Adipokines as Prognostic Biomarkers in Multiple Myeloma: A Case–Control Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Biomarker Measurements
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
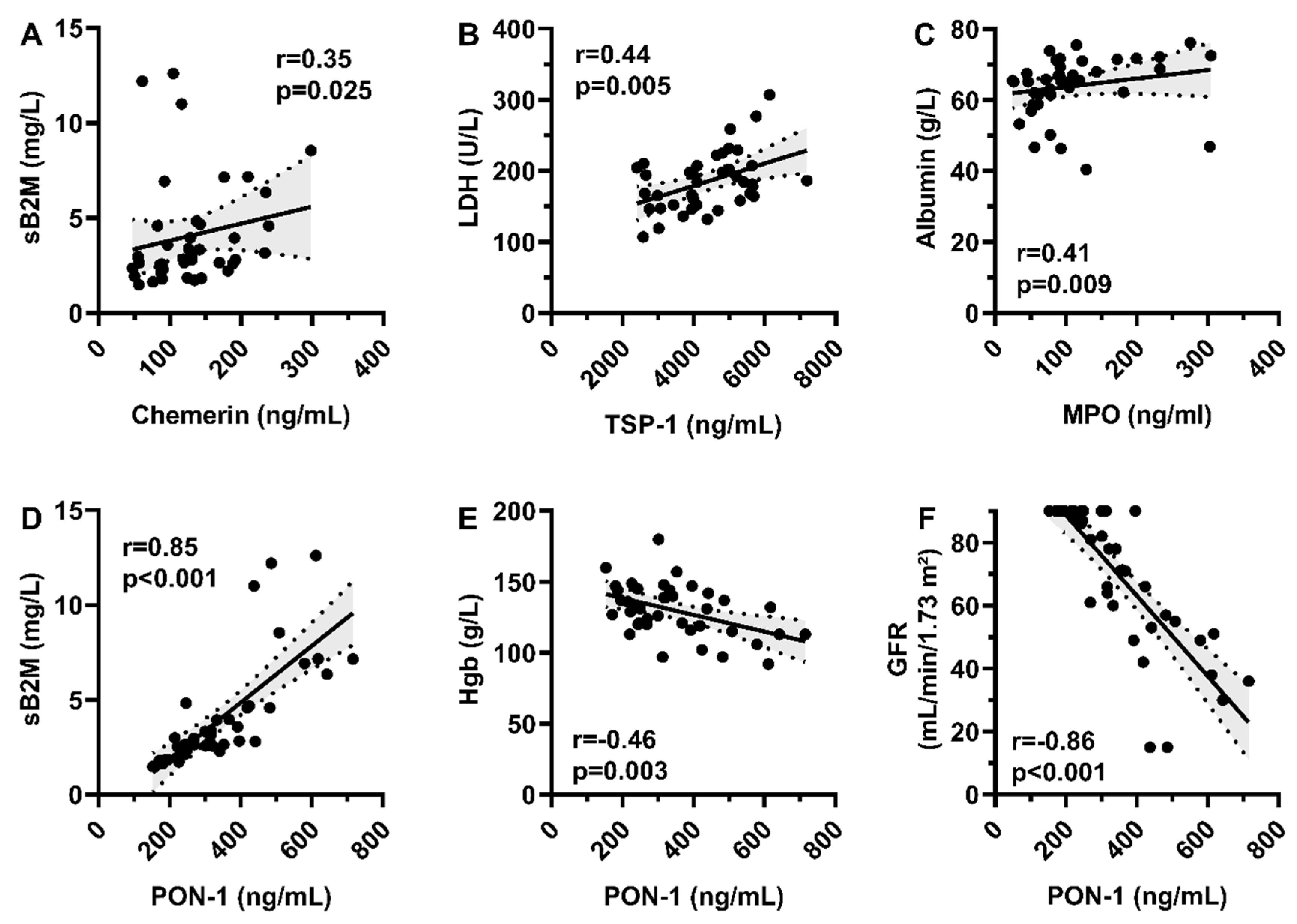
3.2. Correlation with Patient Biology and Disease Characteristics
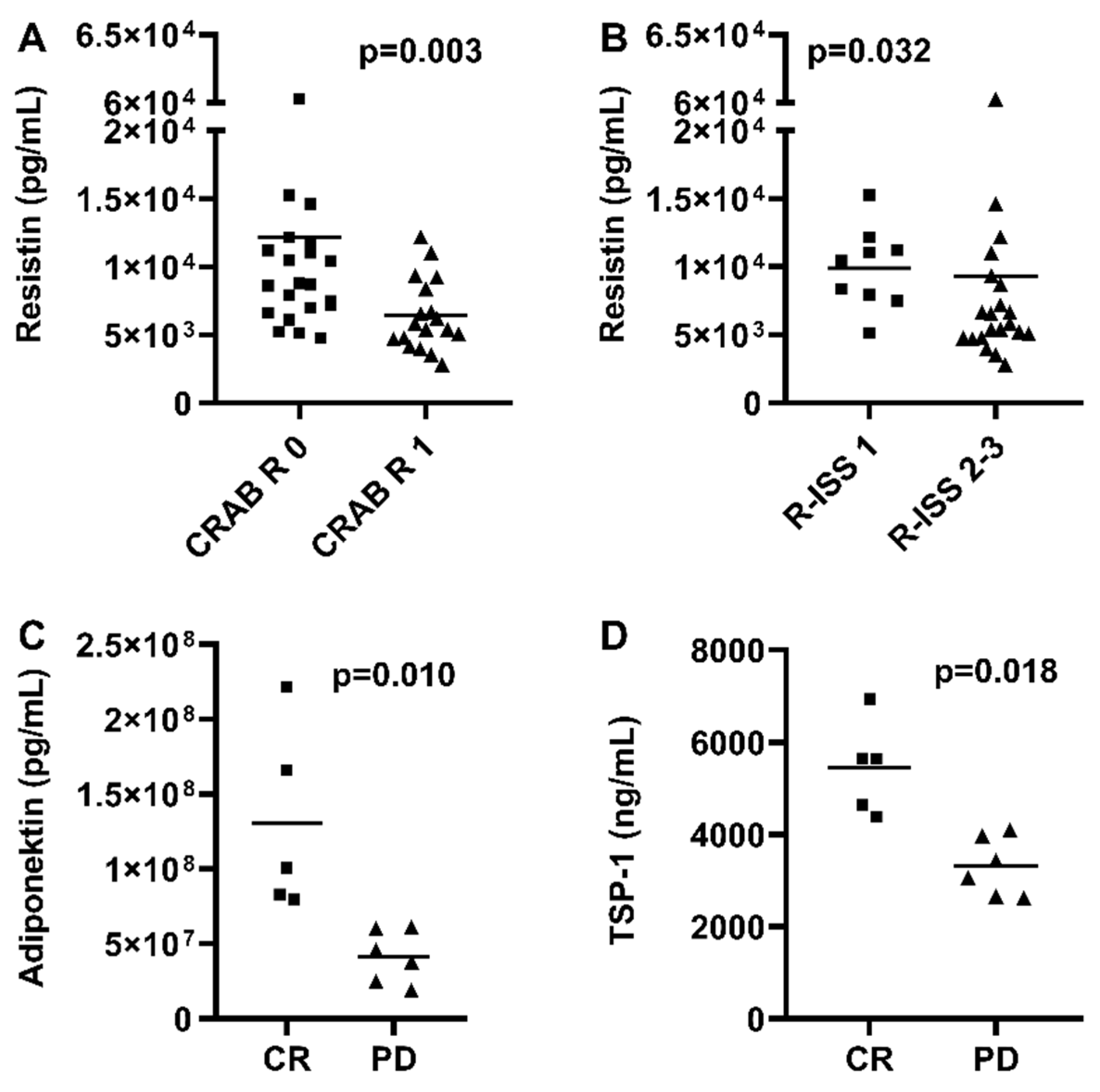
3.3. Correlation with Treatment Response
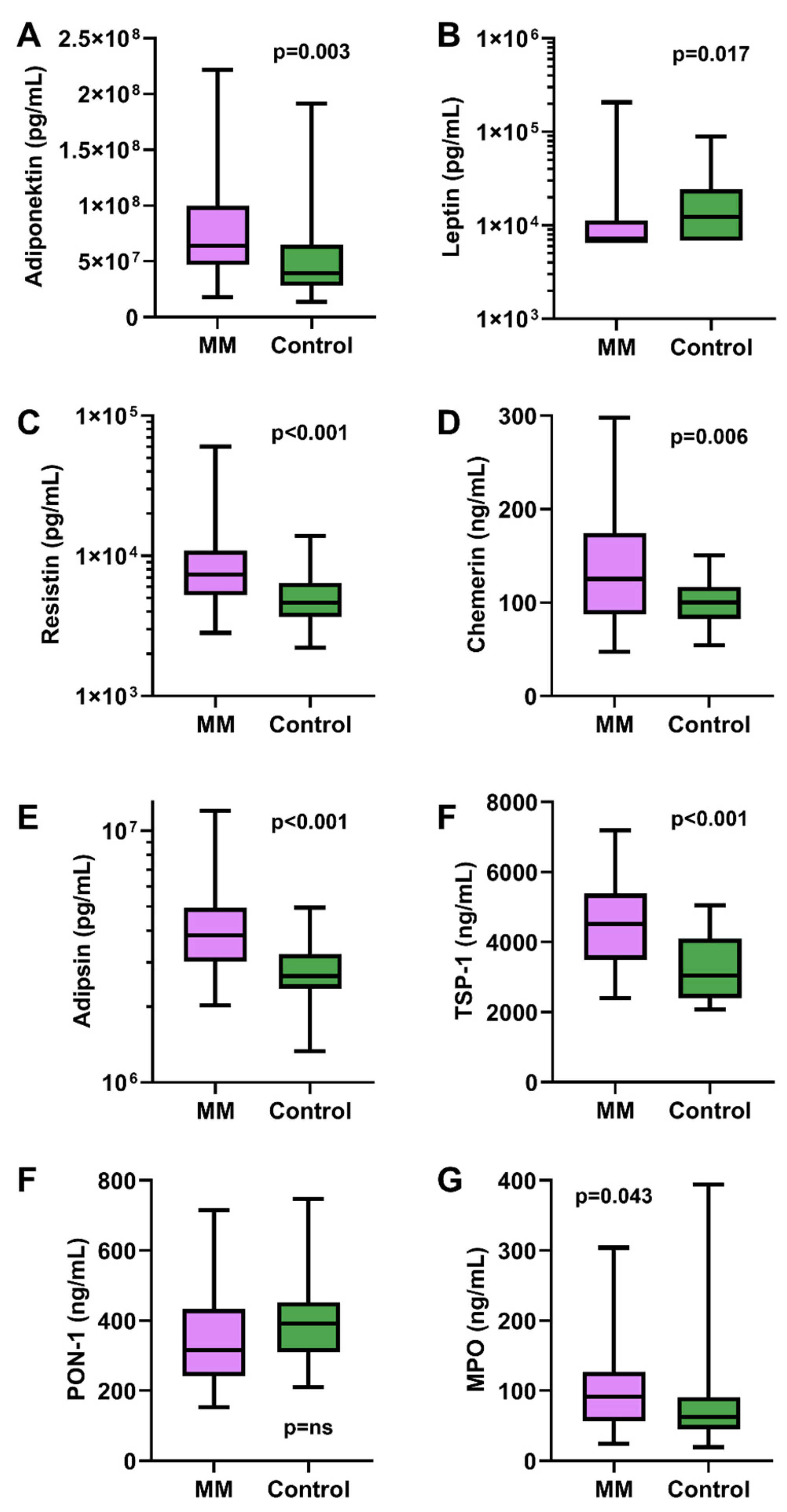
3.4. Biomarker Levels Compared to Controls
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Palumbo, A.; Anderson, K. Multiple myeloma. N. Engl. J. Med. 2011, 364, 1046–1060. [Google Scholar] [CrossRef]
- Rajkumar, S.V. Multiple myeloma: 2024 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2024, 99, 1802–1824. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Avet-Loiseau, H.; Oliva, S.; Lokhorst, H.M.; Goldschmidt, H.; Rosinol, L.; Richardson, P.; Caltagirone, S.; Lahuerta, J.J.; Facon, T.; et al. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. J. Clin. Oncol. 2015, 33, 2863–2869. [Google Scholar] [CrossRef]
- Lee, D.J.; El-Khour, H.; Tramontano, A.C.; Alberge, J.B.; Perry, J.; Davis, M.I.; Horowitz, E.; Redd, R.; Sakrikar, D.; Barnidge, D.; et al. Mass spectrometry-detected MGUS is associated with obesity and other novel modifiable risk factors in a high-risk population. Blood Adv. 2024, 8, 1737–1746. [Google Scholar] [CrossRef]
- Tie, W.; Ma, T.; Yi, Z.; Liu, J.; Li, Y.; Bai, J.; Li, L.; Zhang, L. Obesity as a risk factor for multiple myeloma: Insight on the role of adipokines. Pathol. Oncol. Res. 2023, 29, 1611338. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, Y.; Zhang, X.; Shang, M.; Dong, F. Association of lipid levels, adipokines and multiple myeloma: A two-sample multivariate Mendelian randomization study. Sci. Rep. 2024, 14, 25961. [Google Scholar] [CrossRef]
