Prevalence, Clinical, and Immunological Features of Familial Type 1 Diabetes Among Children and Adolescents: A Retrospective Study from Saudi Arabia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Clinical and Laboratory Variables
2.3. Autoimmune Markers
2.4. Ethical Consideration
2.5. Statistical Analysis
3. Results
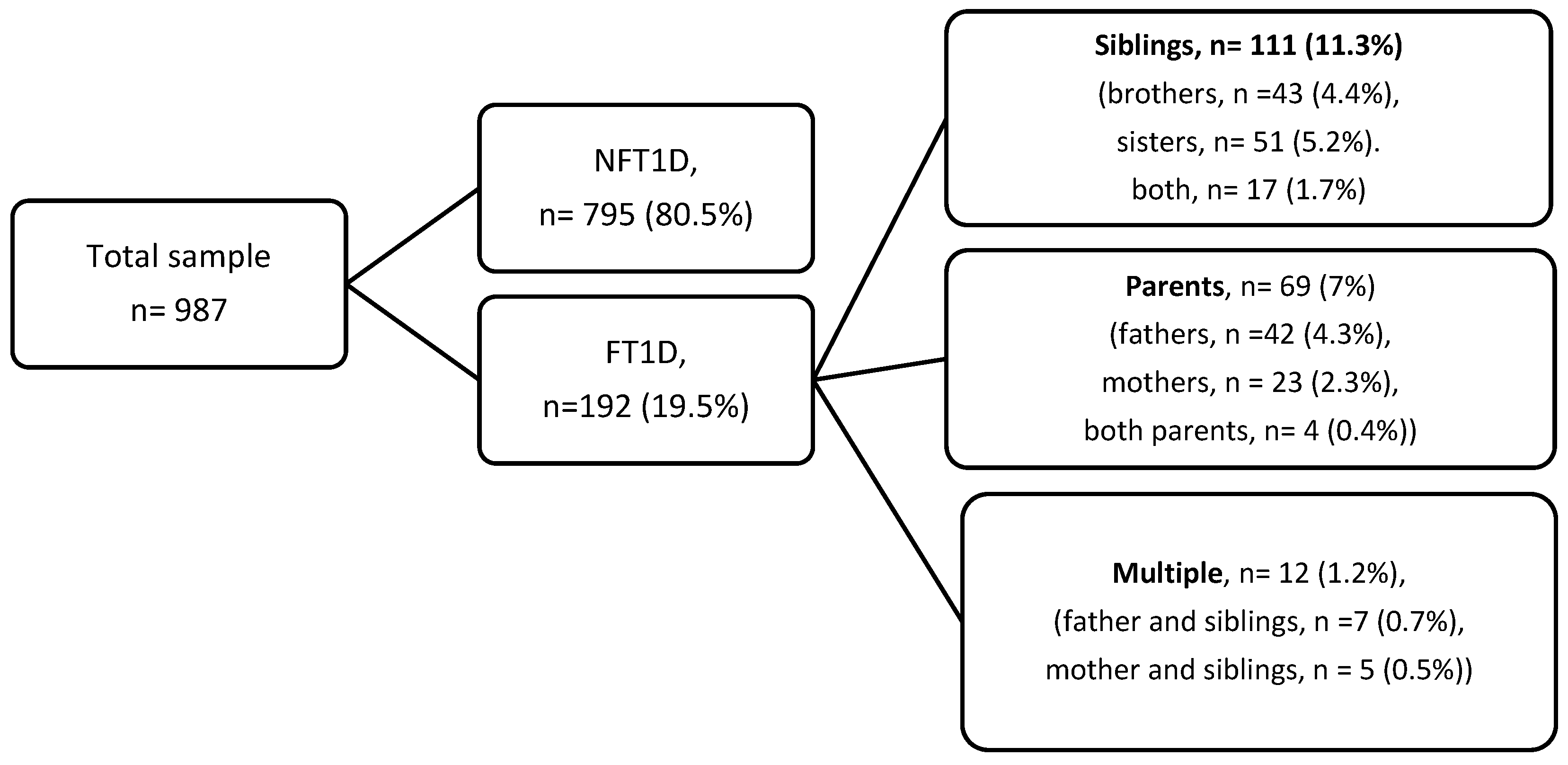
3.1. Prevalence, Sex, and Age Distribution
3.2. Clinical Presentation at Diagnosis
3.3. Autoimmune Markers
3.4. Multivariate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bell, K.J.; Lain, S.J. The Changing Epidemiology of Type 1 Diabetes: A Global Perspective. Diabetes Obes. Metab. 2025, 27 (Suppl. 6), 3–14. [Google Scholar] [CrossRef]
- Parkkola, A.; Härkönen, T.; Ryhänen, S.J.; Ilonen, J.; Knip, M. Extended family history of type 1 diabetes and phenotype and genotype of newly diagnosed children. Diabetes Care 2013, 36, 348–354. [Google Scholar] [CrossRef]
- Redondo, M.J.; Steck, A.K.; Pugliese, A. Genetics of type 1 diabetes. Pediatr. Diabetes 2018, 19, 346–353. [Google Scholar] [CrossRef]
- Turtinen, M.; Härkönen, T.; Parkkola, A.; Ilonen, J.; Knip, M.; Register, F.P.D. Characteristics of familial type 1 diabetes: Effects of the relationship to the affected family member on phenotype and genotype at diagnosis. Diabetologia 2019, 62, 2025–2039. [Google Scholar] [CrossRef]
- Al-Abdulrazzaq, D.; Hudda, M.T.; Hussein, D.K.; Alkandari, H. Are children with familial type 1 diabetes from Kuwait different? Report on prevalence, clinical, biochemical, and immunological characteristics. Prim. Care Diabetes 2025, 19, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Karges, B.; Prinz, N.; Placzek, K.; Datz, N.; Papsch, M.; Strier, U.; Agena, D.; Bonfig, W.; Kentrup, H.; Holl, R.W. A comparison of familial and sporadic type 1 diabetes among young patients. Diabetes Care 2021, 44, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Lebenthal, Y.; Shalitin, S.; Yackobovitch-Gavan, M.; Phillip, M.; Lazar, L. Retrospective comparative analysis of metabolic control and early complications in familial and sporadic type 1 diabetes patients. J. Diabetes Its Complicat. 2012, 26, 219–224. [Google Scholar] [CrossRef]
- Kuo, C.F.; Chou, I.J.; Grainge, M.J.; Luo, S.F.; See, L.C.; Yu, K.H.; Zhang, W.; Doherty, M.; Valdes, A.M. Familial aggregation and heritability of type 1 diabetes mellitus and coaggregation of chronic diseases in affected families. Clin. Epidemiol. 2018, 10, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- AlMutair, A.; AlSabty, N.; AlNuaim, H.; Al Hamdan, R.; Moukaddem, A. Prevalence and special clinical and biochemical characteristics of familial type 1 (insulin dependent) diabetes mellitus in pediatric patients in a tertiary care setting. Int. J. Pediatr. Adolesc. Med. 2021, 8, 107–111. [Google Scholar] [CrossRef]
