Early Rehabilitation Versus Conventional Approaches in Post-Traumatic Hand Injuries with Multiple Lesions: Clinical Outcomes and Future Directions
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
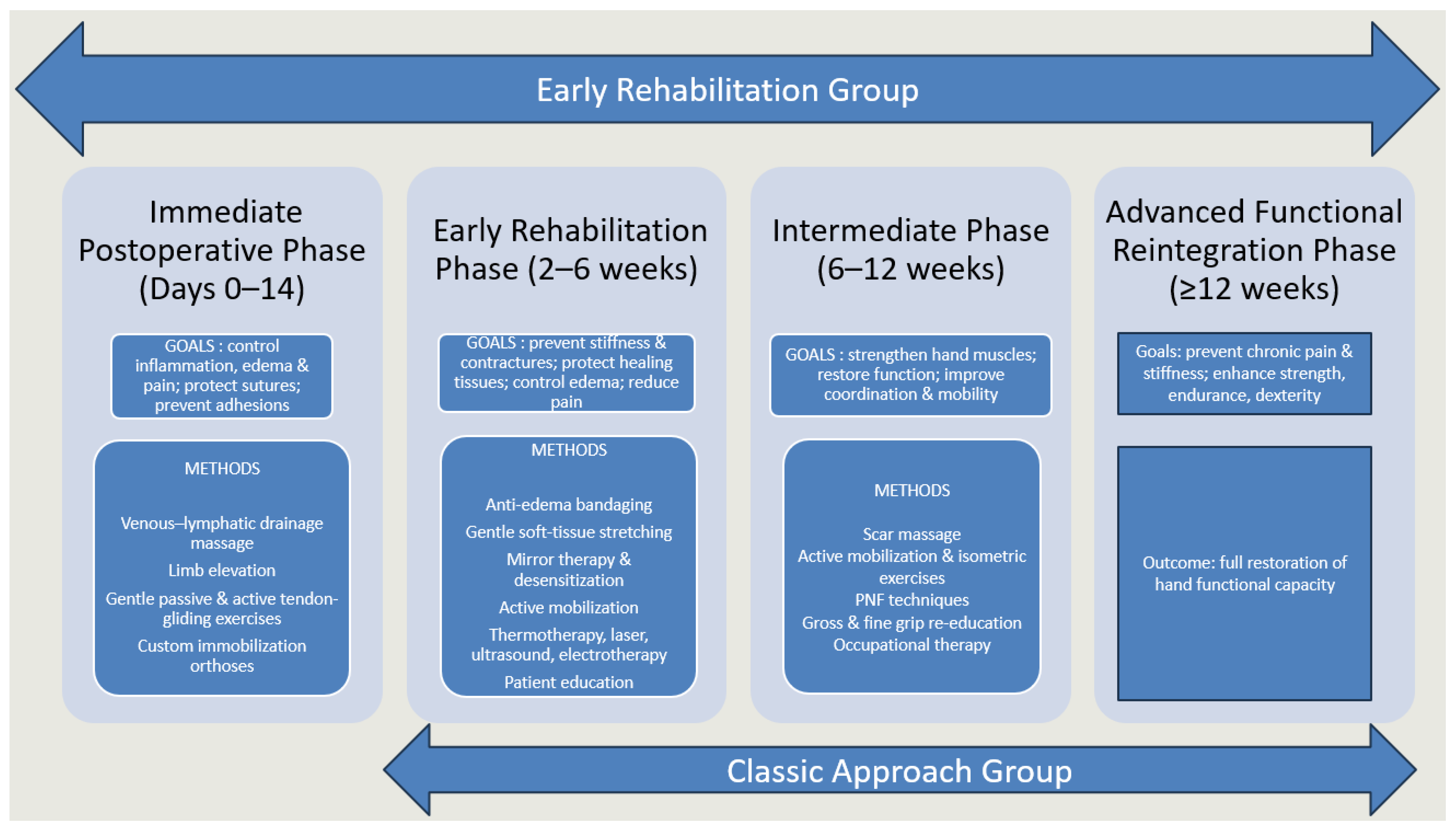
2.2. Therapeutic Protocol
- Immediate postoperative phase (0–14 days): This phase was only applicable to patients who underwent early rehabilitation, initiated shortly after surgical intervention. The priority during the acute phase was to control inflammation, limit edema, protect sutured tissues and prevent complications such as infection, secondary tissue damage or adhesions. Functional goals also included pain reduction and maintenance of mobility in uninvolved joints (elbow, shoulder, cervical spine) to avoid secondary stiffness. Interventions included lymphatic drainage massage (to promote venous and lymphatic return), cryotherapy for edema and pain control, passive and passive-assisted mobilization to maintain joint congruency and early tendon-gliding movements aimed at minimizing adhesions. Customized protective orthoses were used: dorsal blocking splints for flexor tendon repairs, volar blocking splints for extensor injuries and individualized “intrinsic plus” positioning to maintain optimal joint alignment and ligament tension.
- Early rehabilitation phase (2–6 weeks): This phase aimed at preventing stiffness and contractures while allowing controlled mobilization of healing tissues. Techniques focused on maintaining the flexibility of periarticular structures, promoting safe tendon excursion and gradually initiating functional use of the hand. Scar massage and desensitization techniques were introduced as healing permitted, alongside thermotherapy to improve local circulation and tissue pliability. Controlled passive and active-assisted mobilization, tendon-gliding exercises and soft tissue stretching were emphasized to restore motion safely. Complementary modalities such as mirror therapy supported cortical reorganization and pain modulation, while bandaging and physical agents (laser, ultrasound, electrotherapy) enhanced edema reduction and tissue repair. Patient education was an essential component, ensuring adherence to prescribed home exercises and recognition of warning signs (e.g., tendon rupture, excessive inflammation).
