Turabian StylePark, Jiho, Seounghun Lee, Sunyeul Lee, ChaeSeong Lim, and Yeojung Kim. 2025. "Lumbar Interlaminar Ventral Epidural Injection Without Catheter at L5–S1 for Lumbosacral Radicular Pain: A Pilot Feasibility Study" Medicina 61, no. 11: 2069. https://doi.org/10.3390/medicina61112069
APA StylePark, J., Lee, S., Lee, S., Lim, C., & Kim, Y. (2025). Lumbar Interlaminar Ventral Epidural Injection Without Catheter at L5–S1 for Lumbosacral Radicular Pain: A Pilot Feasibility Study. Medicina, 61(11), 2069. https://doi.org/10.3390/medicina61112069