- Falank, C.; Fairfield, H.H.; Reagan, M.R. Signaling interplay between Bone Marrow Adipose Tissue and Multiple Myeloma cells. Front. Endocrinol. 2016, 7, 67. [Google Scholar] [CrossRef] [PubMed]
- Housa, D.; Housová, J.; Vernerová, Z.; Haluzík, M. Adipocytokines and cancer. Physiol. Res. 2006, 55, 233–244. [Google Scholar] [CrossRef]
- Samimi, A.; Ghanavat, M.; Shahrabi, S.; Azizidoost, S.; Saki, N. Role of bone marrow adipocytes in leukemia and chemotherapy challenges. Cell. Mol. Life Sci. 2019, 76, 2489–2497. [Google Scholar] [CrossRef]
- Morris, E.V.; Edwards, C.M. Adipokines, adiposity, and bone marrow adipocytes: Dangerous accomplices in multiple myeloma. J. Cell. Physiol. 2018, 233, 9159–9166. [Google Scholar] [CrossRef]
- Manna, L.; Gelsomino, L.; Martino, E.A.; Gentile, M.; Andò, S.; Bonofiglio, D.; Giordano, C.; Catalano, S.; Barone, I. Unraveling Obesity and Multiple Myeloma: Insights from Epidemiology and Molecular Mechanisms. Curr. Obes. Rep. 2025, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, J.N.; Liao, L.M.; Pollak, M.N.; Wang, Y.; Pfeiffer, R.M.; Baris, D.; Andreotti, G.; Lan, Q.; Landgren, O.; Rothman, N.; et al. A prospective study of circulating adipokine levels and risk of multiple myeloma. Blood 2012, 120, 4418–4420. [Google Scholar] [CrossRef]
- Hofmann, J.N.; Mailankody, S.; Korde, N.; Wang, Y.; Tageja, N.; Costello, R.; Zingone, A.; Hultcrantz, M.; Pollak, M.N.; Purdue, M.P.; et al. Circulating Adiponectin Levels Differ Between Patients with Multiple Myeloma and its Precursor Disease. Obesity 2017, 25, 1317–1320. [Google Scholar] [CrossRef]