- Intermediate phase (6–12 weeks): The objectives in this stage were to consolidate early gains, progressively strengthen the hand and improve dexterity and proprioception. Active free mobilization and isometric strengthening exercises were intensified, while neuroproprioceptive facilitation techniques addressed motor control and coordination. Progressive resistance training and stretching helped restore muscle endurance and joint flexibility. Ergotherapy was introduced to retrain fine and gross motor grips in functional contexts, preparing patients for daily activities. Continued use of customized orthoses was recommended when needed, particularly to maintain joint mobility and alignment or to counteract early stiffness. Mirror therapy and desensitization could be maintained for patients with persistent sensory disturbances.
- Advanced functional reintegration phase (≥12 weeks): The final phase was oriented toward restoring complete functional independence and preventing chronic complications such as pain or stiffness. Rehabilitation progressed to complex, task-oriented exercises requiring force, dexterity and coordination. Activities included advanced strengthening, fine motor training and functional tasks such as gripping, throwing and manipulation, adapted to each patient’s professional and personal needs. Functional reintegration also involved targeted programs for athletes or manual workers, ensuring readiness for high-demand activities. Physical modalities (laser, ultrasound, electrotherapy) and thermoformable orthoses were used selectively in cases of persistent pain, deformity, or instability under stress. The ultimate objective was to reestablish maximal function, allowing safe return to occupational and sports performance while preserving quality of life.

2.3. Evaluation Protocol
2.4. Data Analysis
3. Results
4. Discussion
4.1. Principles of Postoperative Rehabilitation
4.2. Overall Outcomes in the Study Group
4.3. Classic Approach Group (CAG) Analysis
4.4. Early Rehabilitation Group (ERG) Analysis
4.5. Early Versus Classic Rehabilitation: Comparative Findings
4.6. Subgroup Analysis: Flexor Tendon vs. Complex Injuries
4.7. Clinical Implications and Innovative Contributions
4.8. Study Limitations
4.9. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vasdeki, D.; Barmpitsioti, A.; de Leo, A.; Dailiana, Z. How to prevent hand injuries-Review of epidemiological data is the first step in health care management. Injury 2024, 55, 111327. [Google Scholar] [CrossRef] [PubMed]
- Browne, R.; Trockels, A.; Carr, E.; Dolan, R.; Wormald, J.C. Rehabilitation of persistent poor hand function after trauma: A systematic review of randomised controlled trials. Hand Ther. 2025. ahead of print. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arroyo-Berezowsky, C.; Quinzaños-Fresnedo, J. Epidemiology of hand and wrist injuries treated in a reference specialty center over a year. Acta Ortop. Mex. 2021, 35, 429–435. (In English) [Google Scholar] [CrossRef] [PubMed]
- Macrì, M.; Pugliese, P.; Accardo, G.; Clemente, A.; Cecconato, V.; Odella, S.; Tos, P. Current Principles in the Management of a Mangled Hand. J. Hand Surg. Glob. Online 2024, 7, 326–330. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dy, C.J.; Daluiski, A. Update on Zone II Flexor Tendon Injuries. J. Am. Acad. Orthop. Surg. 2014, 22, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Grinsell, D.; Keating, C.P. Peripheral nerve reconstruction after injury: A review of clinical and experimental therapies. BioMed Res. Int. 2014, 2014, 698256. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cacchio, A.; De Blasis, E.; de Blasis, V.; Santilli, V.; Spacca, G. Mirror therapy in complex regional pain syndrome type 1 of the upper limb in stroke patients. Neurorehabilit. Neural Repair 2009, 23, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Dilek, B.; Ayhan, C.; Yagci, G.; Yakut, Y. Effectiveness of the graded motor imagery to improve hand function in patients with distal radius fracture: A randomized controlled trial. J. Hand Ther. 2018, 31, 2–9.e1. [Google Scholar] [CrossRef] [PubMed]
- Yeldan, I.; Huseyınsınoglu, B.E.; Akıncı, B.; Tarakcı, E.; Baybas, S.; Ozdıncler, A.R. The effects of very early mirror therapy on functional improvement of the upper extremity in acute stroke patients. J. Phys. Ther. Sci. 2015, 27, 3519–3524. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Campo-Prieto, P.; Rodríguez-Fuentes, G. Effectiveness of mirror therapy in phantom limb pain: A literature review. Neurologia 2022, 37, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Tofani, M.; Santecchia, L.; Conte, A.; Berardi, A.; Galeoto, G.; Sogos, C.; Petrarca, M.; Panuccio, F.; Castelli, E. Effects of Mirror Neurons-Based Rehabilitation Techniques in Hand Injuries: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 5526. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stonsaovapak, C.; Koonalinthip, N.; Kitisomprayoonkul, W. Efficacy of mirror neuron system-based therapy for rehabilitation of upper limb orthopedic conditions: A systematic review and meta-analysis. PM&R 2024, 17, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, P.; Chang, W.-P.; Fisher, T.F.; Andreae, B. Client buy-in: An essential consideration for graded motor imagery in hand therapy. J. Hand Ther. 2021, 34, 348–350. [Google Scholar] [CrossRef] [PubMed]
- Kleinert, H.E.; Verdan, C. Report of the Committee on Tendon Injuries (International Federation of Societies for Surgery of the Hand). J. Hand Surg. 1983, 8, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Tobler-Ammann, B.C.; Beckmann-Fries, V.; Calcagni, M.; Kämpfen, A.; Schrepfer, L.; Vögelin, E. Outcomes of Primary Flexor Tendon Repairs in Zones 2 and 3: A Retrospective Cohort Study. J. Hand Surg. Glob. Online 2023, 5, 445–453. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, J.S.; Kim, Y.H. Factors associated with limited hand motion after hand trauma. Medicine 2019, 98, e14183. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Żyluk, A.; Janowski, P. Results of the treatment of major, complex hand injuries. Pol. Prz. Chir. 2011, 83, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Bechtol, C.O. Grip test; the use of a dynamometer with adjustable handle spacings. J. Bone Joint Surg. Am. 1954, 36, 820–832. [Google Scholar] [CrossRef] [PubMed]