- Kakleas, K.; Soldatou, A.; Karachaliou, F.; Karavanaki, K. Associated autoimmune diseases in children and adolescents with type 1 diabetes mellitus (T1DM). Autoimmun. Rev. 2015, 14, 781–797. [Google Scholar] [CrossRef]
- Veijola, R.; Reijonen, H.; Vähäsalo, P.; Sabbah, E.; Kulmala, P.; Ilonen, J.; Akerblom, H.K.; Knip, M. HLA-DQB1-defined genetic susceptibility, beta cell autoimmunity, and metabolic characteristics in familial and nonfamilial insulin-dependent diabetes mellitus. Childhood Diabetes in Finland (DiMe) Study Group. J. Clin. Investig. 1996, 98, 2489–2495. [Google Scholar] [CrossRef] [PubMed]
- Phillip, M.; Achenbach, P.; Addala, A.; Albanese-O’Neill, A.; Battelino, T.; Bell, K.J.; Besser, R.E.J.; Bonifacio, E.; Colhoun, H.M.; Couper, J.J.; et al. Consensus Guidance for Monitoring Individuals with Islet Autoantibody–Positive Pre-Stage 3 Type 1 Diabetes. Diabetes Care 2024, 47, 1276–1298. [Google Scholar] [CrossRef]
- Kahaly, G.J.; Hansen, M.P. Type 1 diabetes associated autoimmunity. Autoimmun. Rev. 2016, 15, 644–648. [Google Scholar] [CrossRef]
- Wägner, A.M.; Santana, Á.; Herńndez, M.; Wiebe, J.C.; Nóvoa, J.; Mauricio, D.; The T1DGC6. Predictors of associated autoimmune diseases in families with type 1 diabetes: Results from the Type 1 Diabetes Genetics Consortium. Diabetes/Metab. Res. Rev. 2011, 27, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Lebenthal, Y.; de Vries, L.; Phillip, M.; Lazar, L. Familial type 1 diabetes mellitus-gender distribution and age at onset of diabetes distinguish between parent-offspring and sib-pair subgroups. Pediatr. Diabetes 2010, 11, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Milluzzo, A.; Falorni, A.; Brozzetti, A.; Pezzino, G.; Tomaselli, L.; Tumminia, A.; Frittitta, L.; Vigneri, R.; Sciacca, L. Risk for Coexistent Autoimmune Diseases in Familial and Sporadic Type 1 Diabetes is Related to Age at Diabetes Onset. Endocr. Pract. 2021, 27, 110–117. [Google Scholar] [CrossRef]
- Lebenthal, Y.; Yackobovitch-Gavan, M.; de Vries, L.; Phillip, M.; Lazar, L. Coexistent Autoimmunity in Familial Type 1 Diabetes: Increased Susceptibility in Sib-Pairs? Horm. Res. Paediatr. 2011, 75, 284–290. [Google Scholar] [CrossRef]
- Usher-Smith, J.A.; Thompson, M.J.; Sharp, S.J.; Walter, F.M. Factors associated with the presence of diabetic ketoacidosis at diagnosis of diabetes in children and young adults: A systematic review. Bmj 2011, 343, d4092. [Google Scholar] [CrossRef] [PubMed]
- Yafei, S.; Hummadi, A.; Badedi, M.; Darraj, H.; Khawaji, A.; Alzughbi, T.; Abutaleb, R.; Alhagawy, A.J.; Alnami, A.; Kudam, B.; et al. Disordered Eating Behaviors and Insulin Restriction in Saudi Adolescents and Young Adults with Type 1 Diabetes. Medicina 2023, 59, 345. [Google Scholar] [CrossRef]