- Hofmann, J.N.; Birmann, B.M.; Teras, L.R.; Pfeiffer, R.M.; Wang, Y.; Albanes, D.; Baris, D.; Colditz, G.A.; De Roos, A.J.; Giles, G.G.; et al. Low Levels of Circulating Adiponectin Are Associated with Multiple Myeloma Risk in Overweight and Obese Individuals. Cancer Res. 2016, 76, 1935–1941. [Google Scholar] [CrossRef]
- Avcu, F.; Ural, A.U.; Yilmaz, M.I.; Bingol, N.; Nevruz, O.; Caglar, K. Association of plasma adiponectin concentrations with chronic lymphocytic leukemia and myeloproliferative diseases. Int. J. Hematol. 2006, 83, 254–258. [Google Scholar] [CrossRef]
- Fitter, S.; Vandyke, K.; Schultz, C.G.; White, D.; Hughes, T.P.; Zannettino, A.C.W. Plasma adiponectin levels are markedly elevated in imatinib-treated chronic myeloid leukemia (CML) patients: A mechanism for improved insulin sensitivity in type 2 diabetic CML patients? J. Clin. Endocrinol. Metab. 2010, 95, 3763–3767. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Cao, D.D.; Li, Q.B.; Mei, H.; Hu, Y.; Guo, T. Adipocytes secreted leptin is a pro-tumor factor for survival of multiple myeloma under chemotherapy. Oncotarget 2016, 7, 86075–86086. [Google Scholar] [CrossRef]
- Alexandrakis, M.G.; Passam, F.H.; Sfiridaki, A.; Pappa, C.A.; Moschandrea, J.A.; Kandidaki, E.; Tsirakis, G.; Kyriakou, D.S. Serum Levels of Leptin in Multiple Myeloma Patients and Its Relation to Angiogenic and Inflammatory Cytokines. Int. J. Biol. Markers 2004, 19, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, H.; Li, Y.; Wang, Y.; Xing, R.; Mi, F.; Xiang, C.; Fu, R. Adiponectin inhibits the differentiation and maturation of osteoclasts via the mTOR pathway in multiple myeloma. Int. J. Mol. Med. 2020, 45, 1112–1120. [Google Scholar] [CrossRef]