- Minasian, R.A.; Kuschner, S.H.; Lane, C.S. A review of handgrip strength and its role as a herald of health. Open Orthop. J. 2022, 16, e2201100. [Google Scholar] [CrossRef]
- Lee, S.H.; Gong, H.S. Grip Strength Measurement for Outcome Assessment in Common Hand Surgeries. Clin. Orthop. Surg. 2022, 14, 1–12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mafi, P.; Mafi, R.; Hindocha, S.; Griffin, M.; Khan, W. A systematic review of dynamometry and its role in hand trauma assessment. Open Orthop. J. 2012, 6, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.C. Measurement of feelings using visual analogue scales. Proc. R. Soc. Med. 1969, 62, 989–993. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Navone, P.; Uboldi, F.M.; Domeniconi, G.; Conti, C.; Piscitelli, A.; Nobile, M. Hand and wrist surgery: Study of efficacy about a new way for one-day low-complexity surgery. Ann. Ig. 2015, 27, 784–788. [Google Scholar] [CrossRef]
- Walker, K.L.; Johnson, A.N.; Marchessault, J.A. Dorsal Capsule Interpositional Arthroplasty of the Metacarpophalangeal Joint. Hand 2022, 17, 68–73. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kablanoğlu, S.; Sade, S.I. The relationship between pain and activity participation, quality of life and depression symptoms in traumatic hand injuries. Acta Orthop. Traumatol. Turc. 2025, 59, 146–151. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Van Gorp, B.; Krastman, P.; Kraan, G.; van den Ende, C.; Staal, J.B. Psychometric qualities of the Patient Rated Wrist/Hand Evaluation (PRWHE) in Dutch primary care patients with wrist complaints. BMC Prim. Care 2022, 23, 274. [Google Scholar] [CrossRef]
- Pellecchia, G.L. Figure-of-eight method of measuring hand size: Reliability and concurrent validity. J. Hand Ther. 2003, 16, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Nadar, M.S.; Al-Kandari, D.; Taaqi, M. Reliability of occupational therapy students using the figure-of-eight technique of measuring hand volume. Hong Kong J. Occup. Ther. 2013, 23, 20–25. [Google Scholar] [CrossRef]
- Gebruers, N.; Van Soom, T.; Verbelen, H.; de Vrieze, T. Reliability of jeweler rings and a revised figure of eight circumference protocol for the assessment of finger and hand circumferences. Lymphat. Res. Biol. 2021, 19, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Hudak, P.L.; Amadio, P.C.; Bombardier, C. Development of an upper extremity outcome measure: The DASH (disabilities of the arm, shoulder and hand) [corrected]. Am. J. Ind Med. 1996, 29, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Beaton, D.E.; Wright, J.G.; Katz, J.N. Upper Extremity Collaborative Group. Development of the QuickDASH: Comparison of three item-reduction approaches. J. Bone Jt. Surg. Am. 2005, 87, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Gummesson, C.; Ward, M.M.; Atroshi, I. The shortened disabilities of the arm, shoulder and hand questionnaire (QuickDASH): Validity and reliability based on responses within the full-length DASH. BMC Musculoskelet. Disord. 2006, 7, 44. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kennedy, C.A.; Beaton, D.E.; Smith, P.; Van Eerd, D.; Tang, K.; Inrig, T.; Hogg-Johnson, S.; Linton, D.; Couban, R. Measurement properties of the QuickDASH (disabilities of the arm, shoulder and hand) outcome measure and cross-cultural adaptations of the QuickDASH: A systematic review. Qual. Life Res. 2013, 22, 2509–2547. [Google Scholar] [CrossRef] [PubMed]
- Marinkovic, M.; Janjic, Z.; Nikolic, J. Estimating disability and quality of life after different degrees of hand and forearm trauma. Vojn. Pregl. 2015, 72, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Thuau, F.; Lancien, U.; Tiry, E.; Crenn, V.; Perrot, P. Impact of surgical first excision delay on function for heat-press hand injury by the Quick-DASH questionnaire: Series over 20 years. Burns 2022, 49, 1422–1431. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Janela, D.; Molinos, M.; Moulder, R.G.; Lains, J.; Francisco, G.E.; Bento, V.; Yanamadala, V.; Cohen, S.P.; Correia, F.D. Digital rehabilitation for hand and wrist pain: A single-arm prospective longitudinal cohort study. PAIN Rep. 2022, 7, e1026. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.W.; Löwe, B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch. Intern. Med. 2006, 166, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Castel, L.-C.; Hurst, S.A.; Masmejean, E.; Gregory, T.M. Hand wounds: An analysis of topography and related damage to underlying structures. Injury 2022, 53, 4048–4053. [Google Scholar] [CrossRef] [PubMed]
- Ghițan, A.F.; Gheorman, V.; Pîrvu, D.; Gheorman, V.; Udriștoiu, I.; Ciurea, M.E. A Review of Psychological Outcomes in Patients with Complex Hand Trauma: A Multidisciplinary Approach. Curr. Health Sci. J. 2023, 49, 143–150. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cederlund, R.I.; Ramel, E.; Rosberg, H.E.; Dahlin, L.B. Outcome and clinical changes in patients 3, 6, 12 months after a severe or major hand injury-can sense of coherence be an indicator for rehabilitation focus? BMC Musculoskelet. Disord. 2010, 11, 286. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hansen, A.Ø.; Kristensen, H.K.; Cederlund, R.; Möller, S.; Tromborg, H. An occupation-based intervention in patients with hand-related disorders grouped using the sense of coherence scale-A randomized controlled trial. J Hand Ther. 2020, 33, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, E.A.; Eldesoky, M.T.; Saweres, J.W.; Abdelhaleem, M.D. Effect of Adding Shoulder Stabilization Exercises to the Rehabilitation of Postoperative Hand Injuries in Young Adult Patients. Hand 2024, 20, 778–784. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cantu, C.A.; Myhand, M.; Hazime, A.A.; Yedulla, N.R.; Day, C.S. Patient-Reported Outcomes Can Serve as a Functional Substitute for Grip Strength. J. Wrist Surg. 2023, 13, 427–431. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kazmers, N.H.; Hung, M.; Rane, A.A.; Bounsanga, J.; Weng, C.; Tyser, A.R. Association of Physical Function, Anxiety, and Pain Interference in Nonshoulder Upper Extremity Patients Using the PROMIS Platform. J. Hand Surg. 2017, 42, 781–787. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sorensen, A.A.; Howard, D.; Tan, W.H.; Ketchersid, J.; Calfee, R.P. Minimal clinically important differences of 3 patient-rated outcomes instruments. J. Hand Surg. 2013, 38, 641–649. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beumer, A.; Lindau, T.R. Grip strength ratio: A grip strength measurement that correlates well with DASH score in different hand/wrist conditions. BMC Musculoskelet. Disord. 2014, 15, 336. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Starr, H.M.; Snoddy, M.; Hammond, K.E.; Seiler, J.G., 3rd. Flexor tendon repair rehabilitation protocols: A systematic review. J. Hand Surg. 2013, 38, 1712–1717.e14. [Google Scholar] [CrossRef] [PubMed]
- Galardini, L.; Coppari, A.; Pellicciari, L.; Ugolini, A.; Piscitelli, D.; La Porta, F.; Bravini, E.; Vercelli, S. Minimal Clinically Important Difference of the Disabilities of the Arm, Shoulder and Hand (DASH) and the Shortened Version of the DASH (QuickDASH) in People With Musculoskeletal Disorders: A Systematic Review and Meta-Analysis. Phys. Ther. 2024, 104, pzae033. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kadakia, Z.; Lansang, R.P.; Ball, P.; Kuspinar, A.; VanderKaay, S.; Packham, T. Reliability, validity and responsiveness of composite finger flexion in patients with traumatic hand injuries: A clinical measurement study. J. Hand Ther. 2024, 37, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Saini, N.; Kundnani, V.; Patni, P.; Gupta, S.P. Outcome of early active mobilization after flexor tendons repair in zones II–V in hand. Indian J. Orthop. 2010, 44, 314–321. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Renberg, M.; Svingen, J.; Arner, M.; Farnebo, S. Range of Motion Following Flexor Tendon Repair: Comparing Active Flexion and Extension with Passive Flexion Using Rubber Bands Followed by Active Extension. J. Hand Surg. 2024, 49, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.K.; Jerosch-Herold, C.; Shepstone, L. Effectiveness of edema management techniques for subacute hand edema: A systematic review. J. Hand Ther. 2017, 30, 432–446. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ladds, E.; Redgrave, N.; Hotton, M.; Lamyman, M. Systematic review: Predicting adverse psychological outcomes after hand trauma. J. Hand Ther. 2017, 30, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Tubiana, R. Early mobilization of fractures of the metacarpals and phalanges. Ann. Chir. Main 1983, 2, 293–297, (In English and French). [Google Scholar] [CrossRef] [PubMed]
- Margles, S.W. Early motion in the treatment of fractures and dislocations in the hand and wrist. Hand Clin. 1996, 12, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Hung, L.; Pang, K.; Yeung, P.; Cheung, L.; Wong, J.; Chan, P. Active mobilisation after flexor tendon repair: Comparison of results following injuries in zone 2 and other zones. J. Orthop. Surg. 2005, 13, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Hundozi, H.; Murtezani, A.; Hysenaj, V.; Hysenaj, V.; Mustafa, A. Rehabilitation after surgery repair of flexor tendon injuries of the hand with Kleinert early passive mobilization protocol. Med Arch. 2013, 67, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.E.; Jha, B.; Ross, M. Rehabilitation following surgery for flexor tendon injuries of the hand. Cochrane Database Syst. Rev. 2017, 2017, CD012479. [Google Scholar] [CrossRef] [PubMed Central]
- Chevalley, S.; Tenfält, M.; Åhlén, M.; Strömberg, J. Passive Mobilization with Place and Hold Versus Active Motion Therapy After Flexor Tendon Repair: A Randomized Trial. J. Hand Surg. 2022, 47, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Chesney, A.; Chauhan, A.; Kattan, A.; Farrokhyar, F.; Thoma, A. Systematic review of flexor tendon rehabilitation protocols in zone II of the hand. Plast. Reconstr. Surg. 2011, 127, 1583–1592. [Google Scholar] [CrossRef] [PubMed]
- Skirven, T.M.; DeTullio, L.M. Therapy after Flexor Tendon Repair. Hand Clin. 2023, 39, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Gibson, P.D.; Sobol, G.L.; Ahmed, I.H. Zone II Flexor Tendon Repairs in the United States: Trends in Current Management. J. Hand Surg. 2017, 42, e99–e108. [Google Scholar] [CrossRef] [PubMed]
- Karacam, S.; Gultekinler, B.D. Flexor tendon repair rehabilitation in Turkiye, therapists’ current management trends: A cross-sectional survey study. BMC Musculoskelet. Disord. 2025, 26, 705. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’BRien, L.; Robinson, L.; Parsons, D.; Glasgow, C.; McCarron, L. Hand therapy role in return to work for patients with hand and upper limb conditions. J. Hand Ther. 2022, 35, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Karunadasa, K. Management of the injured hand: Basic principles in rehabilitation. Sri Lanka J. Surg. 2015, 33, 25. [Google Scholar] [CrossRef]
- Chevalley, S.; Wängberg, V.; Åhlén, M.; Strömberg, J.; Björkman, A. Passive Mobilization with Place-and-Hold Versus Active Mobilization Therapy After Flexor Tendon Repair: 5-Year Minimum Follow-Up of a Randomized Controlled Trial. J. Hand Surg. 2024, 49, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Puderbaugh, M.; Emmady, P.D. Neuroplasticity. [Updated 2023 May 1]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557811/ (accessed on 28 September 2025).