- Reddy, S.; Reinert, S.E.; Gopalakrishnan, G.; Plante, W.; Boney, C.M.; Quintos, J.B. Glycemic control in familial vs. sporadic type 1 diabetes patients over 5 years. J. Pediatr. Endocrinol. Metab. 2014, 27, 31–35. [Google Scholar] [CrossRef]
- O’Leary, L.A.; Dorman, J.S.; LaPorte, R.E.; Orchard, T.J.; Becker, D.J.; Kuller, L.H.; Eberhardt, M.S.; Cavender, D.E.; Rabin, B.S.; Drash, A.L. Familial and sporadic insulin-dependent diabetes: Evidence for heterogeneous etiologies? Diabetes Res. Clin. Pract. 1991, 14, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Vakharia, J.D.; Agrawal, S.; Molino, J.; Topor, L.S. Family History of Diabetes is Associated with Increased Risk of Recurrent Diabetic Ketoacidosis in Pediatric Patients. Endocr. Pract. 2020, 26, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Robert, A.A.; Al-Dawish, A.; Mujammami, M.; Dawish, M.A.A. Type 1 Diabetes Mellitus in Saudi Arabia: A Soaring Epidemic. Int. J. Pediatr. 2018, 2018, 9408370. [Google Scholar] [CrossRef] [PubMed]
- Eltayeb-Elsheikh, N.; Khalil, E.; Mubasher, M.; AlJurayyan, A.; AlHarthi, H.; Omer, W.H.; Elghazali, I.; Sherbeeni, S.M.; Alghofely, M.A.; Ilonen, J.; et al. Association of alleles, haplotypes, and diplotypes with type 1 diabetes in Saudis. Diabetes/Metab. Res. Rev. 2020, 36, e3345. [Google Scholar] [CrossRef]
- Manan, H.; Angham, A.M.; Sitelbanat, A. Genetic and diabetic auto-antibody markers in Saudi children with type 1 diabetes. Hum. Immunol. 2010, 71, 1238–1242. [Google Scholar] [CrossRef]
- Algadi, I.S.; AlRuthia, Y.; Mujammami, M.H.; Aburisheh, K.H.; Alotaibi, M.; Al Issa, S.; Al-Saif, A.A.; Seftel, D.; Tsai, C.T.; Al Khalifah, R.A. Early Detection of Type 1 Diabetes in First-Degree Relatives in Saudi Arabia (VISION-T1D): Protocol for a Pilot Implementation Study. JMIR Res. Protoc. 2025, 14, e70575. [Google Scholar] [CrossRef]
- Alyafei, F.; Soliman, A.; Alkhalaf, F.; Sabt, A.; De Sanctis, V.; Elsayed, N.; Waseef, R. Clinical and biochemical characteristics of familial type 1 diabetes mellitus (FT1DM) compared to non-familial type 1 DM (NFT1DM). Acta Bio Medica Atenei Parm. 2018, 89, 27–31. [Google Scholar]
- Libman, I.; Haynes, A.; Lyons, S.; Pradeep, P.; Rwagasor, E.; Tung, J.Y.; Jefferies, C.A.; Oram, R.A.; Dabelea, D.; Craig, M.E. ISPAD Clinical Practice Consensus Guidelines 2022: Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr. Diabetes 2022, 23, 1160–1174. [Google Scholar] [CrossRef]