- Santo, L.; Teras, L.R.; Giles, G.G.; Weinstein, S.J.; Albanes, D.; Wang, Y.; Pfeiffer, R.M.; Lan, Q.; Rothman, N.; Birmann, B.M.; et al. Circulating resistin levels and risk of multiple myeloma in three prospective cohorts. Br. J. Cancer 2017, 117, 1241–1245. [Google Scholar] [CrossRef]
- Thommesen, L.; Stunes, A.K.; Monjo, M.; Grøsvik, K.; Tamburstuen, M.V.; Kjøbli, E.; Lyngstadaas, S.P.; Reseland, J.E.; Syversen, U. Expression and regulation of resistin in osteoblasts and osteoclasts indicate a role in bone metabolism. J. Cell. Biochem. 2006, 99, 824–834. [Google Scholar] [CrossRef]
- Liu, R.; Gao, D.; Lv, Y.; Zhai, M.; He, A. Importance of circulating adipocytokines in multiple myeloma: A systematic review and meta-analysis based on case-control studies. BMC Endocr. Disord. 2022, 22, 29. [Google Scholar] [CrossRef]
- Pang, J.; Shi, Q.; Liu, Z.; He, J.; Liu, H.; Lin, P.; Cui, J.; Yang, J. Resistin induces multidrug resistance in myeloma by inhibiting cell death and upregulating ABC transporter expression. Haematologica 2017, 102, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Zabel, B.A.; Kwitniewski, M.; Banas, M.; Zabieglo, K.; Murzyn, K.; Cichy, J. Chemerin regulation and role in host defense. Am. J. Clin. Exp. Immunol. 2014, 3, 1–19. [Google Scholar]
- Westhrin, M.; Moen, S.H.; Kristensen, I.B.; Buene, G.; Mylin, A.K.; Turesson, I.; Abildgaard, N.; Waage, A.; Standal, T. Chemerin is elevated in multiple myeloma patients and is expressed by stromal cells and pre-adipocytes. Biomark. Res. 2018, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Behnoush, A.H.; Shobeiri, P.; Bahiraie, P.; Amirkhani, N.; Khalaji, A.; Peiman, S. Chemerin levels in chronic kidney disease: A systematic review and meta-analysis. Front. Endocrinol. 2023, 14, 1120774. [Google Scholar] [CrossRef]