- Westlake, K.P.; Byl, N.N. Neural plasticity and implications for hand rehabilitation after neurological insult. J. Hand Ther. 2013, 26, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.; Hollis, E.R., 2nd. Cortical Reorganization of Sensorimotor Systems and the Role of Intracortical Circuits After Spinal Cord Injury. Neurotherapeutics 2018, 15, 588–603. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Capriotti, A.; Moret, S.; Del Bello, E.; Federici, A.; Lucertini, F. Virtual Reality: A New Frontier of Physical Rehabilitation. Sensors 2025, 25, 3080. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Farsi, A.; Cerone, G.L.; Falla, D.; Gazzoni, M. Emerging Applications of Augmented and Mixed Reality Technologies in Motor Rehabilitation: A Scoping Review. Sensors 2025, 25, 2042. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, C.; Li, J.; Zeng, L.; Zhang, P. Effect of different modalities of artificial intelligence rehabilitation techniques on patients with upper limb dysfunction after stroke-A network meta-analysis of randomized controlled trials. Front. Neurol. 2023, 14, 1125172. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bressler, M.; Merk, J.; Gohlke, T.; Kayali, F.; Daigeler, A.; Kolbenschlag, J.; Prahm, C. A Virtual Reality Serious Game for the Rehabilitation of Hand and Finger Function: Iterative Development and Suitability Study. JMIR Serious Games 2024, 12, e54193. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Banyai, A.D.; Brișan, C. Robotics in Physical Rehabilitation: Systematic Review. Healthcare 2024, 12, 1720. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amirbekova, M.; Kispayeva, T.; Adomaviciene, A.; Eszhanova, L.; Bolshakova, I.; Ospanova, Z. Systematic review and meta-analysis of effectiveness of robotic therapy in the recovery of motor functions after stroke. Front. Hum. Neurosci. 2025, 19, 1622661. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gherman, B.; Zima, I.; Vaida, C.; Tucan, P.; Pisla, A.; Birlescu, I.; Machado, J.; Pisla, D. Robotic Systems for Hand Rehabilitation—Past, Present and Future. Technologies 2025, 13, 37. [Google Scholar] [CrossRef]
- Pitzalis, R.F.; Park, D.; Caldwell, D.G.; Berselli, G.; Ortiz, J. State of the Art in Wearable Wrist Exoskeletons Part I: Background Needs and Design Requirements. Machines 2023, 11, 458. [Google Scholar] [CrossRef]
- Chen, B.; Wu, Y.; Ni, X.; Qin, L.; Li, E. Robotic hand exoskeletons for rehabilitation and assistance: A state-of-the-art. J. Med Devices 2025, 19, 010802. [Google Scholar] [CrossRef]
- Feng, S.; Tang, M.; Huang, G.; Wang, J.; He, S.; Liu, D.; Gu, L. EMG biofeedback combined with rehabilitation training may be the best physical therapy for improving upper limb motor function and relieving pain in patients with the post-stroke shoulder-hand syndrome: A Bayesian network meta-analysis. Front. Neurol. 2023, 13, 1056156. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Y.K.; Erickson, J.O.; Garries, J.M.; Arabian, A.K.; Boone, D.A.; Bunnell, A.E. 14. A Pilot Trial of Home-based Hand Therapy Using Surface Electromyography. Plast. Reconstr. Surg. Glob. Open 2025, 13 (Suppl. S1), 9–10. [Google Scholar] [CrossRef] [PubMed Central]
- Liu, Y.M.; Silva, R.M.L.M.; Friedrich, J.B.M.; Kao, D.S.; Mourad, P.D.; Bunnell, A.E. Surface Electromyography-Driven Therapeutic Gaming for Rehabilitation of Upper Extremity Weakness: A Pilot Study. Plast. Reconstr. Surg. 2022, 150, 125–131. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ni, L.; Yao, Z.; Zhao, Y.; Zhang, T.; Wang, J.; Li, S.; Chen, Z. Electrical stimulation therapy for peripheral nerve injury. Front. Neurol. 2023; 14, 1081458. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kennedy, V.; Long, M.D.; Walters, J.; Adewuyi, A.A.; Franz, C.K. Applications of advances in therapeutic electrical stimulation techniques and technologies in precision peripheral nerve repair: A narrative review. Adv. Technol. Neurosci. 