- Ogle, G.D.; Wang, F.; Haynes, A.; Gregory, G.A.; King, T.W.; Deng, K.; Dabelea, D.; James, S.; Jenkins, A.J.; Li, X.; et al. Global type 1 diabetes prevalence, incidence, and mortality estimates 2025: Results from the International diabetes Federation Atlas, 11th Edition, and the T1D Index Version 3.0. Diabetes Res. Clin. Pract. 2025, 225, 112277. [Google Scholar] [CrossRef]
- Al-Yaarubi, S.; Ullah, I.; Sharef, S.W.; Al Shidhani, A.; Al Hanai, S.; Al Kalbani, R.; Al Jamoodi, S. Demographic and clinical characteristics of type 1 diabetes mellitus in omani children-single center experience. Oman Med. J. 2014, 29, 119–122. [Google Scholar] [CrossRef]
- Hemminki, K.; Li, X.; Sundquist, J.; Sundquist, K. Familial association between type 1 diabetes and other autoimmune and related diseases. Diabetologia 2009, 52, 1820–1828. [Google Scholar] [CrossRef] [PubMed]
- Roche, E.F.; Menon, A.; Gill, D.; Hoey, H. Clinical presentation of type 1 diabetes. Pediatr. Diabetes 2005, 6, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Khayat, A.M.; Alshareef, B.G.; Alharbi, S.F.; AlZahrani, M.M.; Alshangity, B.A.; Tashkandi, N.F. Consanguineous Marriage and Its Association with Genetic Disorders in Saudi Arabia: A Review. Cureus 2024, 16, e53888. [Google Scholar] [CrossRef] [PubMed]
- Albishi, L.A.; AlAmri, E.; Mahmoud, A.A. Relationships among consanguinity, family history, and the onset of type 1 diabetes in children from Saudi Arabia. Prim. Care Diabetes 2022, 16, 102–106. [Google Scholar] [CrossRef]
- Parkkola, A.; Laine, A.P.; Karhunen, M.; Härkönen, T.; Ryhänen, S.J.; Ilonen, J.; Knip, M. HLA and non-HLA genes and familial predisposition to autoimmune diseases in families with a child affected by type 1 diabetes. PLoS ONE 2017, 12, e0188402. [Google Scholar] [CrossRef]
- Warram, J.H.; Krolewski, A.S.; Gottlieb, M.S.; Kahn, C.R. Differences in risk of insulin-dependent diabetes in offspring of diabetic mothers and diabetic fathers. N. Engl. J. Med. 1984, 311, 149–152. [Google Scholar] [CrossRef]
- Dahlquist, G.G.; Mustonen, L.R. Clinical Onset Characteristics of Familial Versus Nonfamilial Cases in a Large Population-based Cohort of Childhood-Onset Diabetes Patients. Diabetes Care 1995, 18, 852–854. [Google Scholar] [CrossRef]
- Al-Abdulrazzaq, D.; Othman, F.; Qabazard, S.; Al-Tararwa, A.; Ahmad, D.; Al-Sanae, H.; Al-Kandari, H. Epidemiological trends in the presentation of diabetic ketoacidosis in children newly diagnosed with type 1 diabetes from 2011 to 2017 in Kuwait. Front. Endocrinol. 2022, 13, 908458. [Google Scholar] [CrossRef]
- Małachowska, B.; Baranowska-Jaźwiecka, A.; Hogendorf, A.; Szadkowska, A.; Fendler, W.; Młynarski, W. Unequal contribution of familial factors to autoimmunity and clinical course of childhood diabetes. Pediatr. Endocrinol. Diabetes Metab. 2012, 18, 130–136. [Google Scholar] [PubMed]