- Acchiardo, S.; Kraus, A.P.; Jennings, B.R. β 2-Microglobulin Levels in Patients With Renal Insufficiency. Am. J. Kidney Dis. 1989, 13, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xu, J.; He, J.; Liu, H.; Lin, P.; Wan, X.; Navone, N.M.; Tong, Q.; Kwak, L.W.; Orlowski, R.Z.; et al. Mature adipocytes in bone marrow protect myeloma cells against chemotherapy through autophagy activation. Oncotarget 2015, 6, 34329–34341. [Google Scholar] [CrossRef]
- Sun, S.; Dong, H.; Yan, T.; Li, J.; Liu, B.; Shao, P.; Li, J.; Liang, C. Role of TSP-1 as prognostic marker in various cancers: A systematic review and meta-analysis. BMC Med. Genet. 2020, 21, 139. [Google Scholar] [CrossRef]
- Wu, X.; Guo, J.; Deng, H.; Chen, W. Serum proteome profiling identified thrombospondin-1 and lactoferrin as biomarkers of relapsed multiple myeloma. Front. Med. 2025, 12, 1640245. [Google Scholar] [CrossRef]
- Costa, L.G.; Giordano, G.; Furlong, C.E. Pharmacological and dietary modulators of paraoxonase 1 (PON1) activity and expression: The hunt goes on. Biochem. Pharmacol. 2011, 81, 337–344. [Google Scholar] [CrossRef]
- Klebanoff, S.J. Myeloperoxidase: Friend and foe. J. Leukoc. Biol. 2005, 77, 598–625. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulos, V.; Zinonos, I.; Leach, D.A.; Hay, S.J.; Liapis, V.; Zysk, A.; Ingman, W.V.; DeNichilo, M.O.; Evdokiou, A. Uncovering a new role for peroxidase enzymes as drivers of angiogenesis. Int. J. Biochem. Cell Biol. 2015, 68, 128–138. [Google Scholar] [CrossRef]