2025, 2, 97–101. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Coroneos, C.J.; Levis, C.; Willand, M.P.; So, K.J.W.; Bain, J.R. Pilot Study: A Multicenter, Prospective Study Demonstrating Safety, Usability, and Feasibility of Perioperative 1-hour Electrical Stimulation Therapy for Enhancing Peripheral Nerve Regeneration. Plast. Reconstr. Surg.-Glob. Open 2025, 13, e6729. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Waldburger, L.; Schaller, R.; Furthmüller, C.; Schrepfer, L.; Schaefer, D.J.; Kaempfen, A. 3D-Printed Hand Splints versus Thermoplastic Splints: A Randomized Controlled Pilot Feasibility Trial. Int. J. Bioprinting 2021, 8, 474. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Devanand, D.B.; Gardiner, M.D.; Kedgley, A.E. A Compact Orthosis Compliance Monitoring Device Using Pressure Sensors and Accelerometers: Design and Proof-of-Concept Testing. Sensors 2025, 25, 1352. [Google Scholar] [CrossRef] [PubMed]
| Parameter Evaluated | Assessment Tool/Method | Details |
|---|---|---|
| 1. Active Range of Motion (AROM) | Total Active Motion (TAM) Score | Sum of MCP, PIP, and DIP flexion minus total extension deficit; bilateral measurement with digital goniometer. |
| 2. Grip Strength (GS) | Jamar Hydraulic Hand Dynamometer | Standard ASHT protocol; three maximal 5 s contractions per hand; highest value recorded. |
| 3. Pain Intensity | Visual Analogue Scale (VAS) | 10 cm horizontal line from “no pain” to “worst imaginable pain”; patient marked pain level from last 24 h. |
| 4. Edema (EDM) | Figure-of-Eight Measurement | Standardized tape measurement route around hand joints; reliable for hand swelling assessment. |
| 5. Hand Function | QuickDASH Questionnaire (QD) | 11 items rated 0–5; assesses difficulty in daily activities and functional limitations. |
| 6. Anxiety Level | Generalized Anxiety Disorder Questionnaire (GAD-7) | 7 items evaluating anxiety symptoms in the past 2 weeks; facilitates early detection of psychological impact. |
| Total | ERG | CAG | |
|---|---|---|---|
| Mean Age (years) ± SD | 47.7 ± 14.9 | 50.2 ± 15.6 | 41.7 ± 11.2 |
| Male | 86.70% | 89.10% | 80.80% |
| Female | 13.30% | 10.90% | 19.20% |
| Sharp injury | 20.00% | 21.90% | 15.40% |
| Crush injury | 7.80% | 4.70% | 15.40% |
| Avulsion | 4.40% | 1.60% | 11.50% |
| Combined mechanism | 67.80% | 71.90% | 57.70% |
| Vascular injury | 54.40% | 50.00% | 65.40% |
| Nerve injury | 55.60% | 51.60% | 65.40% |
| Fracture | 68.90% | 68.80% | 69.20% |
| Flexor tendon injury | 71.10% | 65.60% | 84.60% |
| Extensor tendon injury | 64.40% | 64.10% | 65.40% |
| Parameter | Group | Baseline (Mean ± SD) | 4 Weeks (Mean ± SD) | 12 Weeks (Mean ± SD) | p-Value |
|---|---|---|---|---|---|
| VAS | Total | 8.91 ± 1.85 | 5.24 ± 1.98 | 2.13 ± 1.76 | <0.001 |
| CAG | 7.46 ± 2.64 | 5.15 ± 2.47 | 3.08 ± 2.10 | ||
| ERG | 9.50 ± 0.94 | 5.28 ± 1.76 | 1.75 ± 1.45 | ||
| EDM | Total | 61.48 ± 7.18 | 60.23 ± 6.98 | 59.09 ± 6.76 | <0.001 |
| CAG | 61.31 ± 8.05 | 59.58 ± 7.58 | 58.23 ± 7.24 | ||
| ERG | 61.55 ± 6.87 | 60.50 ± 6.76 | 59.44 ± 6.59 | ||
| GS | Total | 1.06 ± 3.15 | 3.77 ± 5.23 | 13.14 ± 8.69 | <0.001 |
| CAG | 2.52 ± 5.43 | 6.57 ± 7.94 | 14.69 ± 13.28 | ||
| ERG | 0.47 ± 1.05 | 2.64 ± 3.01 | 12.51 ± 5.95 | ||
| QD | Total | 85.08 ± 10.88 | 71.65 ± 18.18 | 35.54 ± 24.60 | <0.001 |
| CAG | 82.10 ± 13.28 | 67.82 ± 23.92 | 47.98 ± 28.84 | ||
| ERG | 86.24 ± 9.66 | 73.14 ± 15.36 | 30.68 ± 21.03 | ||
| GAD-7 | Total | 18.31 ± 4.05 | 14.32 ± 5.64 | 7.92 ± 6.59 | <0.001 |
| CAG | 17.31 ± 5.75 | 14.65 ± 6.02 | 10.04 ± 6.00 | ||
| ERG | 18.72 ± 3.07 | 14.19 ± 5.53 | 7.06 ± 6.67 | ||
| TAM | Total | 35.92 ± 26.63 | 49.08 ± 26.55 | 70.51 ± 24.22 | <0.001 |
| CAG | 21.70 ± 24.58 | 39.22 ± 28.80 | 58.13 ± 29.18 | ||
| ERG | 41.70 ± 25.39 | 53.08 ± 24.70 | 75.53 ± 20.05 |
| Group/Dif VAS I-II | Mean ± SD | p |
|---|---|---|
| CAG (p = 0.151) | 2.3 ± 1.43 | <0.001 |
| ETG (p = 0.001) | 4.21 ± 1.71 | |