- Hakami, M.; Yafei, S.; Hummadi, A.; Abutaleb, R.; Khawaji, A.; Solan, Y.; Aljohani, T.; Alhagawy, A.J.; Ali, A.A.; Bakkari, S.; et al. Clinical Characteristics and Prevalence of Celiac Disease in a Large Cohort of Type 1 Diabetes from Saudi Arabia. Medicina 2024, 60, 1940. [Google Scholar] [CrossRef]
| Overall n = 987 | FT1D (n = 192) | NFT1D (n = 795) | p * | Adjusted p ** | |
|---|---|---|---|---|---|
| Males, n (%) | 452 (45.8) | 90 (46.9) | 362 (45.5) | 0.74 | 0.58 |
| Females, n (%) | 535 (54.2) | 102 (53.1) | 433 (54.5) | ||
| Consanguinity, n (%) | 389 (39.4) | 92 (47.9) | 297 (37.4) | 0.007 | 0.011 |
| Age at diagnosis, years, mean (SD) | 9.1 (3.6) | 8.2 (3.4) | 9.3 (3.7) | 0.001 | <0.001 |
| Clinical and metabolic data at diagnosis | |||||
| Serum blood glucose, mg/dL | 452 (87) | 431 (84) | 457 (87) | <0.001 | 0.001 |
| C-peptide level, nmol/liter, median (IQR) | 0.07 (0.05–0.11) | 0.11 (0.08–0.15) | 0.07 (0.05–0.10) | <0.001 | <0.001 |
| DKA at diagnosis, n (%) | 460 (46.6) | 65 (33.9) | 395 (49.7) | <0.001 | <0.001 |
| ICU admission, n (%) | 191 (19.4) | 26 (13.5) | 165 (20.8) | 0.023 | 0.019 |
| HbA1c, mean (SD) | 11.8 (1.6) | 10.9 (1.5) | 12 (1.5) | <0.001 | <0.001 |
| Initial Insulin dose, U/kg/d | 0.8 (0.2) | 0.6 (0.1) | 0.8 (0.2) | <0.001 | <0.001 |
| Clinical and metabolic data at inclusion | |||||
| Age at inclusion, years, mean (SD) | 13.8 (3.5) | 13.5 (3.2) | 13.8 (3.5) | 0.26 | 0.26 |
| Duration, years, mean (SD) | 4.7 (2.7) | 5.3 (2.8) | 4.6 (2.7) | 0.001 | 0.001 |
| HbA1c at inclusion, mean (SD) | 8.6 (2) | 8.7 (2.1) | 8.5 (2) | 0.28 | 0.44 |
| DKA history in the last year, n (%) | 69 (7) | 16 (80.2) | 53 (6.7) | 0.42 | 0.6 |
| Severe hypoglycemia in the last year, n (%) | 181 (18.3) | 41 (21.4) | 140 (17.6) | 0.23 | 0.32 |
| Management modality | |||||
| Carb counting, n (%) | 328 (33.2) | 56 (29.2) | 272 (34.2) | 0.20 | 0.12 |
| Insulin pump users, n (%) | 87 (8.8) | 19 (9.9) | 68 (8.7) | 0.86 | |
| Basal bolus insulin, n (%) | 841 (85.2) | 162 (84.4) | 679 (85.4) | 0.56 | |
| Pre-mixed insulin, n (%) | 58 (5.9) | 11 (5.7) | 47 (5.9) | ||
| Overall n = 987 | FT1D (n = 192) | NFT1D (n = 795) | p * | Adjusted p ** | |
|---|---|---|---|---|---|
| Pancreatic autoantibody positivity rate, n (%) | 656 (66.5) | 134 (69.8) | 522 (65.7) | 0.28 | 0.25 |
| Glutamic acid decarboxylase antibody (GADA), n (%) | 605 (61.3) | 112 (58.3) | 493 (62) | 0.35 | 0.36 |
| Insulin auto-antibody (IAA), n (%) | 397 (40.2) | 83 (43.2) | 314 (39.5) | 0.34 | 0.27 |
| Anti-islet cell antibody (ICA), n (%) | 312 (31.6) | 51 (26.6) | 261 (32.8) | 0.09 | 0.12 |
| Overall extra-pancreatic autoantibody positivity, n (%) | 187 (18.9) | 50 (26) | 137 (17.2) | 0.005 | 0.003 |
| Thyroid peroxidase antibody, n (%) | 135 (13.7) | 34 (17.7) | 101 (12.7) | 0.07 | 0.058 |