- Panagopoulos, V.; Leach, D.A.; Zinonos, I.; Ponomarev, V.; Licari, G.; Liapis, V.; Ingman, W.V.; Anderson, P.; DeNichilo, M.O.; Evdokiou, A. Inflammatory peroxidases promote breast cancer progression in mice via regulation of the tumour microenvironment. Int. J. Oncol. 2017, 50, 1191–1200. [Google Scholar] [CrossRef]
- Oberley, M.J.; Li, S.; Orgel, E.; Wee, P.; Hagiya, A.; O’Gorman, M.R.G. Clinical Significance of Isolated Myeloperoxidase Expression in Pediatric B-Lymphoblastic Leukemia. Am. J. Clin. Pathol. 2017, 147, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.M.D.; Noll, J.E.; Bradey, A.L.; Duggan, J.; Wilczek, V.J.; Masavuli, M.G.; Grubor-Bauk, B.; Panagopoulos, R.A.; Hewett, D.R.; Mrozik, K.M.; et al. Myeloperoxidase creates a permissive microenvironmental niche for the progression of multiple myeloma. Br. J. Haematol. 2023, 203, 614–624. [Google Scholar] [CrossRef]
| Variable | Data | |
|---|---|---|
| Age, median, years (range) | 69 (39–81) | |
| Male/female, n | 23/17 | |
| CRAB criteria, n (%) | ||
| Hypercalcaemia | 6 (15) | |
| Renal failure | 18 (45) | |
| Anemia | 25 (63) | |
| Bone lesion | 27 (68) | |
| Cytogenetics, n (%) | ||
| Standard risk | 26 (65) | |
| High risk | 5 (13) | |
| n/a | 9 (22) | |
| R-ISS stage, n (%) | ||
| I | 9 (22) | |
| II | 11 (28) | |
| III | 10 (25) | |
| n/a | 10 (25) | |
| Previous therapy, n (%) | ||
| Immunomodulatory drug | 37 (93) | |
| Proteasome inhibitor | 38 (95) | |
| Steroid | 40 (100) | |
| Daratumumab | 6 (15) | |
| Cyclophosphamide | 10 (25) | |
| APSCT | 30 (75) | |
| Disease status, n (%) | ||
| CR | 5 (13) | |
| VGPR/PR | 26 (65) | |
| SD | 3 (8) | |
| PD | 6 (15) | |
| Adipokines | ||