| Group/Dif VAS I-III | Mean ± SD | p |
| CAG (p = 0.090) | 4.38 ± 2.11 | <0.001 |
| ERG (p = 0.003) | 7.75 ± 1.35 | |
| Group/Dif EDM I-II | Mean ± SD | p |
| Classic (p = 0.010) | 1.73 ± 1.18 | 0.004 |
| Early (p < 0.001) | 1.05 ± 0.51 | |
| Group/Dif EDM I-III | Mean ± SD | p |
| CAG (p = 0.166) | 3.08 ± 1.81 | 0.007 |
| ERG (p < 0.001) | 2.11 ± 1 | |
| Group/Dif GS I-II | Mean ± SD | p |
| CAG (p = 0.001) | −4.06 ± 4.3 | 0.061 |
| ERG (p < 0.001) | −2.17 ± 2.5 | |
| Group/Dif GS I-III | Mean ± SD | p |
| CAG (p = 0.025) | −12.17 ± 10.9 | 0.487 |
| ERG (p = 0.436) | −12 ± 5.55 | |
| Group/Dif QD I-II | Mean ± SD | p |
| CAG (p = 0.001) | 14.38 ± 13.4 | 0.456 |
| ERG (p < 0.001) | 13.1 ± 10 | |
| Group/Dif QD I-III | Mean ± SD | p |
| CAG (p = 0.001) | 34.11 ± 22.2 | <0.001 |
| ERG (p = 0.001) | 55.56 ± 17 | |
| Group/Dif GAD-7 I-II | Mean ± SD | p |
| CAG (p = 0.001) | 2.65 ± 2.92 | 0.011 |
| ERG (p = 0.003) | 4.53 ± 3.18 | |
| Group/Dif GAD-7 I-III | Mean ± SD | p |
| CAG (p = 0.367) | 7.27 ± 4.73 | <0.001 |
| ERG (p < 0.001) | 11.66 ± 5.21 | |
| Group/Dif TAM I-II | Mean ± SD | p |
| CAG (p = 0.174) | −17.53 ± 11.98 | 0.016 |
| ERG (p < 0.001) | −11.38 ± 8.66 | |
| Group/Dif TAM I-III | Mean ± SD | p |
| CAG (p = 0.048) | −36.43 ± 22.19 | 0.862 |
| ERG (p = 0.002) | −33.84 ± 16.16 |
| Parameter | Time Point | Flexor Tendon Injuries (Mean ± SD) | Other Complex Hand Injuries (Mean ± SD) | p-Value |
|---|---|---|---|---|
| VAS | Baseline | 9.05 ± 1.2 | 9.72 ± 0.7 | <0.001 |
| 4 weeks | 5.38 ± 1.62 | 5.23 ± 1.85 | ||
| 12 weeks | 2 ± 1.58 | 1.63 ± 1.39 | ||
| EDM | Baseline | 58.48 ± 7 | 63.05 ± 6.36 | <0.001 |
| 4 weeks | 57.43 ± 6.87 | 62 ± 6.25 | ||
| 12 weeks | 56.43 ± 6.78 | 60.91 ± 6.04 | ||
| GS | Baseline | 0.33 ± 0.79 | 0.53 ± 1.16 | <0.001 |
| 4 weeks | 3.14 ± 2.74 | 2.39 ± 3.14 | ||
| 12 weeks | 12.33 ± 5.52 | 12.6 ± 6.21 | ||
| QD | Baseline | 81.65 ± 11.75 | 88.48 ± 7.65 | <0.001 |
| 4 weeks | 67.6 ± 14.52 | 75.85 ± 15.18 | ||
| 12 weeks | 27.69 ± 19.8 | 32.15 ± 21.68 | ||
| GAD-7 | Baseline | 16.71 ± 3.6 | 19.7 ± 2.24 | <0.001 |
| 4 weeks | 10.57 ± 5.46 | 15.95 ± 4.68 | ||
| 12 weeks | 4.38 ± 5.39 | 8.37 ± 6.89 | ||
| TAM | Baseline | 35.81 ± 26 | 44.57 ± 24.85 | <0.001 |
| 4 weeks | 52.13 ± 24.9 | 53.54 ± 24.88 | ||
| 12 weeks | 77.6 ± 20 | 74.51 ± 20.2 |
| Injury/Dif VAS I-II | Mean ± SD | p |
|---|---|---|
| Flexor (p = 0.029) | 3.67 ± 1.19 | 0.136 |
| Other (p = 0.012) | 4.49 ± 1.87 | |
| Injury/Dif VAS I-III | Mean ± SD | p |
| Flexor (p = 0.053) | 7.05 ± 1.07 | 0.004 |
| Other (p = 0.006) | 8.09 ± 1.36 | |
| Injury/Dif EDM I-II | Mean ± SD | p |
| Flexor (p < 0.001) | 1.05 ± 0.49 | 1.000 |
| Other (p < 0.001) | 1.05 ± 0.53 | |
| Injury/Dif EDM I-III | Mean ± SD | p |
| Flexor (p = 0.064) | 2.05 ± 1.2 | 0.668 |
| Other (p < 0.001) | 2.14 ± 0.89 | |
| Injury/Dif GS I-II | Mean ± SD | p |
| Flexor (p = 0.020) | −2.81 ± 2.4 | 0.039 |
| Other (p < 0.001) | −1.86 ± 2.51 | |
| Injury/Dif GS I-III | Mean ± SD | p |
| Flexor (p = 0.458) | −12 ± 5.45 | 0.070 |
| Other (p = 0.438) | −12.07 ± 5.65 | |
| Injury/Dif QD I-II | Mean ± SD | p |
| Flexor (p = 0.015) | 14.07 ± 8.14 | 0.246 |
| Other (p < 0.001) | 12.63 ± 10.95 | |
| Injury/Dif QD I-III | Mean ± SD | p |
| Flexor (p = 0.985) | 53.96 ± 13.65 | 0.232 |
| Other (p < 0.001) | 56.33 ± 18.52 | |
| Injury/Dif GAD-7 I-II | Mean ± SD | p |
| Flexor (p = 0.460) | 6.14 ± 2.79 | 0.006 |
| Other (p = 0.003) | 3.74 ± 3.08 | |
| Injury/Dif GAD-7 I-III | Mean ± SD | p |
| Flexor (p = 0.005) | 12.33 ± 3.83 | 0.874 |
| Other (p < 0.001) | 11.33 ± 5.78 | |
| Injury/Dif TAM I-II | Mean ± SD | p |
| Flexor (p = 0.020) | −16.32 ± 10.8 | 0.003 |
| Other (p = 0.016) | −8.97 ± 6.23 | |
| Injury/Dif TAM I-III | Mean ± SD | p |
| Flexor (p = 0.143) | −41.82 ± 16.28 | 0.004 |
| Other (p = 0.002) | −29.94 ± 14.77 |
| Technology Type | Working Mode/Functionality Addressed | Advantages | Limitations/Challenges |
|---|---|---|---|