| Anti-tissue transglutaminase antibody, n (%) | 121 (12.3) | 32 (16.7) | 89 (11.2) | 0.038 | 0.031 |
| Hypothyroidism, n (%) | 66 (6.7) | 17 (8.9) | 49 (6.2) | 0.18 | 0.11 |
| Hyperthyroidism, n (%) | 19 (1.9) | 5 (2.6) | 14 (1.8) | 0.45 b | 0.44 |
| Celiac disease, n (%) | 91 (9.2) | 25 (13) | 66 (8.3) | 0.043 | 0.045 |
| Vitiligo, n (%) | 9 (0.9) | 3 (1.6%) | 6 (0.8) | 0.29 b | 0.29 |
| Logistic Regression Model | β = Odds Ratios, 95% Confidence Interval | p |
|---|---|---|
| DKA at diagnosis | 0.540 (0.387, 0.755) | <0.001 |
| ICU admission at diagnosis | 0.583 (0.371, 0.916) | 0.019 |
| Overall extra-pancreatic autoantibodies | 1.778 (1.1214, 2.605) | 0.003 |
| Anti-tissue transglutaminase antibody | 1.637 (1.046, 2.563 | 0.031 |
| Linear Regression Model | B = Unstandardized Coefficient, 95% CI | |
| Age at diagnosis, years * | −1 (−1.573, −0.428) | <0.001 |
| Serum blood glucose, mg/dL | −22.588 (−39.531, −12.209) | <0.001 |
| C peptide, nmol/liter | 0.053 (0.042, 0.064) | <0.001 |
| Hemoglobin A1c, % | −1.01 (−1.251, −0.768) | <0.001 |
| Initial insulin dose, U/kg/d | −0.19 (−0.219, −0.170) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abutaleb, R.; Yafei, S.; Hummadi, A.; Solan, Y.; Khawaji, A.; Hakami, M.; Alhagawy, A.J.; Al Ali, A.; Adawi, M.; Makrami, A.; et al. Prevalence, Clinical, and Immunological Features of Familial Type 1 Diabetes Among Children and Adolescents: A Retrospective Study from Saudi Arabia. Medicina 2025, 61, 2066. https://doi.org/10.3390/medicina61112066
Abutaleb R, Yafei S, Hummadi A, Solan Y, Khawaji A, Hakami M, Alhagawy AJ, Al Ali A, Adawi M, Makrami A, et al. Prevalence, Clinical, and Immunological Features of Familial Type 1 Diabetes Among Children and Adolescents: A Retrospective Study from Saudi Arabia. Medicina. 2025; 61(11):2066. https://doi.org/10.3390/medicina61112066
Chicago/Turabian StyleAbutaleb, Raed, Saeed Yafei, Abdulrahman Hummadi, Yahia Solan, Abdullah Khawaji, Mohammed Hakami, Ali Jaber Alhagawy, Amer Al Ali, Morghema Adawi, Azizah Makrami, and et al. 2025. "Prevalence, Clinical, and Immunological Features of Familial Type 1 Diabetes Among Children and Adolescents: A Retrospective Study from Saudi Arabia" Medicina 61, no. 11: 2066. https://doi.org/10.3390/medicina61112066
APA StyleAbutaleb, R., Yafei, S., Hummadi, A., Solan, Y., Khawaji, A., Hakami, M., Alhagawy, A. J., Al Ali, A., Adawi, M., Makrami, A., Bahsan, F., Mashhour, M., Khardaly, L., Zahrani, D., Johar, R., & Algohani, N. (2025). Prevalence, Clinical, and Immunological Features of Familial Type 1 Diabetes Among Children and Adolescents: A Retrospective Study from Saudi Arabia. Medicina, 61(11), 2066. https://doi.org/10.3390/medicina61112066