| Adiponectin, median, ×107 ug/mL (range) | 6.4 (1.8–22.2) | |
| Leptin, median, ng/mL (range) | 6850 (6535–207,502) | |
| Resistin, median, ng/mL (range) | 7336 (2819–60,272) | |
| Chemerin, median, ng/mL (range) | 125 (48–298) | |
| Adipsin, median, ×106 ug/mL (range) | 3.8 (2.0–12.0) | |
| Thrombospondin-1, median, ug/mL (range) | 4516 (2399–7189) | |
| Paraoxanase-1, median, ng/mL (range) | 316 (153–715) | |
| Myeloperoxidase, median, mg/L (range) | 91 (24–304) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obajed Al-Ali, N.; Csige, D.; Pinczés, L.I.; Farkas, K.; Rebenku, I.; Domján, A.; Panyi, G.; Szekanecz, Z.; Szűcs, G.; Illés, Á.; et al. Adipokines as Prognostic Biomarkers in Multiple Myeloma: A Case–Control Study. Medicina 2025, 61, 2065. https://doi.org/10.3390/medicina61112065
Obajed Al-Ali N, Csige D, Pinczés LI, Farkas K, Rebenku I, Domján A, Panyi G, Szekanecz Z, Szűcs G, Illés Á, et al. Adipokines as Prognostic Biomarkers in Multiple Myeloma: A Case–Control Study. Medicina. 2025; 61(11):2065. https://doi.org/10.3390/medicina61112065
Chicago/Turabian StyleObajed Al-Ali, Nóra, Dóra Csige, László Imre Pinczés, Katalin Farkas, István Rebenku, Andrea Domján, György Panyi, Zoltán Szekanecz, Gabriella Szűcs, Árpád Illés, and et al. 2025. "Adipokines as Prognostic Biomarkers in Multiple Myeloma: A Case–Control Study" Medicina 61, no. 11: 2065. https://doi.org/10.3390/medicina61112065
APA StyleObajed Al-Ali, N., Csige, D., Pinczés, L. I., Farkas, K., Rebenku, I., Domján, A., Panyi, G., Szekanecz, Z., Szűcs, G., Illés, Á., & Váróczy, L. (2025). Adipokines as Prognostic Biomarkers in Multiple Myeloma: A Case–Control Study. Medicina, 61(11), 2065. https://doi.org/10.3390/medicina61112065