| Virtual/Augmented Reality (VR/AR) | Immersive, task-specific environments; real-time visual and kinematic feedback; gamified training. | Enhances engagement and motivation; enables high-repetition practice; supports pain modulation (phantom pain, CRPS). | Requires equipment and technical support; risk of cybersickness; transfer to daily activities still variable. |
| Robotics | End-effector systems guiding hand/arm movement; repetitive assist-as-needed training. | Increases therapy volume; ensures consistent dosing; provides objective data on performance. | High cost; bulky devices; limited availability in smaller centers; ADL transfer not always demonstrated. |
| Exoskeletons (Soft/Hard) | Wearable devices assisting or resisting finger/wrist motion. | Portable (especially soft devices); adaptable assistance; potential for home-based use. | Comfort, durability, and cost remain barriers; clinical evidence still limited. |
| Surface EMG (sEMG) Biofeedback | Records muscle activity; provides visual/game feedback; trains selective recruitment and control. | Improves voluntary activation; supports cortical reorganization; feasible for home-based training. | Requires patient understanding and calibration; may be less effective in cases with very weak or absent signals. |
| Brief Therapeutic Electrical Stimulation (TES) | Short intra-/post-operative nerve stimulation to accelerate regeneration. | Enhances axonal regrowth; improves motor/sensory recovery after nerve repair; only one session needed. | Currently experimental in many centers; requires surgical integration; long-term clinical data still emerging. |
| Orthoses (3D-printed, dynamic) | Custom immobilization or guided motion; scan-to-fit printing. | Lightweight, ventilated, higher comfort/compliance; customizable with hinges/sensors. | Access to 3D printing technology; reprinting needed for swelling changes; not yet universally available. |
| Telerehabilitation/Remote rehabilitation | Remote supervision via video, digital exercise platforms, and sensors. | Expands access; maintains adherence; outcomes comparable to in-person in selected conditions. Exercise program can be performed on a touchscreen tablet-based app in combination with face-to-face physiotherapy | Limited for complex cases needing hands-on therapy; digital literacy and internet access required. |
| Sensory-Motor Re-education | Tactile discrimination, mirror therapy, graded motor imagery. | Improves sensory recovery after nerve trauma; addresses cortical remapping; helpful for phantom pain. | Time-intensive; variable efficacy across studies; requires high patient engagement. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serban, A.; Grosu-Bularda, A.; Bordeanu-Diaconescu, E.-M.; Tache, G.-O.; Stoica, M. Early Rehabilitation Versus Conventional Approaches in Post-Traumatic Hand Injuries with Multiple Lesions: Clinical Outcomes and Future Directions. Medicina 2025, 61, 2063. https://doi.org/10.3390/medicina61112063
Serban A, Grosu-Bularda A, Bordeanu-Diaconescu E-M, Tache G-O, Stoica M. Early Rehabilitation Versus Conventional Approaches in Post-Traumatic Hand Injuries with Multiple Lesions: Clinical Outcomes and Future Directions. Medicina. 2025; 61(11):2063. https://doi.org/10.3390/medicina61112063
Chicago/Turabian StyleSerban, Adriana, Andreea Grosu-Bularda, Eliza-Maria Bordeanu-Diaconescu, Georgiana-Ozana Tache, and Marius Stoica. 2025. "Early Rehabilitation Versus Conventional Approaches in Post-Traumatic Hand Injuries with Multiple Lesions: Clinical Outcomes and Future Directions" Medicina 61, no. 11: 2063. https://doi.org/10.3390/medicina61112063
APA StyleSerban, A., Grosu-Bularda, A., Bordeanu-Diaconescu, E.-M., Tache, G.-O., & Stoica, M. (2025). Early Rehabilitation Versus Conventional Approaches in Post-Traumatic Hand Injuries with Multiple Lesions: Clinical Outcomes and Future Directions. Medicina, 61(11), 2063. https://doi.org/10.3390/medicina61112063






